Abstract
Objective
The aims of the Korean Neuro-Trauma Data Bank System (KNTDBS) are to evaluate and improve treatment outcomes for brain trauma, prevent trauma, and provide data for research. Our purpose was to examine the mortality rates following traumatic brain injury (TBI) in a retrospective study and to investigate the sociodemographic variables, characteristics, and causes of TBI-related death based on data from the KNTDBS.
Methods
From 2010 to 2014, we analyzed the data of 2617 patients registered in the KNTDBS. The demographic characteristics of patients with TBI were investigated. We divided patients into 2 groups, survivors and nonsurvivors, and compared variables between the groups to investigate variables that are related to death after TBI. We also analyzed variables related to the interval between TBI and death, mortality by region, and cause of death in the nonsurvivor group.
Results
The frequency of TBI in men was higher than that in women. With increasing age of the patients, the incidence of TBI also increased. Among 2617 patients, 688 patients (26.2%) underwent surgical treatment and 125 patients (4.7%) died. The age distributions of survivors vs. nonsurvivor groups and mortality rates according the severity of the brain injury, surgical treatment, and initial Glasgow Coma Scale (GCS) scores were statistically significantly different. Among 125 hospitalized nonsurvivors, 70 patients (56%) died within 7 days and direct brain damage was the most common cause of death (80.8%). The time interval from TBI to death differed depending on the diagnosis, surgical or nonsurgical treatment, severity of brain injury, initial GCS score, and cause of death, and this difference was statistically significant.
Conclusion
Using the KNTDBS, we identified epidemiology, mortality, and various factors related to nonsurvival. Building on our study, we should make a conscious effort to increase the survival duration and provide rapid and adequate treatment for TBI patients.
Keywords: Korean Neuro-Trauma Data Bank System, Mortality, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is defined as intracranial injury resulting from a blunt or penetrating trauma or from acceleration-deceleration of an external force causing neurological or neuropsychological problems, such as altered mentality, intracranial hematomas, memory impairment, skull fracture, or death8,14). TBI is a major cause of death and disability around the world and a leading global socioeconomic and health problem14). In the United States, approximately 17000000 people experienced some kind of TBI each year during 2002–2007, resulting in 275000 hospitalizations (16%), 52000 deaths (3.0%), and 124000 disabilities4,8). The fatality rate has decreased owing to improved management and systems for treating TBI in societies wealthy enough to provide modern emergency and neurosurgical services. This decrease in death after TBI has resulted in a concomitant increase in the number of patients living with disabilities11).
Although many studies in the international literature have reported aspects of the epidemiology and mortality after TBI, there are few published data from Korean studies9). The Korean Society of Neurotraumatology established Korean guidelines for treatment of severe brain injury in 2003, and the need for patient data treated according to these guidelines is increasing. The aims of the Korean Neuro-Trauma Data Bank System (KNTDBS) are to evaluate and improve treatment outcomes for brain trauma, prevent trauma, and provide data for research. Epidemiological studies of TBI are essential to the targeted prevention and effective treatment of brain-injured patients. Our purpose was to examine the mortality rates following TBI in a retrospective study and to investigate the sociodemographic variables and characteristics related to TBI-related hospitalization and the characteristics and causes of TBI-related death based on data from the KNTDBS.
MATERIALS AND METHODS
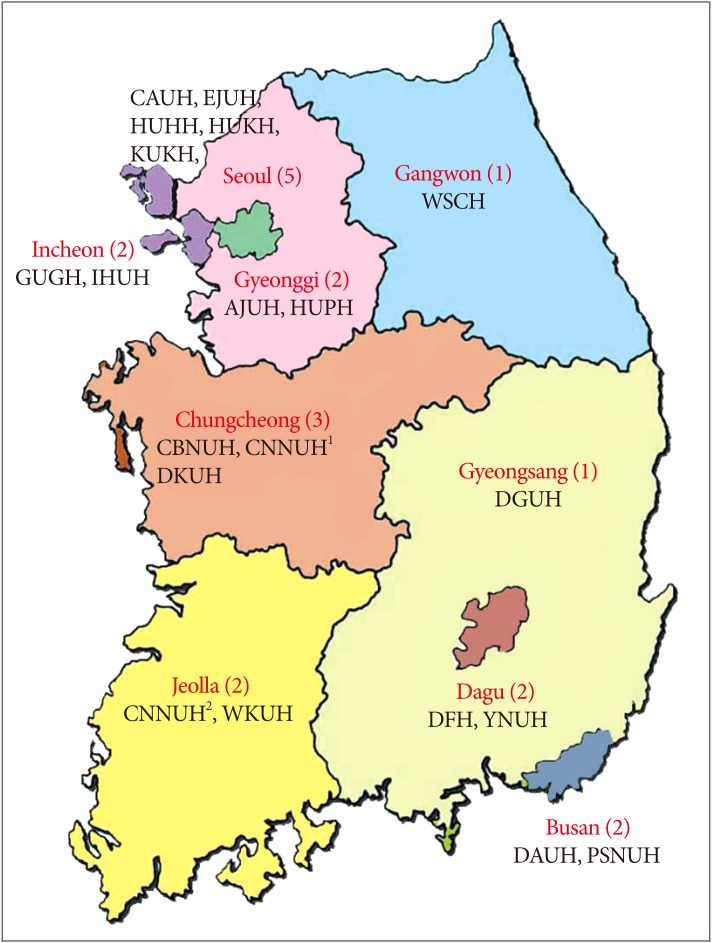
From 2010 to 2014, we analyzed the data of 2617 patients registered in the KNTDBS and retrospectively reviewed the medical records of patients admitted for TBI. The Korean Society of Neurotraumatology collected data from 20 institutions from September 2010 to March 2014 (Fig. 1). They established a KNTDBS website and provided identifications and passwords to the individuals at each institution assigned to input the data of trauma patients. All admitted head trauma patients with a Glasgow Coma Scale (GCS) score over 3 were included. Exclusion criteria were severe extracranial injury, cerebral concussion, and chronic subdural hematoma. The demographic characteristics of patients with TBI, including sex, age distribution, diagnosis, surgical versus vs. nonsurgical treatment, and outcome, were investigated. Diagnoses included acute epidural hematoma (A-EDH), acute subdural hematoma (A-SDH), traumatic subarachnoid hemorrhage (T-SAH), contused intracranial hemorrhage (C-ICH), diffuse axonal injury (DAI), and other. Additionally, we divided patients into 2 groups, survivors and nonsurvivors, and compared variables such as sex, age distribution, severity of brain injury, diagnosis, treatment, and initial GCS between the groups to investigate variables that are related to death after TBI. The severity of brain injury was divided into 3 grades : mild, moderate, and severe. We also analyzed variables related to the interval between TBI and death, mortality by region, and cause of death in the nonsurvivor group.
Fig. 1. Participating institutions. AJUH : Ajou University Hospital, CAUH : Chung-Ang University Hospital, CBNUH : Chungbuk National University Hospital, CNNUH1 : Chungnam National University Hospital1, CNNUH2 : Chonnam national University Hospital2, DAUH : Dong-A University Hospital, DGUH : Dongguk University Hospital, DKUH : Dankook University Hospital, DFH : Daegu Fatima Hospital, EJUH : Eulji University Hospital, GUGH : Gachon University Gill Hospital, HUHH : Hallym University Hangang Sacred Heart Hospital, HUKH : Hallym University Kangdong Sacred Heart Hospital, HUPH : Hallym University Pyeongchon Sacred Heart Hospital, IHUH : Inha University Hospital, KUKH : Korea University Guro Hospital, PSNUH : Pusan National University Hospital, WKUH : Wonkwang University Hospital, WSCH : Wonju Severance Christian Hospital, YNUH : Yeungnam University Hospital. The Korean Society of Neurotraumatology collected data from 20 institutions from September 2010 to March 2014.
All statistical calculations were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). The frequencies of variables were identified using descriptive statistics. Student's t-tests, or nonparametric tests when necessary, were employed for analysis of quantitative variables, and Pearson's χ2 and Fisher's exact tests were used for analysis of categorical variables between survivors and nonsurvivors. We also used analysis of variance for continuous variables. Differences were considered statistically significant if p values were <0.5.
RESULTS
Demographic characteristics of patients with TBI registered in the KTBDS
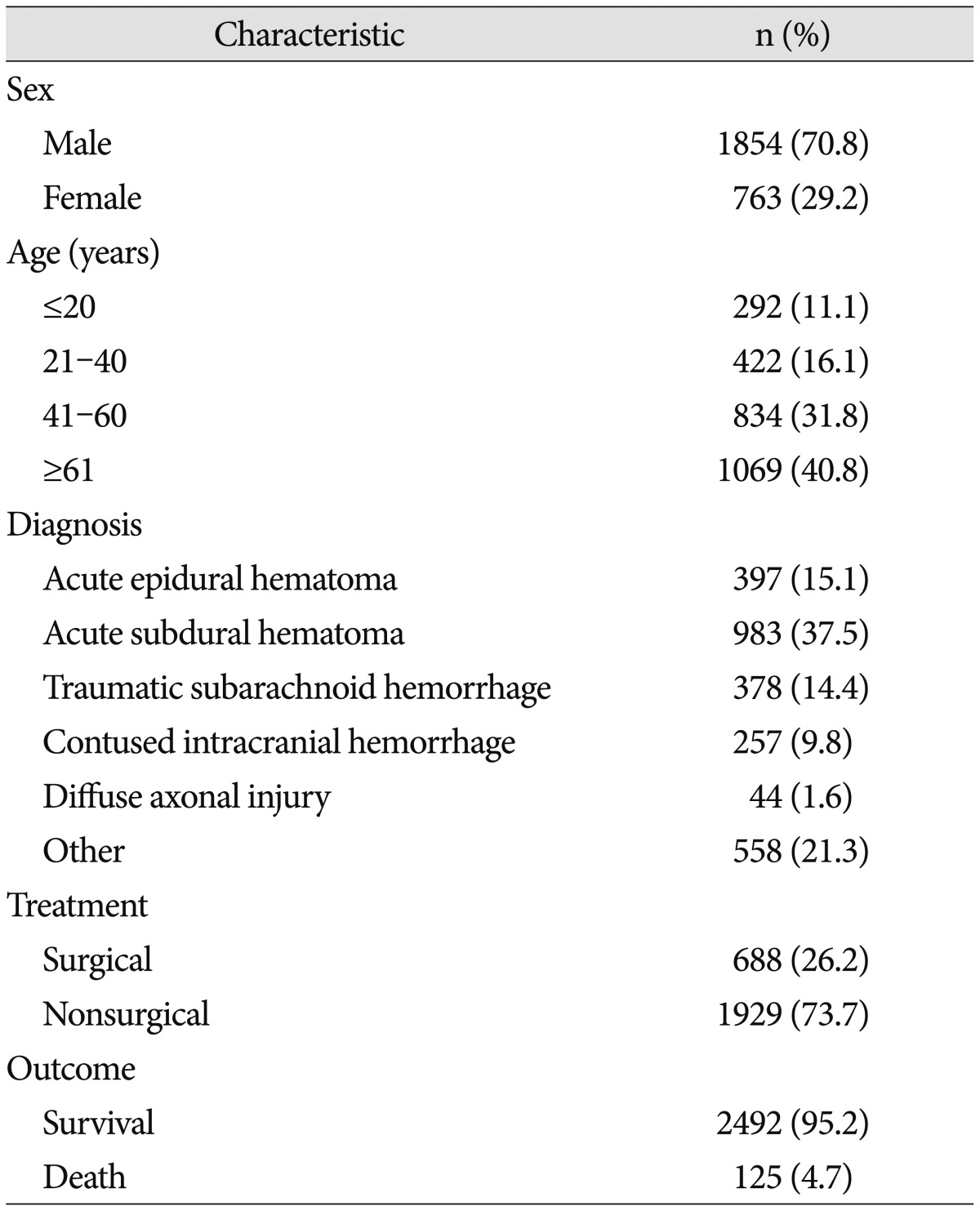
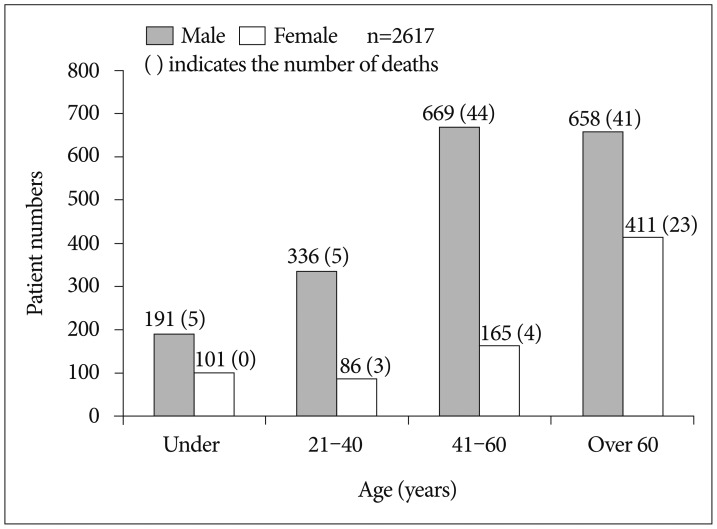
A total of 2617 patients with TBI were included in this study. The demographic characteristics of patients are summarized in Table 1. There were 1854 men (70.8%) and 763 women (29.2%), and the frequency of TBI in men was higher than that in women. The incidence of TBI according to age was lowest in those <20 years of age (11.1%; n=292). With increasing age of the patients, the incidence of TBI also increased. There were 422 patients (16.1%) aged 21–40 years and 834 patients (31.9%) aged 41–60 years. The highest incidence occurred in those aged ≥61 (40.8%, n=1069). The diagnosis of admitted patients was determined by the most prominent injury that led to admission. The most common diagnosis was A-SDH (37.5%; n=983), followed by other (including skull fractures, massive scalp flap wound, and mild head injury with a GCS of ≤14) (21.3%; n=558), A-EDH (15.1%; n=397), T-SAH (14.4%; n=378), C-ICH (9.8%; n=257), and DAI (1.6%; n=44). A total of 688 patients (26.2%) underwent surgical treatment, and 1929 patients (73.7%) underwent nonsurgical treatment. Among 2617 patients, 125 patients (4.7%) died and 2492 patients (95.2%) survived. The distribution of patients with TBI according to age and sex is shown in Fig. 2. For all age groups, the incidence of TBI in men was higher than that in women. Among the male patients, the trauma incidence was highest in those 41–60 years of age (36.1%; n=669), but this incidence was similar to that in those aged ≥61 (35.5%; n=658). Among the female patients, the incidence in those under age 20 (13.2%; n=101) was higher than that in those aged 21–40 (11.3%; n=86).
Table 1. Demographic characteristics of patients with traumatic brain injury.
Fig. 2. Distribution of patients with traumatic brain injury according to age and sex.
Comparison of clinical characteristics between survivors and nonsurvivors
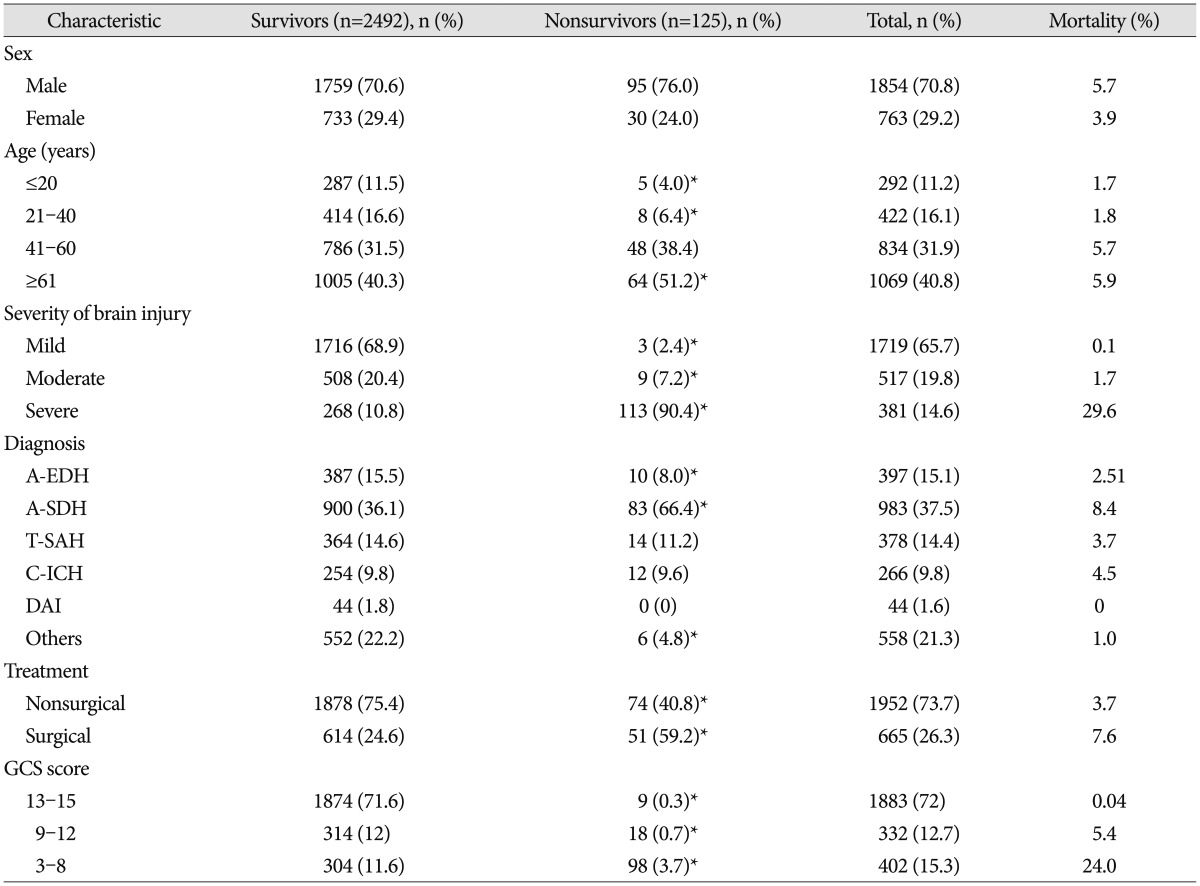
The clinical characteristics of both groups are summarized in Table 2. The mortality of male patients (5.7%) was slightly higher than that of the female patients (3.9%), but this difference was not statistically significant (p=0.194). The age distributions of survivors vs. nonsurvivor groups were statistically significantly different, with mortality rates of 1.7% in those under age 20 (survivors vs. nonsurvivors : 11.5% vs. 4%, p=0.009), 1.8% in those aged 21–40 (16.6% vs. 6.4%, p=0.002), 5.7% in those aged 41–60 (31.5% vs. 38.4%, p=0.108), and 5.9% in those aged ≥61 (40.3% vs. 51.2%, p=0.016). As the age of the patients with TBI increased, so did the mortality rate. The mortality rates according the severity of the brain injury show that as the severity increased, the mortality increased, and the difference was statistically significant (p=0.000). Regarding mortality according to diagnosis, no patient diagnosed with DAI died, and the mortality rates in those diagnosed with A-SDH or other groups were statistically significant (p=0.215). The difference in mortality rates between nonsurgical (3.7%) and surgical (7.6%) treatment was statistically significant (p=0.101). Regarding GCS scores at the time of diagnosis, mortality increased as GCS score decreased, and the difference was statistically significant (p=0.000).
Table 2. Comparison of clinical characteristics between survivors and nonsurvivors.
*p<0.05 vs. survivor group. A-EDH : acute epidural hematoma, A-SDH : acute subdural hematoma, T-SAH : traumatic subarachnoid hemorrhage, C-ICH : contused intracranial hemorrhage, DAI : diffuse axonal injury, GCS : Glasgow Coma Scale
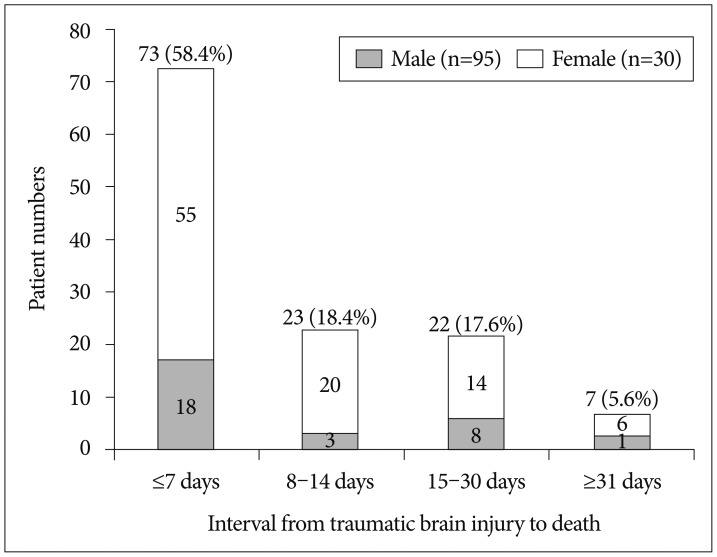
The interval from TBI to death in nonsurvivors
Among 125 hospitalized nonsurvivors, 73 patients (58.4%) died within 7 days, 23 patients (18.4%) died within 8–14 days, 22 patients (17.6%) died within 15–30 days, and 7 patients (5.6%) died after 1 month (Fig. 3).
Fig. 3. The interval from traumatic brain injury to death in nonsurvivors.
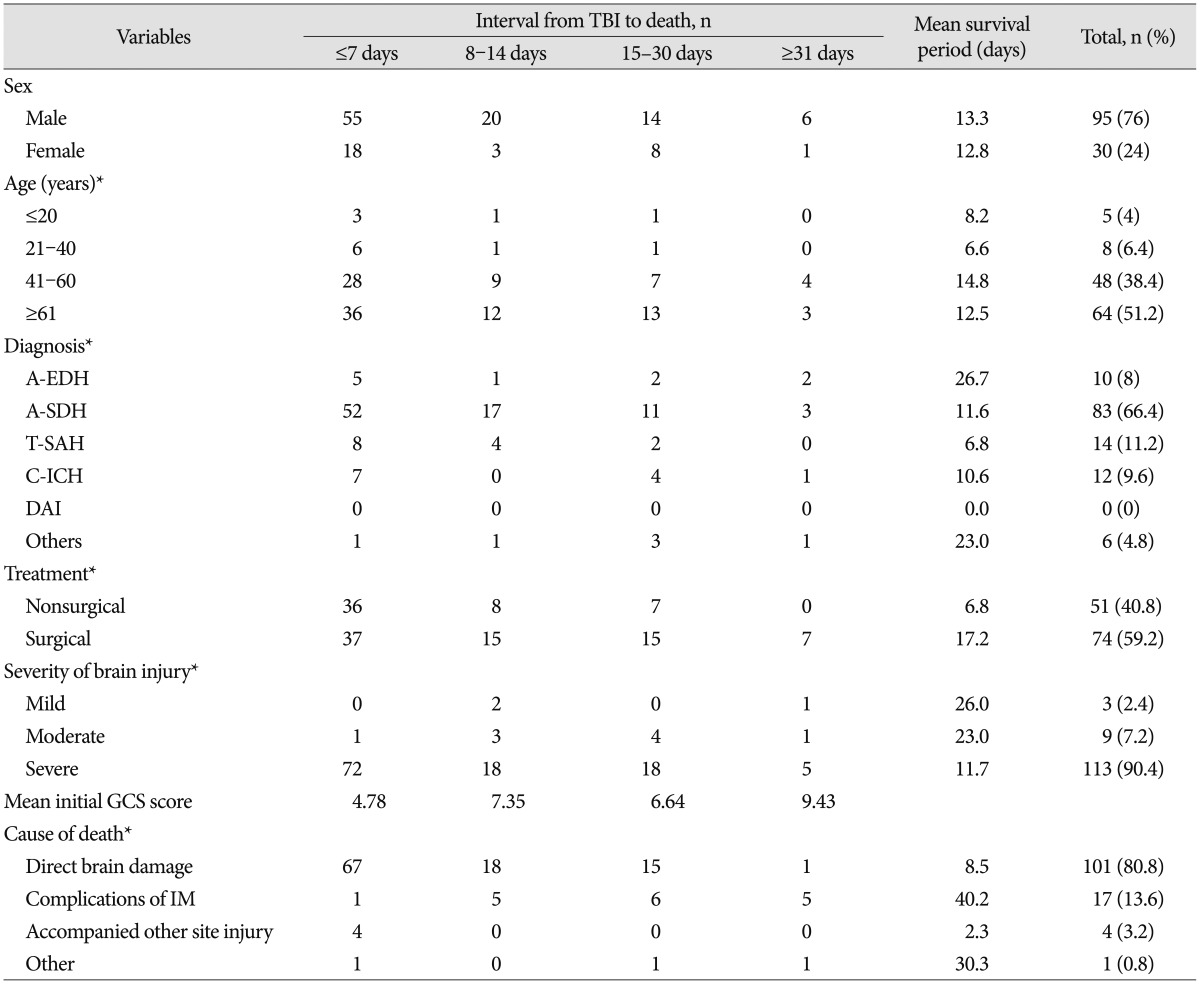
Variables related to the interval from TBI and death in nonsurvivors
Table 3 shows the clinical characteristics according to the interval from TBI to death in nonsurvivors. The largest number of patients died within 7 days in both male and female groups. In the male group, as the time interval (≤7, 8–14, 15–30, and ≥31 days) increased, the number of deaths decreased (n=55, 20, 14, and 6, respectively) with a mean survival period (MSP) of 13.3 days. In the female group, the number of deaths within 15–30 days was more than that within 8–14 days (n=18, 3, 8, and 1, respectively) with a MSP of 12.8 days, but this difference was not statistically significant (p=0.291). For all age groups (≤20, 21–40, 41–60, and ≥61 years), the frequency of death was highest within 7 days (n=3, 6, 28, and 36, respectively). Those aged 41–60 had the longest MSP (14.8 days), followed by those aged ≥61 (12.5 days), those aged ≤20 (8.2 days), and those aged 21–40 (6.6%). As the time interval increased, a nonsignificant trend of a decrease in the number of deaths was observed in all age groups (p=0.991). The diagnoses in decreasing order of MSP were A-EDH (26.7 days), other (23 days), A-SDH (11.6 days), C-ICH (10.6 days), and T-SAH (6.8 days). The time interval from TBI to death differed depending on the diagnosis, and this difference was statistically significant (p=0.049). The time interval from TBI to death (≤7, 8–14, 15–30, and ≥31 days) differed depending on whether patients received surgical treatment (n=37, 15, 15, and 7, respectively; MSP, 17.2 days) or nonsurgical treatment (n=36, 8, 7, and 0, respectively; MSP, 6.8 days). This difference was statistically significant (p=0.044). The higher the severity of brain injury (mild, moderate, and severe), the shorter the interval from TBI to death (MSP : 26, 23, and 11.7 days, respectively), and this difference was statistically significant (p=0.107). The longer the interval from TBI to death (≤7, 8–14, 15–30, and ≥31 days), the higher the initial GCS score (4.78, 7.35, 6.64, and 9.43, respectively), and this difference was statistically significant (p=0.000). The causes of death in descending order of MSP were complications of internal medicine (40.2 days), other (30.3 days), direct brain injury (8.5 days), and accompanied other site injury (2.3 days). The time interval from TBI to death differed depending on the cause of death, and this difference was statistically significant (p=0.001).
Table 3. Variables related to the interval from TBI to death.
*p<0.05. TBI : traumatic brain injury, ISC : injury severity score, A-EDH : acute epidural hematoma, A-SDH : acute subdural hematoma, T-SAH : traumatic subarachnoid hemorrhage, C-ICH : contused intracranial hemorrhage, DAI : diffuse axonal injury, GCS : Glasgow Coma Scale, IM : internal medicine
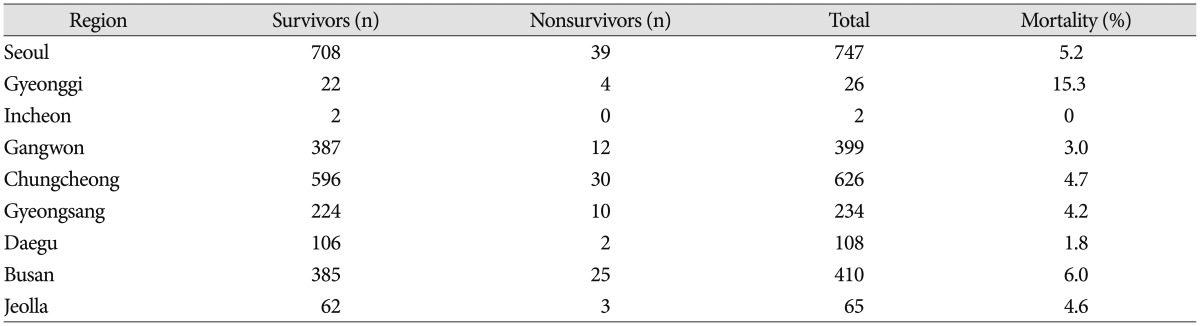
Mortality by regional group
Not including the 2 regions of Gyeonggi (n=26; mortality 15.3%) and Incheon (n=2, mortality 0%), because there were so few data available from these regions, mortality according to region was highest in Busan (n=410, mortality 6.0%) and lowest in Daegu (n=108, mortality 1.8%) (Table 4).
Table 4. Mortality by region.
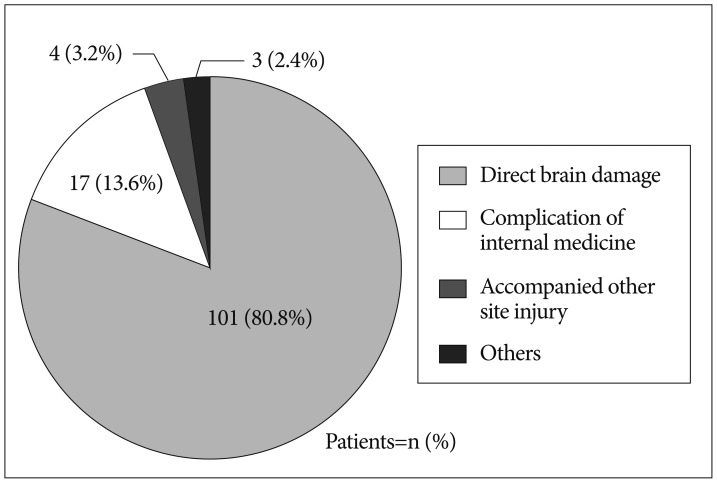
Cause of TBI in nonsurvivors
Among nonsurvivors, direct brain damage was the most common cause of death (80.8%, n=101) followed by complications of internal medicine (13.6%, n=17), accompanied other site injury (3.2%; n=4), and other (2.4%; n=3) (Fig. 4).
Fig. 4. The cause of traumatic brain injury in nonsurvivors.
DISCUSSION
Irrespective of age, TBI rates are reported as higher in male patients throughout the literature2,3,4,7,8,9,10,11,12,14,15,16). Males undergo twice as many TBIs as females do and have a 4-fold higher risk of lethal TBI. Males account for two-thirds of childhood and adolescent TBI12). Our study also showed that there were nearly 2.4 times as many male patients as female patients, and the mortality of male patients was 1.5 times higher than that of female patients. However, the number of female patients increased rapidly in those aged ≥61, which may be because of an increasing life expectancy and women's social activities. TBI is known to be more common in young people, and the highest incidence occurs in young adults aged 15–24 years5). Peak age is similar for males and females. Twenty percent of TBIs occur in the pediatric age group (birth to 17 years). The highest mortality rate (32.8 cases per 100000 people) is found in persons aged 15–24 years. The mortality rate in patients who are elderly (65 years or older) is approximately 31.4 individuals per 100000 people7). Haring et al.6) found that between 2000 and 2010, 950132 TBI-related hospitalizations occurred in adults aged 65 years or older in the United States, along with 107666 TBI-related deaths. Our study showed that as the age of patients increased, the incidence of TBI also increased, and the proportion of middle-aged and older adults was higher compared with that reported from other countries. This difference may result from the different socioeconomic statuses of different countries. Socioeconomic status appears to affect the incidence of TBI. The median age of people with head injuries has increased in first-world countries, because the fall-related TBI rate increases as the population ages10).
In our study, the diagnoses in decreasing order of the number of TBI patients admitted were A-SDH, other, A-EDH, T-SAH, and C-ICH, and this result may be owing to the role of University Hospital that has to admit patients who have more serious injury first. Development of traumatic ICH is the most important complication of TBI. Traumatic ICH occurs in 25–45% of patients with severe TBI, 3–12% of patients with moderate TBI, and approximately 1 in 500 patients with mild TBI15). Among the most serious complications of TBI might be A-SDH and A-EDH. The difference in the severity of the diagnosis is without a doubt a leading reason for the difference in mortality, which was also confirmed by our study. Since brain computed tomography has been widely used for TBI, most studies have reported mortality rates of approximately 3–12% for A-EDH, 41–70% for A-SDH, and 28% for overall mortality for A-EDH and A-SDH13). The results of our study showed an improvement in terms of mortality with 2.5% for A-EDH, 8.4% for A-SDH, and 6.7% overall for A-EDH and A-SDH. These improved outcomes can be ascribed to the rapid patient transport system from accident to hospital and improvements in rescue and surgical practices in Korea.
In Korea in 1998, Lee9) reported that the total number of patients with head injury was 109462, and surgery was performed in 20.2% with an average mortality of 4.0%. Our study showed that surgery was performed in 26.2%, and the mortality of the surgical treatment group (7.6%) was higher than that of the nonsurgical treatment group (3.7%). Despite recent advances in medical technology, selection bias with respect to registration of patients in the KTBDS and broad indications for surgical treatment of severely injured patients may account for the higher surgical mortality rate of our study compared with that of 17 years ago. The initial GCS score has been used to classify TBI into levels of severity and predict the prognosis, with mild TBI (GCS score 13–15) reported to have 0.1% mortality, moderate TBI (GCS score 9–12) reported to have 10% mortality, and severe TBI (GCS score <9) reported to have 40% mortality1). However, our study showed that the mortalities of mild, moderate, and severe head injury were 0.04%, 5.4%, and 24%. Although the mortality rate was also high in severe TBI and was low in moderate and mild TBI in our study, we showed improvement in terms of mortality for all 3 severity categories. The reason for these improved outcomes may be the same as the aforementioned reasons for the recent decrease of mortality.
Among the many studies about death after TBI, to the best of our knowledge, there is no paper that evaluated the time interval from TBI to death. Because the complex pathophysiological mechanisms of TBI and various variables are multifactorial and the opinions on preferred strategies may vary, precise and reasonable results and comparisons are difficult. Our study also showed that MSP was not related to mortality in the nonsurvivor group. MSP was similar in male and female patients. MSP was longer in those who were aged 41–60, had A-EDH, underwent surgical treatment, or had a mild brain injury and in those whose cause of death was complications of internal medicine. Deaths from TBI are more common in rural than in urban areas, because the outcome largely depends on prompt and quality care to such patients, which is deficient in rural areas16). Our study showed similar levels of mortalities according to region, which may be accounted for by equalized medical treatment for TBI and hospital accessibility. The incidence of TBI has been decreasing because of the introduction of preventive measures and as a result of better enforcement of drunk driving laws. The length of stay in acute hospitals and rehabilitation facilities has been declining because of the increased demand for facilities and because of the resources that are available in the community for patients who are discharged early3,15).
Study limitations
There are several limitations of this study. First, we could not identify the number of TBI patients who were not registered in the KNTDBS. Therefore, we cannot rule out the possibility of selection bias in our data. Furthermore, we have some doubt about the reliability and accuracy of the data because some incomplete data were identified. Second, there is a lack of precise criteria and definitions of multiple or combined injuries in the diagnosis categories as well as in diagnoses classified as other. Third, there are also no precise criteria for the separate category of severity of brain injury (mild, moderate, and severe), even though there was a category of initial GCS score. Therefore, when data were registered, there is a possibility that the severity of brain injury was decided subjectively. Fourth, the socioeconomic conditions, traffic systems, demographic characteristics, and educational attainment according to region were not considered in the analysis of mortality. Fifth, we need more precise and detailed classification with respect to the causes of TBI in nonsurvivors.
CONCLUSION
Traumatic events can be prevented through public relations campaigns when various causes of trauma are collected by a TDBS. Data extracted from the TDBS can be used in research and can contribute to new treatment guideline and attempts. Using the KNTDBS, we identified epidemiology, mortality, and various factors related to nonsurvival. Inadequate treatment may be a cause of increased morbidity and mortality in TBI patients. Although there are limitations, we believe this study is worthwhile for considering the survival period of nonsurvivors for the first time. Building on our study, we should make a conscious effort to increase the survival duration and provide rapid and adequate treatment for TBI patients. We also hope our study may play a role in improving and compensating for the defect of the KNTDBS in the future.
Acknowledgements
This work was supported by Wonkwang University in 2015. Authors are thankful to Professor Cheol Lee of Wonkwang University Hospital for statistical advice and members of the KNTDB investigators include Bo-Ra Seo (CNNUH2), Byeong-Cheol Rim (CBNUH), Byung-Moon Cho (HUKH), Hyun-Ho Jung (WSCH), Jae-Hoon Kim (EJUH), Jeong-Ho Lee (DFH), Jong-Hyun Kim (KUKH), Min-Su Kim (YNUH), Seung-Won Choi (CNNUH1), Taek-Kyun Nam (CAUH), and Young-Jin Song (DAUH).
References
- 1.BMJ Best Practice. Evaluation of traumatic brain injury, acute. Available at: http://bestpractice.bmj.com/best-practice/monograph/515.html.
- 2.Brown AW, Elovic EP, Kothari S, Flanagan SR, Kwasnica C. Congenital and acquired brain injury. 1. Epidemiology, pathophysiology, prognostication, innovative treatments, and prevention. Arch Phys Med Rehabil. 2008;89(3 Suppl 1):S3–S8. doi: 10.1016/j.apmr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Colantonio A, Croxford R, Farooq S, Laporte A, Coyte PC. Trends in hospitalization associated with traumatic brain injury in a publicly insured population, 1992-2002. J Trauma. 2009;66:179–183. doi: 10.1097/TA.0b013e3181715d66. [DOI] [PubMed] [Google Scholar]
- 4.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States : Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 5.Hardman JM, Manoukian A. Pathology of head trauma. Neuroimaging Clin N Am. 2002;12:175–187. doi: 10.1016/s1052-5149(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Haring RS, Narang K, Canner JK, Asemota AO, George BP, Selvarajah S, et al. Traumatic brain injury in the elderly : morbidity and mortality trends and risk factors. J Surg Res. 2015;195:1–9. doi: 10.1016/j.jss.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Kraus JF, Black MA, Hessol N, Ley P, Rokaw W, Sullivan C, et al. The incidence of acute brain injury and serious impairment in a defined population. Am J Epidemiol. 1984;119:186–201. doi: 10.1093/oxfordjournals.aje.a113737. [DOI] [PubMed] [Google Scholar]
- 8.Lagbas C, Bazargan-Hejazi S, Shaheen M, Kermah D, Pan D. Traumatic brain injury related hospitalization and mortality in California. Biomed Res Int. 2013;2013:143092. doi: 10.1155/2013/143092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KS. Estimation of the incidence of head injury in Korea : an approximation based on national traffic accident statistics. J Korean Med Sci. 2001;16:342–346. doi: 10.3346/jkms.2001.16.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 11.Marshall LF. Head injury : recent past, present, and future. Neurosurgery. 2000;47:546–561. doi: 10.1097/00006123-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Lyketsos C. Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics. 2000;41:95–103. doi: 10.1176/appi.psy.41.2.95. [DOI] [PubMed] [Google Scholar]
- 13.Taussky P, Widmer HR, Takala J, Fandino J. Outcome after acute traumatic subdural and epidural haematoma in Switzerland : a single-centre experience. Swiss Med Wkly. 2008;138:281–285. doi: 10.4414/smw.2008.12056. [DOI] [PubMed] [Google Scholar]
- 14.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United State : a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 16.Yattoo G, Tabish A. The profile of head injuries and traumatic brain injury deaths in Kashmir. J Trauma Manag Outcomes. 2008;2:5. doi: 10.1186/1752-2897-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]