Abstract
Objective
To assess the maternal demographic characteristics and uterine artery (UA) Doppler parameters at first and second trimesters of pregnancy as predictors for hypertensive disorders (HDs) and adverse perinatal outcomes.
Methods
This prospective cohort study comprised 162 singleton low-risk women undergoing routine antenatal care. The left and right UA were assessed by color and pulsed Doppler and the mean pulsatility and resistance indices as well as the presence of a bilateral protodiastolic notch were recorded at 11 to 14 and 20 to 24 weeks' gestation. Multilevel regression analysis was used to determine the effects of maternal characteristics and abnormal UA Doppler parameters on the incidence of HD, small for gestational age newborn, cesarean section rate, Apgar score <7 at 1st and 5th minute, and admission to the neonatal intensive care unit.
Results
Fifteen women (9.2%) developed HD. UA mean resistance index (RI), UA mean pulsatility index, and parity were independent predictors of HD. Compared to the pregnancies with a normal UA mean RI at the first and second trimesters, pregnancies with UA mean RI >95th percentile only at the first trimester showed an increased risk for HD (odds ratio, 23.25; 95% confidence interval, 3.47 to 155.73; P<0.01). Similar result was found for UA mean pulsatility index >95th percentile (odds ratio, 9.84; 95% confidence interval, 1.05 to 92.10; P=0.05). The model including maternal age, maternal and paternal ethnicity, occupation, parity and UA mean RI increased the relative risk for HD (area under receiver operating characteristics, 0.81).
Conclusion
A first-trimester screening combining maternal characteristics and UA Doppler parameters is useful to predict HD in a low-risk population.
Keywords: Doppler ultrasonography, First trimester of pregnancy, Pregnancy-induced hypertension, Second trimester of pregnancy, Uterine artery
Introduction
Nowadays, arterial hypertension is one of the most prevalent obstetric complications with high rates of maternal and perinatal morbidity and mortality worldwide [1]. In the USA, the prevalence of obstetric hospitalizations related to hypertensive disorders (HDs) increased from 67.000 to 81.000 between 1998 and 2006 [2]. As a result of this increase in the number of cases, there was also severe increase in rates of serious maternal and perinatal complications such as abruption placentae, thrombocytopenia, acute pulmonary edema, stroke, intrauterine growth restriction, fetal death, and elective prematurity.
Pregnancy-related HD are thought to be the consequence of impaired trophoblastic invasion of the maternal spiral arteries, resulting in maintenance of vessels of high resistance, inadequate perfusion of the placenta, tissue injury, and increased production of vasoconstrictive substances [3]. In these cases, there are qualitative and quantitative changes in the maternal uterine artery (UA) Doppler waveforms [4,5]. Accordingly, several studies have examined the potential value of the UA Doppler velocimetry as screening test for HD during pregnancy [6,7,8].
Some maternal UA Doppler studies have shown that this method is significantly better than traditional screening by maternal demographic characteristics and medical history in predicting HD [9]. However, differences among UA Doppler study protocols, measured Doppler parameters, gestational age at screening and clinical outcomes considered contribute to the delay in adopting the test universally. Conde-Agudelo et al. [10] have concluded that "there is not a clinically useful test to be applied in the clinical practice to predict HDs of pregnancy".
The majority of the studies evaluated the UA Doppler in the second trimester of pregnancy, supposedly because the trophoblastic invasion of the maternal spiral arteries has finished at this point. On the other hand, there is now strong evidence demonstrating that abnormal UA Doppler in the first trimester of pregnancy is also associated with abnormalities in trophoblast invasion [11]. Consequently, some authors have been proposed the assessment of UA Doppler in the first and second trimester of pregnancy in order to increase the sensitivity for predicting HD. Gomez et al. [12] have assessed the UA Doppler of 870 singleton pregnancies over two gestational age intervals: 11 to 14 weeks and 19 to 22 weeks. They showed that the UA mean pulsatility index (PI) significantly decreased within each of the two intervals considered and the persistence of an abnormal mean PI from the first to the second trimester identified pregnant women with the greatest risk for HD and/or fetal growth restriction.
Therefore, the objective of this study was to assess the UA Doppler at the first and second trimester of pregnancy as screening test for HD and adverse perinatal outcomes in a low-risk population since more than half of women who develop such complications have no risk factors in their history.
Materials and methods
We conducted a prospective cohort study with 174 low-risk pregnant women undergoing routine antenatal care. All participants were recruited from a group of women admitted to the Reference Centre of Women's Health of Ribeirão Preto (MATER), state of São Paulo, Brazil, from March 2011 to November 2012. This is a 40-bed unit that serves approximately 3,600 low-risk pregnant women per year in Brazil's public health system. The aim and methodology of the study was explained to all recruited women. Voluntary participation was requested, and informed consent was obtained. This study was approved by the local Ethics Research Committee (protocol number 14366/2009) in agreement with the current procedures and according to the internationally acknowledged STROBE criteria.
Gestational age was calculated from the last menstrual period and confirmed by crown-rump length measurement at the time of the first-trimester scan. The eligibility criteria included a singleton pregnancy at 11+0 to 13+6 weeks of gestation with a normal fetus, body mass index <30 kg/m2, no smoking, alcoholism or maternal drug addiction, and absence of chronic diseases and of treatment with aspirin, heparin or antihypertensive drugs before enrolment. The exclusion criteria were fetal malformation or in the newborn, abortion, loss to follow up, and failure to acquire data from the medical records.
UA artery Doppler recordings were obtained prospectively twice, first at 11 to 14 weeks of gestation and second at 20 to 24 weeks. All scans were performed by only one experienced sonographer (RMS) using a Voluson 730 Expert ultrasound machine (General Electric Medical Systems, Milwaukee, WI, USA) equipped with both transvaginal (5 to 9 MHz) and transabdominal (4 to 8 MHz) probe. In the first trimester, transabdominal ultrasound was used to perform UA Doppler examination. A mid-sagittal section of the uterus with visualization of the cervical canal and internal cervical os was obtained. The transducer was gently tilted from side to side, and the right and left UA were identified by color flow mapping along the side of the uterine cervix at the level of the internal os. After ensuring that the insonation angle was <30°, pulsed-wave Doppler with the sampling gate set at 3mm was used to capture the entire vessel width. The signal was updated until at least three similar consecutive waveforms were obtained, and both the PI and resistance index (RI) of the right and left UA was measured electronically once and recorded as well as the presence and/or absence of protodiastolic notch. In the second trimester, a transvaginal transducer was used to obtain a sagittal section of the cervix. Patients were oriented to empty their bladders and were placed in the dorsal lithotomy position. The probe was then introduced into the vagina and placed in the anterior fornix. Subsequently, the probe was moved into the lateral fornix and the UA was identified using color Doppler at the level of the internal cervical os as described by Papageorghiou et al. [13]. Pulsed-wave Doppler was then applied to obtain waveforms of both UA similarly to the first trimester of pregnancy. Abnormal UA Doppler findings were the presence of bilateral protodiastolic notch and/or a mean PI or RI values >95th percentile [14].
Maternal demographic characteristics, medical history and ultrasonographic measurements were recorded in a computer database. Details regarding pregnancy outcomes were added to the database as soon as they became available.
1. Outcome measures
1) Primary outcome
The primary outcome of our study was the development of HD as defined by the Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy [15]. Preeclampsia was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, recorded after 20th week of gestation at least 4 hours apart in previously normotensive, and proteinuria ≥300 mg in 24 hours or one reading of at least + on dipstick analysis of midstream urine specimen. Gestational hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, also recorded after 20th week of gestation at least 4 hours apart in previously normotensive without proteinuria.
2) Secondary outcome
The secondary outcomes of our study were: small for gestational age newborn (birth weight <10th percentile according to the Battaglia and Lubchenco curve [16]), cesarean section rate, Apgar score <7 at 1st and 5th minute, and admission to the neonatal intensive care unit (NICU).
2. Sample size calculation
Considering a significance level of 5%, power of the test of 80%, and a 6-fold increase in the risk of perinatal adverse outcomes (especially preeclampsia) in the group of patients with abnormal findings of UA Doppler [13], a sample size of 160 would be enough to perform this study.
3. Statistical analysis
Mean, standard deviation (SD), median, minimum, and maximum were calculated to describe quantitative variables. Percentages were used to describe qualitative variables. The 5th and 95th percentile of the mean UA PI and RI were calculated according to the following formula: percentile=mean+(k×DP), where k corresponded to the percentile of the standard normal distribution, and SD is the sample standard deviation. In this study, k=±1.64 [14]. The Mann-Whitney or χ2 test where appropriate were applied to verify the association between maternal and ultrasound parameters and outcome measures. Simple and multiple logistic regression analysis were used to determine the effects of maternal and ultrasound parameters on the occurrence of the outcomes [17]. A P<0.05 was considered statistically significant. All analyses were performed using SAS ver. 9.0 (SAS Institute Inc., Cary, NC, USA).
Results
Initially, we selected 174 pregnant women, however 12 were excluded by the following reasons: 5 lost to follow-up, 2 abortions, 5 inability to obtain all data from medical records. Of the 162 patients, 15 patients (9.2%) developed HD (preeclampsia or gestational hypertension), 10 (6.1%) had small for gestational age newborns, 65 (40.1%) had cesarean section, 5 newborns (3.1%) had Apgar score <7 at 5th minute, and 10 (6.1%) were admitted to the NICU.
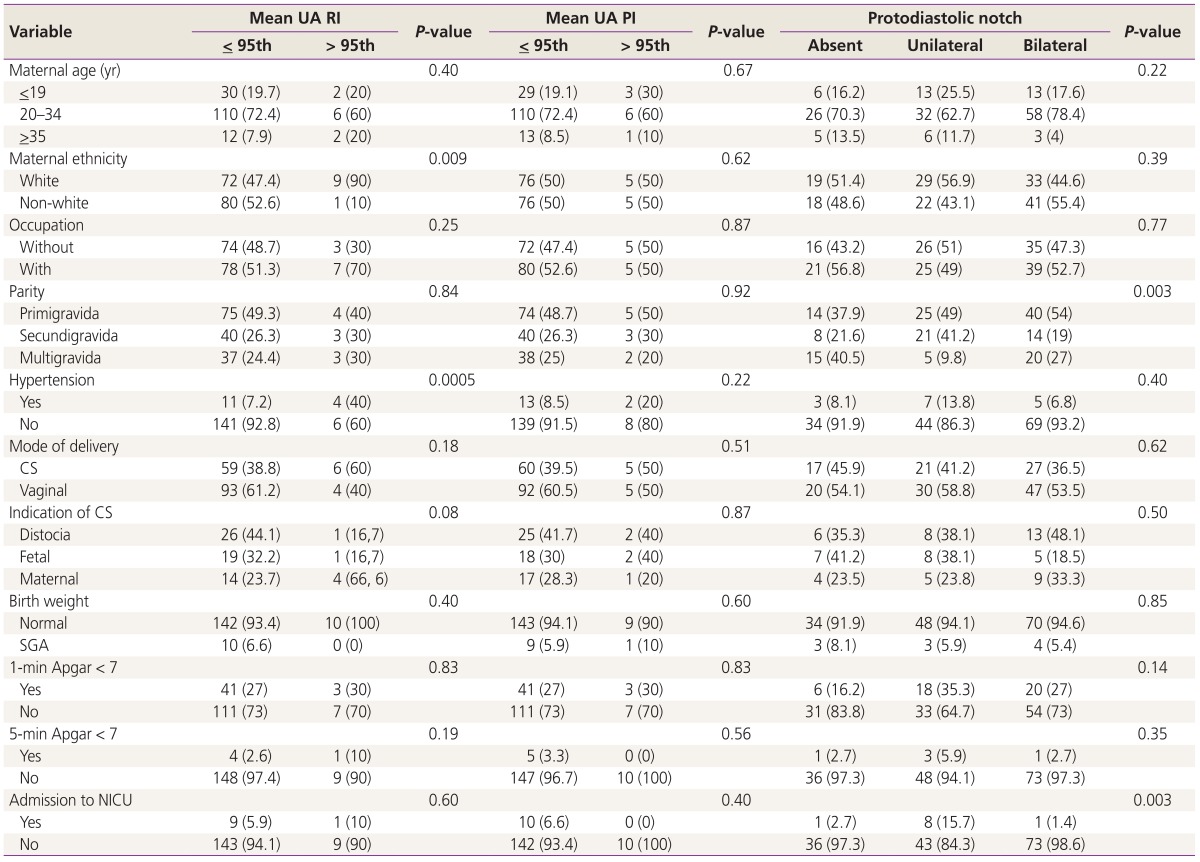
Table 1 shows the relative frequencies of maternal demographic characteristics, and obstetric and perinatal outcomes considering the UA Doppler parameters in the first trimester of pregnancy. Mean±SD of maternal age (years) were 25.2±6.0. Regarding the ethnicity, 81 women (51.0%) were white. Considering the parity, 79 patients (48.7%) were primigravidae, 43 (26.5%) secundigravidae and 40 (24.7%) multigravidae. Table 1 also shows a significant association between mean UA RI and the following variables: HD (P=0.0005) and maternal ethnicity (P=0.009). Still, this table shows a significant association between protodiastolic notch and the following variables: parity (P=0.003) and admission to the NICU (P=0.003).
Table 1. Maternal demographic characteristics, obstetric and perinatal outcomes considering the UA Doppler parameters in the first trimester of pregnancy.
Values are presented as number (%).
UA, uterine artery; RI, resistance index; PI, pulsatility index; CS, cesarean section; SGA, small for gestational age; NICU, neonatal intensive care unit.
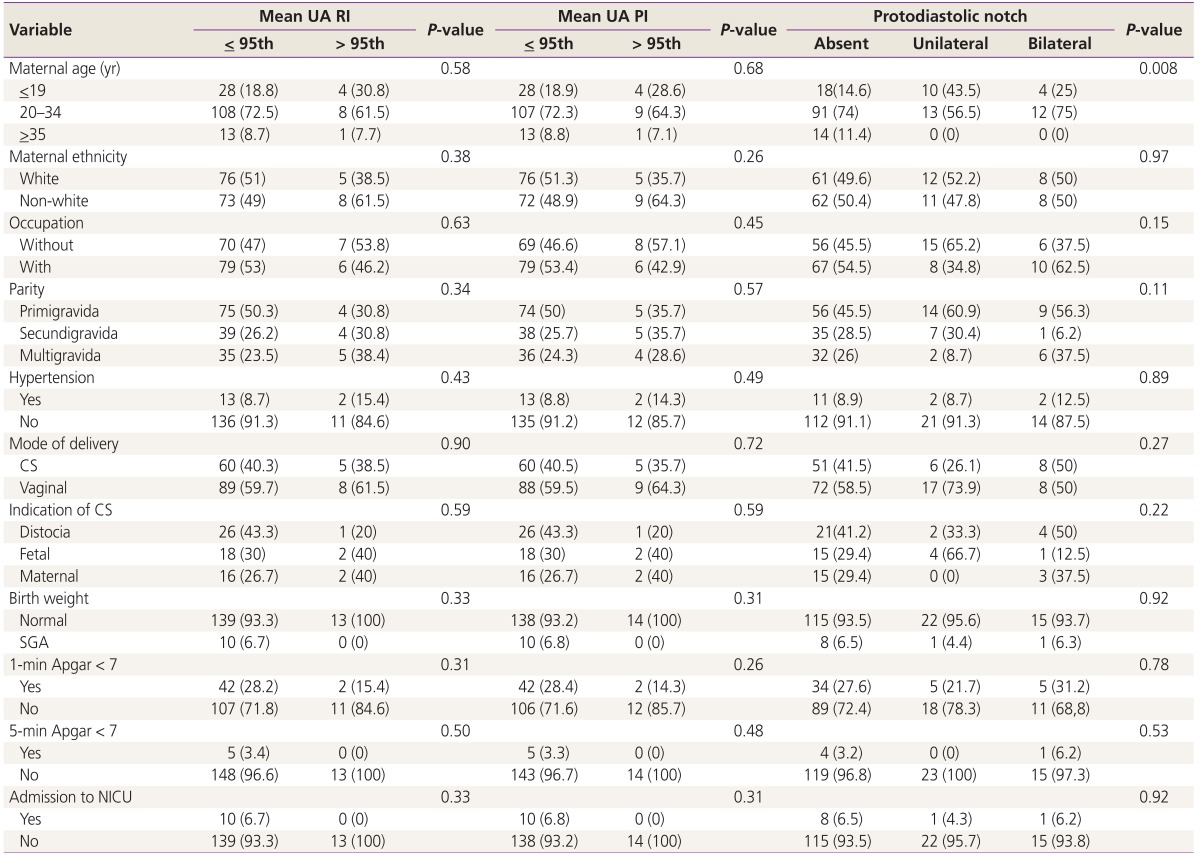
Table 2 shows the relative frequencies of maternal demographic characteristics, and obstetric and perinatal outcomes considering the UA Doppler parameters in the second trimester of pregnancy. This table shows a significant association between maternal age and protodiastolic notch (P=0.008).
Table 2. Maternal demographic characteristics, obstetric and perinatal outcomes considering the UA Doppler parameters in the second trimester of pregnancy.
Values are presented as number (%).
UA, uterine artery; RI, resistance index; PI, pulsatility index; CS, cesarean section; SGA, small for gestational age; NICU, neonatal intensive care unit.
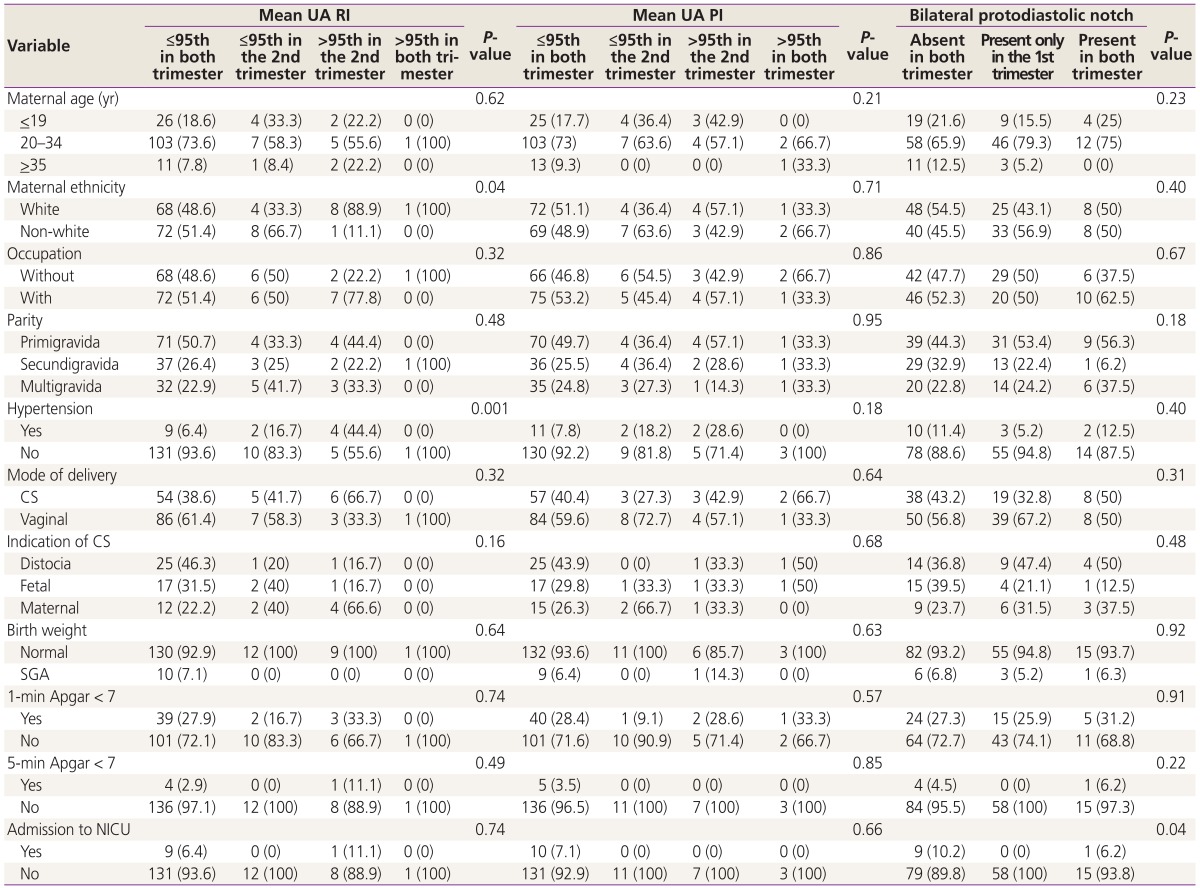
Table 3 shows the relative frequencies of maternal demographic characteristics, and obstetric and perinatal outcomes considering the UA Doppler parameters in both first and second trimester of pregnancy. Again, there is a significant association between mean UA RI and the following variables: HD (P=0.001) and maternal ethnicity (P=0.04). The association between protodiastolic notch and admission to the NICU (P=0.04) also is demonstrated in Table 3.
Table 3. Maternal demographic characteristics, obstetric and perinatal outcomes considering the UA Doppler parameters in both first and second trimester of pregnancy.
Values are presented as number (%).
UA, uterine artery; RI, resistance index; PI, pulsatility index; trim, trimester; CS, cesarean section; SGA, small for gestational age; NICU, neonatal intensive care unit.
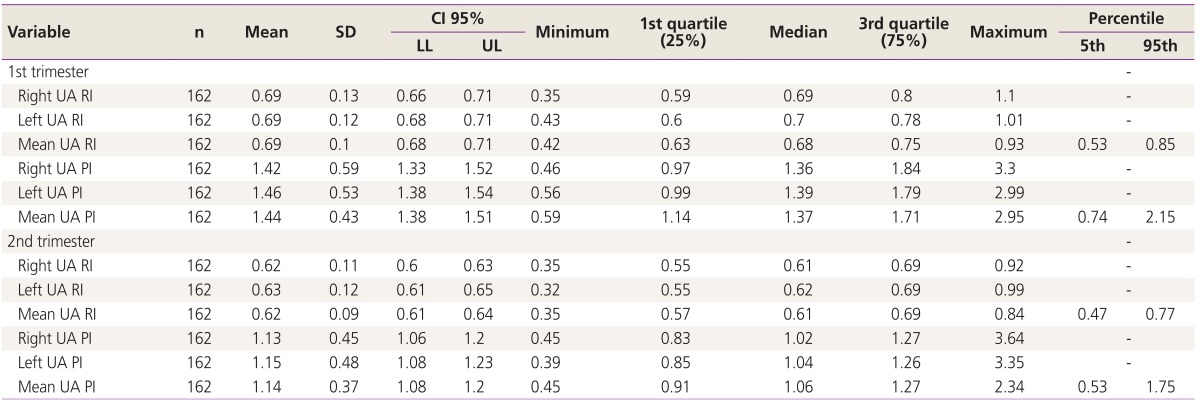
Table 4 shows the mean, SD, median, 1st and 3rd quartile, minimum and maximum values of the UA Doppler parameters in both first and second trimester of pregnancy. Furthermore, the 5th and 95th percentile of the mean UA RI and PI are also demonstrated.
Table 4. Mean, standard deviation, median, 1st and 3rd quartile, minimum, maximum values of the UA Doppler parameters, and 5th and 95th percentile of the mean UA RI and PI in both first and second trimester of pregnancy.
UA, uterine artery; RI, resistance index; PI, pulsatility index; SD, standard deviation; CI, confidence interval; LL, lower limit; UL, upper limit.
Simple and multiple logistic regression analysis were used to determine the effects of maternal and ultrasound parameters on the occurrence of the outcomes. A mean UA RI or PI >95th percentile in the first trimester of pregnancy increased the risk of HD by 23 (odds ratio [OR], 23.25; 95% confidence interval [CI], 3.47 to 155.73; P<0.01) and 10 fold (OR, 9.84; 95% CI, 1.05 to 92.10; P=0.05), respectively when compared to the mean UA RI or PI ≤95th percentile in both trimesters. The presence of bilateral protodiastolic notch or any other maternal parameter showed no effects on the outcome measures such as HD, cesarean section rate, Apgar score <7 at 1st and 5th minute, and admission to the NICU.
Discussion
In our study, we proposed a combined screening for HD and other maternal and perinatal adverse outcomes using the UA Doppler parameters both in the first and second trimester of pregnancy. In the present study, the maternal ethnicity seems to influence the reshuffle of maternal spiral arteries in the first trimester since there is a higher proportion of white women with abnormal values of mean UA RI at this point of pregnancy. This result is in disagreement with that observed by some authors [18,19] who have demonstrated that non-white women have an increased risk for HD and/or other obstetric complications. On the other hand, after logistic regression analyses the maternal white race did not appear as risk factor for any outcome measures.
Parity was also a maternal characteristic that could interfere in the trophoblastic invasion of the maternal spiral arteries in the present study. Primigravidae represented more than half (56.3%) of pregnant women who presented a bilateral protodiastolic notch in both first and second trimester of pregnancy. Nulliparity is a known risk factor for HD as well as multiparity with an adverse obstetric history [20,21].
In the second trimester, maternal age also showed effect on the UA protodistolic notch. In UK, the universal screening for preeclampsia using the UA Doppler study is indicated to pregnant women >40 years [22], and significant contributions were obtained when this screening is applied to patients with age over 35 [19,23].
In our study, the mean UA RI was calculated in both trimesters of pregnancy. We considered abnormal values greater than 0.85 and 0.77 in the first and second trimester, respectively. In a prospective study conducted by Alves et al. [24] studying 409 singleton pregnancies between 11 and 14 weeks of gestation, the 95th percentile of the mean UA RI was similar to our study (0.80). Considering the second trimester, Valensise et al. [25] demonstrated that a mean UA RI greater than 0.58 was a good predictor of HD. Kurdi et al. [26] showed increase of the detection rate of preeclampsia in women with bilateral notches with mean UA RI greater than 0.55 measured between 19 and 21 weeks of pregnancy. Albaiges et al. [27] evaluated pregnant women at 23 weeks of gestation and obtained a mean UA RI of 0.69 (95th percentile). The authors showed that UA mean resistance indices perform better than do velocity indices in the prediction of adverse pregnancy outcome such as preeclampsia, small for gestational age newborn, placental abruption and intrauterine death, irrespective of notch status. These differences in values may be attributed to different methodological aspects, sample size, UA Doppler protocols, and heterogeneity of subjects included in the studies.
Regarding the mean UA PI, values greater than 2.15 and 1.75 were considered abnormal in the first and second trimester, respectively. Gomez et al. [28] performed a transversal study assessing 620 pregnant women throughout pregnancy and demonstrated the following values of 95th percentile: 2.7 at 11 weeks and 2.2 at 14 weeks of pregnancy. In our study, the 95th percentile of the mean UA PI was similar to the values recorded by those authors at the end of the first trimester probably because the majority of the Gomez et al. patients were included at this gestational age. The 95th percentile values demonstrated by the Alves et al. [24] ranged from 2.5 to 1.9 at 11 weeks and 14 weeks of pregnancy, respectively. Therefore, the 95th percentile of the mean UA PI of the present study lies within this range of values. In a multicenter study conducted by Papageorghiou et al. [13], the 95th percentile of the mean UA PI in the second trimester was 1.63. Similar finding were published by Gomez et al. [12]. These authors showed 95th percentile values of mean UA PI of 1.77 at 20 weeks and 1.52 at 22 weeks of pregnancy. This result is in agreement with the value recorded by us in the second trimester of pregnancy.
We observed a possible association between development of HD and abnormal mean UA RI in the first trimester of pregnancy. After logistic regression analysis, an abnormal mean UA RI and PI (>95th percentile) only in the first increased the risk of HD by 23 and 10 fold when compared to pregnant women with normal impedance indices in both trimesters of pregnancy. According to Gomez et al. [12], women in whom the UA mean.
PI shifted from abnormal to normal between the two trimesters show a significant risk of HD and intrauterine growth restriction (OR, 5; 95% CI, 2.1 to 10.6). These findings are widely confirmed by numerous studies with similar results [13,21,25,27,29,30].
The strengths of our study include the well-designed prospective follow-up of patients, an adequate interobserver reproducibility, an extensive statistical evaluation, and the strict inclusion criteria of low-risk pregnant women. It is crucial to emphasize that there are some doubts about the validity of the universal screening for HD in this group. So, the results of this research provide more information about this. Furthermore, all scans were performed by only one experienced sonographer using a single ultrasound machine with the same Doppler settings.
Nevertheless, our study has several limitations. First, the sample size may be insufficient for calculating the risk of HD and/or other outcome measures for some maternal and ultrasound parameters. Moreover, we did not separate HD in preeclampsia or gestational hypertension. Furthermore, we did not consider early-onset or late-onset preeclampsia.
In conclusion, the first-trimester screening at 11 to 14 weeks combining maternal characteristics and UA Doppler parameters is useful to predict HD in low-risk pregnant women, particularly in settings in which it is not possible to analyze maternal biochemical markers.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toal M, Chan C, Fallah S, Alkazaleh F, Chaddha V, Windrim RC, et al. Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol. 2007;196:363.e1–363.e7. doi: 10.1016/j.ajog.2006.10.897. [DOI] [PubMed] [Google Scholar]
- 5.Tuuli MG, Odibo AO. The role of serum markers and uterine artery Doppler in identifying at-risk pregnancies. Clin Perinatol. 2011;38:1–19. doi: 10.1016/j.clp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2004;18:383–396. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;30:742–749. doi: 10.1002/uog.5157. [DOI] [PubMed] [Google Scholar]
- 8.Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, et al. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014;43:500–507. doi: 10.1002/uog.13275. [DOI] [PubMed] [Google Scholar]
- 9.Papageorghiou AT, Roberts N. Uterine artery Doppler screening for adverse pregnancy outcome. Curr Opin Obstet Gynecol. 2005;17:584–590. doi: 10.1097/01.gco.0000191898.84567.04. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Villar J, Lindheimer M. World Health Organization systematic review of screening tests for preeclampsia. Obstet Gynecol. 2004;104:1367–1391. doi: 10.1097/01.AOG.0000147599.47713.5d. [DOI] [PubMed] [Google Scholar]
- 11.Prefumo F, Sebire NJ, Thilaganathan B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod. 2004;19:206–209. doi: 10.1093/humrep/deh037. [DOI] [PubMed] [Google Scholar]
- 12.Gomez O, Figueras F, Martinez JM, del Rio M, Palacio M, Eixarch E, et al. Sequential changes in uterine artery blood flow pattern between the first and second trimesters of gestation in relation to pregnancy outcome. Ultrasound Obstet Gynecol. 2006;28:802–808. doi: 10.1002/uog.2814. [DOI] [PubMed] [Google Scholar]
- 13.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening Group. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:441–449. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 14.Royston P, Wright EM. How to construct 'normal ranges' for fetal variables. Ultrasound Obstet Gynecol. 1998;11:30–38. doi: 10.1046/j.1469-0705.1998.11010030.x. [DOI] [PubMed] [Google Scholar]
- 15.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 16.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–163. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York (NY): John Willey & Sons; 2000. [Google Scholar]
- 18.Gallo DM, Poon LC, Akolekar R, Syngelaki A, Nicolaides KH. Prediction of preeclampsia by uterine artery Doppler at 20-24 weeks' gestation. Fetal Diagn Ther. 2013;34:241–247. doi: 10.1159/000356171. [DOI] [PubMed] [Google Scholar]
- 19.Khalil A, Rezende J, Akolekar R, Syngelaki A, Nicolaides KH. Maternal racial origin and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;41:278–285. doi: 10.1002/uog.12313. [DOI] [PubMed] [Google Scholar]
- 20.Harrington K, Fayyad A, Thakur V, Aquilina J. The value of uterine artery Doppler in the prediction of uteroplacental complications in multiparous women. Ultrasound Obstet Gynecol. 2004;23:50–55. doi: 10.1002/uog.932. [DOI] [PubMed] [Google Scholar]
- 21.Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening Group. An integrated model for the prediction of preeclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2005;193:429–436. doi: 10.1016/j.ajog.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Campbell S. First-trimester screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2005;26:487–489. doi: 10.1002/uog.2591. [DOI] [PubMed] [Google Scholar]
- 23.Harutyunyan A, Armenian H, Petrosyan V. Interbirth interval and history of previous preeclampsia: a case-control study among multiparous women. BMC Pregnancy Childbirth. 2013;13:244. doi: 10.1186/1471-2393-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves JA, Silva BY, de Sousa PC, Maia SB, Costa Fda S. Reference range of uterine artery Doppler parameters between the 11th and 14th pregnancy weeks in a population sample from Northeast Brazil. Rev Bras Ginecol Obstet. 2013;35:357–362. doi: 10.1590/s0100-72032013000800004. [DOI] [PubMed] [Google Scholar]
- 25.Valensise H, Bezzeccheri V, Rizzo G, Tranquilli AL, Garzetti GG, Romanini C. Doppler velocimetry of the uterine artery as a screening test for gestational hypertension. Ultrasound Obstet Gynecol. 1993;3:18–22. doi: 10.1046/j.1469-0705.1993.03010018.x. [DOI] [PubMed] [Google Scholar]
- 26.Kurdi W, Campbell S, Aquilina J, England P, Harrington K. The role of color Doppler imaging of the uterine arteries at 20 weeks' gestation in stratifying antenatal care. Ultrasound Obstet Gynecol. 1998;12:339–345. doi: 10.1046/j.1469-0705.1998.12050339.x. [DOI] [PubMed] [Google Scholar]
- 27.Albaiges G, Missfelder-Lobos H, Parra M, Lees C, Cooper D, Nicolaides KH. Comparison of color Doppler uterine artery indices in a population at high risk for adverse outcome at 24 weeks' gestation. Ultrasound Obstet Gynecol. 2003;21:170–173. doi: 10.1002/uog.30. [DOI] [PubMed] [Google Scholar]
- 28.Gomez O, Figueras F, Fernandez S, Bennasar M, Martinez JM, Puerto B, et al. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet Gynecol. 2008;32:128–132. doi: 10.1002/uog.5315. [DOI] [PubMed] [Google Scholar]
- 29.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks' gestation. Obstet Gynecol. 2000;96:559–564. doi: 10.1016/s0029-7844(00)00946-7. [DOI] [PubMed] [Google Scholar]
- 30.Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, et al. The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low-risk population. Obstet Gynecol. 2012;120:815–822. doi: 10.1097/AOG.0b013e31826af7fb. [DOI] [PMC free article] [PubMed] [Google Scholar]