Abstract
Food intake occurs in bouts or meals, and numerous meal-generated signals have been identified that act to limit the size of ongoing meals. Hormones such as cholecystokinin (CCK) are secreted from the intestine as ingested food is being processed, and in addition to aiding the digestive process, they provide a signal to the brain that contributes to satiation, limiting the size of the meal. The potency of CCK to elicit satiation is enhanced by elevated levels of adiposity signals such as insulin. In the present experiments we asked whether CCK and insulin interact at the level of the blood-brain barrier (BBB). We first isolated rat brain capillary endothelial cells that comprise the BBB and found that they express the mRNA for both the CCK1R and the insulin receptor, providing a basis for a possible interaction. We then administered insulin intraperitoneally to another group of rats and 15 min later administered CCK-8 intraperitoneally to half of those rats. After another 15 min, CSF and blood samples were obtained and assayed for immunoreactive insulin. Plasma insulin was comparably elevated above baseline in both the CCK-8 and control groups, indicating that the CCK had no effect on circulating insulin levels given these parameters. In contrast, rats administered CCK had CSF-insulin levels that were more than twice as high as those of control rats. We conclude that circulating CCK greatly facilitates the transport of insulin into the brain, likely by acting directly at the BBB. These findings imply that in circumstances in which the plasma levels of both CCK and insulin are elevated, such as during and soon after meals, satiation is likely to be due, in part, to this newly-discovered synergy between CCK and insulin.
Keywords: Blood-brain barrier, cholecystokinin receptor, CNS insulin transport, satiation, endocrinology, CCK-8
1. INTRODUCTION
Energy intake is orchestrated by numerous signals including those that provide information about the quantity and composition of ingested food (satiation signals), those that report the amount of stored energy (adiposity signals), and nutrients, themselves, that are present in the circulation and extracellular fluid. All of these signals, in turn, converge on circuits in the brain where they are integrated with cognitive, social, hedonic and other situational factors. The calculus of all of this information is complex and provides ample opportunity for synergy, interference or other interactions among the various signals.
Satiation signals arise in the gastrointestinal tract and are secreted acutely in proportion to the quantity and quality of food being consumed; they signal to the brain neurally and hormonally and thereby contribute to meal termination as ingested calories accumulate. Several gastrointestinal peptides/hormones are included in this category including cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), members of the bombesin family of peptides, peptide YY (PYY), amylin, apolipoprotein A-IV and many others (1–4). The exogenous administration of any of these just prior to a meal thus provides a false signal to the brain, implying that more calories have been eaten than actually have been consumed, with the result that a smaller-than-normal meal is consumed. CCK is the most-investigated satiation signal, and its isoforms, such as CCK-8, reduce meal size dose-dependently in experimental animals (5–7). Humans administered CCK-8 report feeling fuller or more satiated, and they eat a smaller meal than occurs in the control condition, without feeling ill (8,9). The physiological relevance of CCK during consumption of a normal meal is demonstrated by the observation that animals eat larger-than-normal meals when administered antagonists of the CCK-1 receptor, implying that endogenous CCK normally contributes to limiting meal size (10,11).
Adiposity signals, including insulin and leptin, are secreted in direct proportion to the amount of fat stored in the body (12,13). Each of these hormones is transported from the blood into the brain, and the administration of either directly into the brain causes animals and humans to eat less; if insulin or leptin is administered chronically into the brain, body weight is also reduced (14–16). The physiological relevance of insulin and leptin in this regard is demonstrated when their respective signals in the brain are blocked by pharmacological or genetic means. In both instances, individuals become hyperphagic and carry more fat than controls (17–19).
Satiation signals and adiposity signals are catabolic, eliciting a net decrease of bodily energy reserves through combinations of reduced energy intake and increased energy expenditure, favoring weight loss (20). An important question is whether or how these various catabolic signals interact to influence food intake. CCK secreted from the duodenum in response to ingested food acts locally in the wall of the intestine to stimulate CCK-1 receptors (CCK-1R) expressed on branches of the vagus nerve that project to the nucleus of the solitary (NST) tract in the hindbrain (21–24). The vagal CCK signal then activates a complex circuit that forwards the satiating signal to several brain areas including the hypothalamus. Severing the vagus nerve proximal to the duodenum, or selectively cutting vagal afferent fibers entering the hindbrain, greatly reduces the ability of exogenous CCK to reduce meal size (24,25).
We and others have found that when insulin is elevated in the brain of animals prior to a meal, the ability of exogenous CCK to reduce meal size is dose-dependently increased (26,27). Similarly, an increased leptin signal also renders animals more sensitive to satiation signals (28,29). The implication is that there is normally a cooperative catabolic action caused by combinations of satiation and adiposity signals. Thus, when an individual has gained weight/fat, this consequently leads to increased levels of circulating insulin, leptin and other adiposity signals that then enter and stimulate the brain. Homeostatic models of body weight regulation would suggest that the individual should eat smaller meals in order to reduce weight to normal. Consistent with this, the elevated adiposity signal in the brain renders the individual more sensitive to meal-generated satiation signals with the consequence that they do in fact consume smaller meals (12,30). In theory, adiposity signals could interact with satiation signals at any point along the circuit from the vagal afferent nerves to the NST to other brain areas.
While all of these examples imply that the interactions among different classes of catabolic signals occurs mainly in the brain, there are other, not mutually exclusive, possibilities as well. Cano et al. reported that exogenous CCK increases the transport of leptin into the CNS (31,32). In the present series of experiments, we demonstrate that CCK also increases the transport of insulin into the CNS and we investigate the mechanism mediating this effect.
2. MATERIALS AND METHODS
2.1. Animals
Adult male Long-Evans rats (12-14 wk of age; Envigo, Indianapolis, IN) were housed individually in tub cages and maintained on a 12/12-h light/dark cycle. Animals were provided ad-libitum access to water and pelleted chow (LabDiet® # TD7012), except where otherwise specified. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.2. Isolation of rat brain microvessels
The brain capillary endothelial cells that comprise the BBB express insulin receptors (13,33,34). Because insulin is too large to passively diffuse through the BBB, the generally-accepted model of insulin transport involves insulin binding its receptors at the BBB (30,33,35), with the bound insulin receptors then being endocytosed within the endothelial cells (35). Following receptor-mediated transport to the abluminal side (brain-side) of the capillary, insulin is then released intact into the CNS (35–37). To determine the feasibility of an interaction of CCK and insulin at the level of the BBB, we first asked whether brain capillary cells express CCK-1 receptors.
Brain microvessels were isolated as previously described (33). Rats were quickly and deeply anesthetized with isoflurane and were sacrificed after rapid collection of the whole brain. After allowing the brain to quickly cool to 4°C in chilled M199 buffer (Gibco, #11150) on ice for ~10-15 min, meninges were removed. Forebrains were isolated and individually homogenized with 10 strokes in a Dounce homogenizer in ~4 mL M199 buffer. Each homogenate was then resuspended in a solution containing a final concentration of 20% dextran (40,000 M.W., Sigma, #31390). Samples were centrifuged at 3,000 × g for 10 min, yielding a pure pellet of microvessels at the bottom of the tube. To isolate microvessels with capillary diameters within a size range of 20-100 microns, pelleted vessels were isolated, resuspended in M199 buffer with 1% BSA, and filtered through a 100-micron nylon mesh (Small Parts) with flow-through then being collected in 20-micron nylon mesh (Millipore). Microvessels that were retained in the filter were then rinsed several times with 1% BSA and briefly vortexed in M199 buffer until detached. After centrifuging the microvessels for 10 min at 1,000 × g and isolation of the pellets, qPCR and Western Blotting or immunohistochemistry were conducted.
2.3. Analysis of brain microvessels
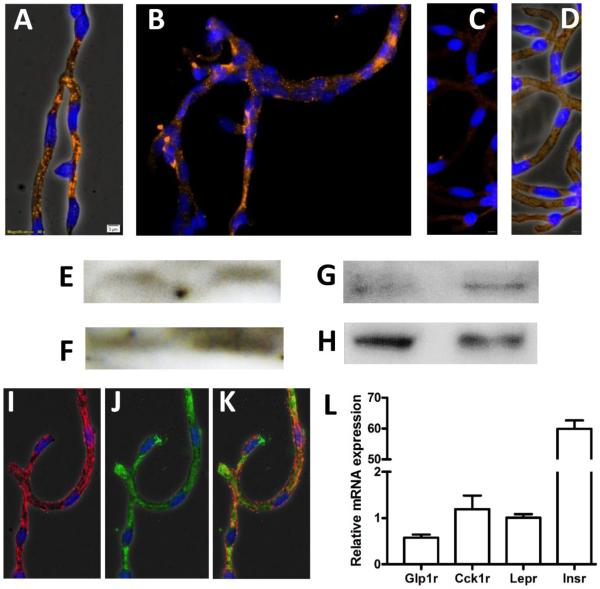
The relative purity of microvessel isolates was first verified with qPCR (TaqMan) using a StepOne™ Plus device (Thermo Scientific), by screening isolates for markers of microvessel mRNA and assessing the extent of contamination from glia and neurons, as described (33). β-actin was used as the reference housekeeping gene. Only samples that were significantly enriched to a comparable extent with previously-defined cutoffs (33) were used for Western blotting (Fig 1). Relative purity of the brain microvessel isolates was further assessed by visual inspection and by immunohistochemistry with a rat-BBB antibody, as described (33). Briefly, aliquots of the fresh microvessel samples were directly spread onto glass slides. Samples were fixed in 4% paraformaldehyde/PBS for 10 min and then washed 5 times in PBS (30 min). After permeabilization with 0.1% Triton in PBS (30 min), rinsing 3 times in PBS (30 min), and blocking in 2% serum (30 min, 26°C), microvessels were incubated in primary antibody for 24 h at 4° C. Antibodies used were targeted to the CCK-1R (Santa Cruz, sc-16172), insulin-receptor- β (IR-β) subunit (Santa Cruz, sc-711) or the rat-BBB antibody (abcam, #24764). After 5 washes with PBS, secondary fluorescent antibodies (Cy-3, AlexaFluor® 594 or AlexaFluor® 488, Molecular Probes, Carlsbad, CA) were incubated with microvessel samples for 1 h in 1% BSA solution. Slides were then rinsed 5x in PBS and tamped to remove excess liquid prior to mounting with SlowFade® Gold reagent (Molecular Probes). After curing for 24 h at 26° C, microvessels were examined with fluorescence microscopy (Olympus) (Fig 1C-E). For verification of specific binding in immunohistochemistry, we processed microvessels side-by-side with the other samples, except that no primary antibodies were added to the incubation solution. Microvessels were then incubated with secondary antibodies and were visualized, as described (Fig 1H-I).
Fig. 1. CCK-1R is expressed in rat brain microvessels.
CCK-1R immunoreactivity was detected in freshly-isolated brain microvessels via fluorescence microscopy (A-B, orange/Alexa Fluor® 594, 40X magnification), imaged with phase contrast (A) and without phase contrast (B). Nuclei were stained with DAPI (blue). No immunoreactivity was observed in control IHC experiments that omitted primary antibody (C-D); images were taken with phase contrast (C) and without phase contrast (D), for comparison. Western blotting was performed with protein lysates from brain microvessel isolates (E) and choroid plexuses (F) using the Santa Cruz CCK- 1R antibody (47-50 kDa) and using the LS Biosciences (~90 kDa) antibody in separate blots of brain microvessel isolates (G) and choroid plexuses (H). Insulin receptor protein expression was detected in brain microvessels (I) and vessels were co-immunoblotted with a rat brain microvessel antibody (J, alone and K, together). CCK-1R mRNA expression (Cck1r) was also detected in rat brain microvessel isolates (L) at levels comparable to that of the full leptin receptor transcript (Lepr). Other genes examined included the GLP-1 receptor (Glp1r) and insulin receptor (Insr). n=6, ± SEM.
Microvessel lysates were analyzed via Western blotting (Fig 1E-G) using previously-validated antibodies for CCK-1R (Santa Cruz, sc-16172) and LS Biosciences (LS-C177096). The insulin-receptor- β (IR-β) subunit antibody (Santa Cruz, sc-711) was validated in a previous publication (33). After running SDS-PAGE with protein lysates prepared using T-PER extraction buffer (Pierce, #78510) and transferring to an Immobilon PVDF membrane (Millipore), Western Blots were incubated with primary antibody for 16-24 h at 4°C. After rinsing and incubating in horseradish peroxidase-conjugated goat anti-rabbit IgG antibody for 1 h at room temperature (1:5000-1:10,000, Dako #P0448) and rinsing again, membranes were incubated in Immobilon® HRP substrate (Millipore Cat. #WBKLS) 5 min at room temperature and blots were visualized with photolithography film (Denville Scientific, #E3018). Interestingly, the Santa Cruz CCK-1R antibody detected bands of the correct size only for freshly-prepared blots and these bands were not apparent after gentle stripping and re-probing.
2.4. Assessing insulin transport into the CNS
Rats were fasted overnight for 16 h. The next day, they were administered intraperitoneal (ip) insulin (0.3 U/kg, NPH, Novo Nordisk) or vehicle (saline) 30 min prior to cerebrospinal fluid (CSF) collection. Precisely 15 min after the insulin or vehicle injection, half of the rats in each group received ip CCK-8 (10 μg/rat; Sigma) and the other half received sterile saline. 10 min after the second injection, rats were anesthetized with isoflurane (Isothesia, Henry Schein, Dublin, Ohio) and positioned into a stereotaxic instrument, with the head maximally ventroflexed. A modified 25-G needle, with a tip prepared at a 30° angle (38), was connected to microrenethane tubing filled with sterile saline and secured in an electrode holder. The scalp and neck were shaved and sterilized. An incision was made to expose the atlanto- occipital membrane. Exactly 30 min after the initial insulin injection, the needle was inserted into the cisternum magnum and CSF flow was initiated by applying slight negative pressure with a 1-mL syringe attached to the microrenathane tubing. CSF was then collected (approximately 200 μL CSF per rat) into a chilled 1.5-mL microcentrifuge tube within a 2-min period. Rats were then sacrificed, and blood was immediately collected in chilled EDTA-coated tubes. Prior to analysis, the CSF samples were screened for blood contamination via spectrophotometric inspection. Only pure samples with less than 0.001% blood contamination were analyzed. Insulin levels were then measured in plasma and pure CSF samples using a previously- validated ultrasensitive rat insulin ELISA kit (Crystal Chem, #90060, Downers Grove, IL) (13,39). In order to determine the absorbance of the human insulin used for this study, we prepared a standard curve with insulin NPH; this was compared to the absorbance of the reference rat insulin standard, as described (13). As previously found, the absorbance of insulin NPH is typically 80% that of rat insulin with the low-range assay. An exact correction was not made in this instance, because we did not have basal CSF insulin readings from the same cohort of rats. If a correction were made, it would increase the reported concentrations of insulin in the plasma and CSF, and would only strengthen the observed effect in this instance (13).
2.5. Statistical Analysis
All statistical analyses were conducted with GraphPad Prism 5 software using a student’s t-test for comparisons between groups. Significance was pre-defined as P≤ 0.05.
3. RESULTS
3.1. CCK-1R is expressed at the BBB
CCK-1R protein expression was detected in microvessels via immunohistochemistry (Fig 1A,B, 40x magnification). Fluorescence was specific to the binding of primary antibody, since samples processed without primary antibody were devoid of nonspecific binding (Fig 1C,D). Western blotting with the CCK-1R antibody revealed solitary bands of the expected size for the truncated type 1 receptor (~47 kDa) (40–42) in protein lysates from brain microvessels (Fig 1E) and from the choroid plexus (Fig 1F), indicating that the immunoreactivity observed with fluorescence microscopy in Fig 1A,B is solely from the truncated form of CCK-1R. For comparison, microvessel protein lysates were blotted with the LS Biosciences CCK-1R antibody (which is solely validated for Western blotting, and not immunohistochemistry). In this case, we detected a band at the ~90 kDa range in brain microvessels (Fig 1G) and choroid plexus samples (Fig 1H), which is the expected size for the full-length CCK-1R (40–42). Due to this discrepancy between antibodies, we verified expression of Cck1r mRNA in freshly-isolated rat brain microvessels via qPCR and found that its expression was comparable to that of the leptin receptor (Fig 1L). The finding that CCK-1R is expressed at the BBB is important because CCK- 1R is the receptor involved in the satiating effects of CCK-8 (21–23). As we and others have seen before (33,34), insulin receptor protein was also expressed throughout microvessels (Fig 1I) in a pattern that was comparable to that of truncated CCK-1R; it appears that both CCK-1R and insulin receptors are expressed in every endothelial cell. A rat-BBB-specific antibody was also used as validation of microvessel specificity in these studies (Fig 1J,K) and the relative purity of microvessel isolates was verified via qPCR using our previously-published approach (33). These findings provide an anatomical foundation for an interaction of CCK-8 with insulin transport at the BBB.
3.2. Acute CCK treatment increases insulin transport into the CNS
Fasted rats receiving ip insulin 30 min earlier had comparable plasma insulin whether they subsequently received ip CCK-8 or not, such that CCK had no effect on circulating insulin (Figure 2A). Nonetheless, ip CCK resulted in CSF insulin levels being more than twice as high as occurred in rats given insulin but no CCK (Figure 2B). A separate experiment was conducted to establish baseline insulin levels in a group of rats injected ip with 0 U/kg insulin (saline, n=6). Because this experiment was conducted on a different day, these data are not incorporated into the graph. As listed in the description for Fig 2, insulin levels in the plasma were 1.11 ± 0.27 ng/mL and baseline concentrations of insulin in the CSF were comparable to those of the group injected with 0.3 U/kg insulin. These values are comparable to previously- published results from our lab (13). Taken together, these results indicate that the low dose of ip insulin did not significantly increase CSF insulin, whereas the administration of CCK significantly increased insulin transport under these conditions.
Figure 2. Acute CCK treatment increases insulin transport into the CNS.
A) No difference in plasma insulin was observed between groups treated with CCK (Ins+CCK) or saline (Ins), following an ip injection of 0.3 U/kg insulin in all rats. For reference, in comparably prepared rats injected with saline (0 U/kg insulin), the basal concentration of insulin in the plasma is 1.11 ± 0.27 ng/mL (not depicted). B) Rats treated with CCK had a significantly higher concentration of insulin in the CSF in comparison to rats administered saline. For comparison, in rats injected with saline (0 U/kg insulin), the basal concentration of CSF insulin is 0.53 ± 0.07 ng/mL n=6, P<0.05, ± SEM.
4. DISCUSSION
Collectively, our findings indicate that the satiation signal, CCK-8, increases the transport of insulin into the CNS and that these effects are likely mediated by CCK-1Rs that are expressed throughout the BBB and blood-CSF barrier. These observations are in agreement with previous reports that CCK-8 increases the appearance of the adiposity signal, leptin, into the CSF of rats (31,32). These intriguing observations suggest that CCK may also promote satiation by increasing the transport of other catabolic signals into the CNS (although CCK itself does not cross the BBB (43)) in addition to sending signals to the CNS via its action in the peripheral nervous system.
One implication of these findings is that this cooperative process would be active during meals when endogenous CCK and insulin are both elevated; it would be anticipated to be greatly enhanced in animals with higher and more prolonged elevations of GI hormones, such as can occur following successful bariatric surgeries (44). In turn, increasing the levels of these hormones is anticipated to lead to a pronounced suppression of food intake and body weight, by increasing the levels of adiposity signals in the brain (45).
Insulin was increased in the CSF within 15 minutes following ip CCK-8 administration. If CCK exerts its effects directly at the BBB as proposed (31,32), then it must diffuse from the peritoneal cavity into the bloodstream, circulate and bind to CCK-1R on endothelial cells of the BBB, and consequently increase insulin’s appearance in the CSF within a very short time-frame (as CCK-8 does not cross the BBB). This process involves the transport of insulin across the BBB and its subsequent release into the brain interstitial fluid (36). Each molecule of insulin may then bind to brain insulin receptors and/or may be degraded. The bulk flow of unbound insulin will enter the CSF, where it then is transported back into the bloodstream (36). Given the many processes that need to occur in this short time frame, it is unlikely that significant increases in insulin receptor expression could have accounted for the increased CSF insulin concentration. Instead, CCK may act to increase the rate of insulin transport through the BBB by increasing the saturation threshold of the insulin receptors already present at the outer cell membranes of endothelial cells comprising the BBB. This could either involve increasing the sensitivity of insulin receptors or increasing the insulin receptor-recycling rate within endothelial cells as they are transported from the abluminal side to the luminal side of BBB-endothelial cells, prior to binding another insulin molecule (30). Future experiments will have to determine if this is the case.
An alternative explanation for our results is that CCK somehow prevents the degradation of insulin (and presumably leptin, as well) within the brain. Since CCK does not cross the BBB (43) and is unlikely to affect the activity of the insulin-degrading enzyme (IDE) therein, CCK would have to substantially reduce the activity of IDE expressed at the BBB in order for such an interaction to take place (46). Further, plasma insulin levels were unaffected by CCK treatment, indicating that CCK does not alter IDE activity acutely in other tissues that are important for insulin degradation via IDE, such as the liver and kidney. Alternatively, CCK might send signals to the CNS that acutely suppress IDE activity, but such a mechanism has yet to be reported in the literature.
In conclusion, our findings support a model in which CCK increases the appearance of insulin into the CNS by acting on CCK-1Rs expressed in the BBB, although the exact mechanism(s) remains to be demonstrated. These findings point to a direct role of CCK at the BBB in the mediation of satiation. Whether other GI hormones can similarly exert these effects at the BBB is not known (45,47). Further studies are warranted to determine the mechanism by which the transport of insulin and leptin are increased following CCK-8 administration.
HIGHLIGHTS.
Cholecystokinin (CCK) increases the transport of insulin into the CNS of rats.
CCK-1 receptors are expressed by blood-brain barrier (BBB)-endothelial cells.
CCK may promote satiation by enhancing insulin transport through the BBB.
5. Acknowledgements
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) awards DK017844, DK92779, DK95440, and DK059803.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Woods SC, Seeley RJ, Porte D, Jr., Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. (80-. ) [DOI] [PubMed] [Google Scholar]
- 2.Tso P, Sun W, Liu M. Gastrointestinal satiety signals IV. Apolipoprotein A-IV. Am.J.Physiol Gastrointest.Liver Physiol. 2004;286:G885–G890. doi: 10.1152/ajpgi.00511.2003. [DOI] [PubMed] [Google Scholar]
- 3.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tso P, Liu M. Ingested fat and satiety. Physiol Behav. 2004;81:275–287. doi: 10.1016/j.physbeh.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. [Internet] 1973;84:488–95. doi: 10.1037/h0034870. [cited 2016 Jan 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/4745816. [DOI] [PubMed] [Google Scholar]
- 6.Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J. Comp. Physiol. Psychol. [Internet] 1975;89:784–90. doi: 10.1037/h0077040. [cited 2016 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1176672. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimichi G, Lo CC, Tamashiro KL, Ma L, Lee DM, Begg DP, et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am.J Physiol Gastrointest.Liver Physiol [Internet] 2012;302:G1336–G1342. doi: 10.1152/ajpgi.00325.2010. Available from: http://ajpgi.physiology.org/content/ajpgi/early/2012/03/26/ajpgi.00325.2010.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacher G, Bauer H, Steinringer H. Cholecystokinin decreases appetite and activation evoked by stimuli arising from the preparation of a meal in man. Physiol. Behav. [Internet] 1979;23:325–331. doi: 10.1016/0031-9384(79)90374-3. [cited 2016 May 25] Available from: http://www.sciencedirect.com/science/article/pii/0031938479903743. [DOI] [PubMed] [Google Scholar]
- 9.Pi-Sunyer X, Kissileff HR, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol. Behav. [Internet] 1982;29:627–30. doi: 10.1016/0031-9384(82)90230-x. [cited 2016 Apr 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/6294699. [DOI] [PubMed] [Google Scholar]
- 10.Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am. J. Physiol. [Internet] 1992;262:R554–61. doi: 10.1152/ajpregu.1992.262.4.R554. [cited 2016 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1373576. [DOI] [PubMed] [Google Scholar]
- 11.Brenner L, Ritter RC. Peptide cholesystokinin receptor antagonist increases food intake in rats. Appetite [Internet] 1995;24:1–9. doi: 10.1016/s0195-6663(95)80001-8. [cited 2016 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/7537951. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nat. 2000 Apr 6;404(6778):661–71. doi: 10.1038/35007534. [Internet]. 2000;404:661–671. Available from: http://www.nature.com/nature/journal/v404/n6778/full/404661a0.html. [DOI] [PubMed] [Google Scholar]
- 13.Begg DP, May AA, Mul JD, Liu M, D’Alessio DA, Seeley RJ, et al. Insulin Detemir Is Transported From Blood to Cerebrospinal Fluid and Has Prolonged Central Anorectic Action Relative to NPH Insulin. Diabetes [Internet] 2015;64:2457–66. doi: 10.2337/db14-1364. [cited 2015 Dec 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25667307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SC, Lotter EC, McKay LD, Porte D, Porte D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature [Internet] 1979;282:503–505. [cited 2016 Jul 27] Available from: http://www.nature.com/nature/journal/v282/n5738/abs/282503a0.html. [Google Scholar]
- 15.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav.Neurosci [Internet] 1995;109:528–531. doi: 10.1037//0735-7044.109.3.528. [cited 2014 Jan 25] Available from: http://psycnet.apa.org/journals/bne/109/3/528.html. [DOI] [PubMed] [Google Scholar]
- 16.Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology [Internet] 2003;144:3789–3798. doi: 10.1210/en.2002-0148. Available from: http://press.endocrine.org/doi/pdf/10.1210/en.2002-0148. [DOI] [PubMed] [Google Scholar]
- 17.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. (80-. ). [Internet] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11000114. [DOI] [PubMed] [Google Scholar]
- 18.Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, et al. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav. [Internet] 2007;92:691–701. doi: 10.1016/j.physbeh.2007.05.043. [cited 2016 Mar 28] Available from: http://www.sciencedirect.com/science/article/pii/S003193840700193X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D’Alessio DA, et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab. [Internet] 2015;21:877–82. doi: 10.1016/j.cmet.2015.04.015. [cited 2016 May 25] Available from: http://www.sciencedirect.com/science/article/pii/S1550413115001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition [Internet] 2000;16:894–902. doi: 10.1016/s0899-9007(00)00454-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11054594. [DOI] [PubMed] [Google Scholar]
- 21.Corwin RL, Gibbs J, Smith GP. Increased food intake after type A but not type B cholecystokinin receptor blockade. Physiol. Behav. [Internet] 1991;50:255–8. doi: 10.1016/0031-9384(91)90529-w. [cited 2016 Apr 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1946726. [DOI] [PubMed] [Google Scholar]
- 22.Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am. J. Physiol. [Internet] 1992;262:R46–50. doi: 10.1152/ajpregu.1992.262.1.R46. [cited 2016 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1733339. [DOI] [PubMed] [Google Scholar]
- 23.Moran TH, Ameglio PJ, Peyton HJ, Schwartz GJ, McHugh PR. Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. Am. J. Physiol. [Internet] 1993;265:R620–4. doi: 10.1152/ajpregu.1993.265.3.R620. [cited 2016 May 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8214156. [DOI] [PubMed] [Google Scholar]
- 24.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am.J.Physiol [Internet] 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9140026. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GJ, Moran TH. CCK elicits and modulates vagal afferent activity arising from gastric and duodenal sites. Ann.N.Y.Acad.Sci. [Internet] 1994;713:121–128. doi: 10.1111/j.1749-6632.1994.tb44058.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8185153. [DOI] [PubMed] [Google Scholar]
- 26.Figlewicz DP, Sipols AJ, Seeley RJ, Chavez M, Woods SC, Porte D., Jr. Intraventricular insulin enhances the meal-suppressive efficacy of intraventricular cholecystokinin octapeptide in the baboon. Behav.Neurosci. [Internet] 1995;109:567–569. doi: 10.1037//0735-7044.109.3.567. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7662167. [DOI] [PubMed] [Google Scholar]
- 27.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 28.Williams DL, Baskin DG, Schwartz MW. Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. Am.J Physiol Regul.Integr.Comp Physiol [Internet] 2009;297:R1238–R1246. doi: 10.1152/ajpregu.00182.2009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19726710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am.J.Physiol [Internet] 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10233050. [DOI] [PubMed] [Google Scholar]
- 30.Begg DP, Woods SC. The central insulin system and energy balance. Handb.Exp.Pharmacol. [Internet] 2012;209:111–129. doi: 10.1007/978-3-642-24716-3_5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22249812. [DOI] [PubMed] [Google Scholar]
- 31.Cano V, Merino B, Ezquerra L, Somoza B, Ruiz-Gayo M. A cholecystokinin-1 receptor agonist (CCK-8) mediates increased permeability of brain barriers to leptin. Br. J. Pharmacol. [Internet] 2008;154:1009–15. doi: 10.1038/bjp.2008.149. [cited 2016 Feb 1] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2451056&tool=pmcentrez&ren dertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cano V, Ezquerra L, Ramos MP, Ruiz-Gayo M. Regulation of leptin distribution between plasma and cerebrospinal fluid by cholecystokinin receptors. Br. J. Pharmacol. [Internet] 2003;140:647–52. doi: 10.1038/sj.bjp.0705477. [cited 2016 Feb 1] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1574067&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May AA, Bedel ND, Shen L, Woods SC, Liu M. Estrogen and insulin transport through the blood-brain barrier. Physiol. Behav. [Internet] 2016 doi: 10.1016/j.physbeh.2016.05.019. [cited 2016 May 24]; Available from: http://www.sciencedirect.com/science/article/pii/S0031938416302517. [DOI] [PMC free article] [PubMed]
- 34.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MRC, Porte D. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides [Internet] 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [cited 2013 Nov 19] Available from: http://www.sciencedirect.com/science/article/pii/0196978190900446. [DOI] [PubMed] [Google Scholar]
- 35.Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits [Internet] 1987 doi: 10.1016/0006-8993(87)90236-8. [cited 2013 Oct 22]. Available from: http://www.sciencedirect.com/science/article/pii/0006899387902368. [DOI] [PubMed]
- 36.Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Fisher LD, Sipols AJ, et al. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J. Clin. Invest. [Internet] 1991;88:1272–81. doi: 10.1172/JCI115431. [cited 2016 Apr 6] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=295596&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baura GD, Foster DM, Porte D, Jr., Kahn SE, Bergman RN, Cobelli C, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J.Clin.Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu M, Shen L, Begg DP, D’alessio DA, Woods SC. Insulin increases central apolipoprotein E levels as revealed by an improved technique for collection of cerebrospinal fluid from rats. J. Neurosci. Methods [Internet] 2012;209:106–12. doi: 10.1016/j.jneumeth.2012.05.034. [cited 2014 Jun 9] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3402581&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen L, Haas M, Wang DQ-H, May A, Lo CC, Obici S, et al. Ginsenoside Rb1 increases insulin sensitivity by activating AMP-activated protein kinase in male rats. Physiol. Rep. [Internet] 2015:3. doi: 10.14814/phy2.12543. [cited 2016 Jan 28] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4600387&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 40.Poirot S, Escrieut C, Dufresne M, Martinez J, Bouisson M, Vaysse N, et al. Photoaffinity labeling of rat pancreatic cholecystokinin type A receptor antagonist binding sites demonstrates the presence of a truncated cholecystokinin type A receptor. Mol. Pharmacol. [Internet] 1994;45:599–607. [cited 2016 May 31] Available from: http://molpharm.aspetjournals.org/content/45/4/599. [PubMed] [Google Scholar]
- 41.Schjoldager B, Molero X, Miller LJ. Gallbladder CCK receptors: species differences in glycosylation of similar protein cores. Regul. Pept. [Internet] 1990;28:265–272. doi: 10.1016/0167-0115(90)90024-q. [cited 2016 May 31] Available from: http://www.sciencedirect.com/science/article/pii/016701159090024Q. [DOI] [PubMed] [Google Scholar]
- 42.Deweerth A, Pisegna JR, Huppi K, Wank SA. Molecular Cloning, Functional Expression and Chromosomal Localization of the Human Cholecystokinin Type A Receptor. Biochem. Biophys. Res. Commun. [Internet] 1993;194:811–818. doi: 10.1006/bbrc.1993.1894. [cited 2016 May 31] Available from: http://www.scopus.com/inward/record.url?eid=2-s2.00027234316&partnerID=tZOtx3y1. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin BA, Parrott RF, Ebenezer IS. Food for thought: a critique on the hypothesis that endogenous cholecystokinin acts as a physiological satiety factor. Prog. Neurobiol. [Internet] 1998;55:477–507. doi: 10.1016/s0301-0082(98)00005-7. [cited 2016 Feb 2] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9670215. [DOI] [PubMed] [Google Scholar]
- 44.Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int. J. Obes. (Lond). [Internet] 2011;35:153–66. doi: 10.1038/ijo.2010.132. [cited 2016 May 31] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3632050&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am.J.Physiol Gastrointest.Liver Physiol [Internet] 2004;286:G7–13. doi: 10.1152/ajpgi.00448.2003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14665437. [DOI] [PubMed] [Google Scholar]
- 46.Gao W, Eisenhauer PB, Conn K, Lynch JA, Wells JM, Ullman MD, et al. Insulin degrading enzyme is expressed in the human cerebrovascular endothelium and in cultured human cerebrovascular endothelial cells. Neurosci. Lett. [Internet] 2004;371:6–11. doi: 10.1016/j.neulet.2004.07.034. [cited 2016 Feb 26] Available from: http://www.sciencedirect.com/science/article/pii/S0304394004008766. [DOI] [PubMed] [Google Scholar]
- 47.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab [Internet] 2009;9:489–498. doi: 10.1016/j.cmet.2009.04.007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19490904. [DOI] [PMC free article] [PubMed] [Google Scholar]