Abstract
Objective
The results of epidemiological studies investigated the association between polycystic ovary syndrome (PCOS) and the breast cancer are inconsistent. This meta-analysis was conducted to estimate the association between PCOS and the breast cancer risk. We searched PubMed, Web of Science, and Scopus for observational studies until June 2015. Data were independently extracted and analyzed using 95% odds ratio, and confidence intervals (CIs) based on the random-effects models.
Methods
We identified 970 references and conducted eight studies with 45,470 participants and 243,064 person- year.
Results
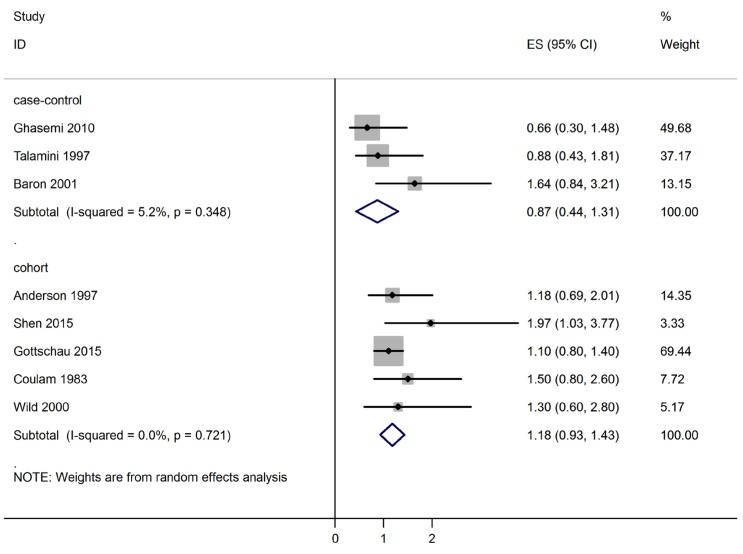
The association between PCOS and the breast cancer risk in case-control studies 0.87 (95% CI, 0.44 to 1.31) and that of cohort studies was estimated 1.18 (95% CI, 0.93 to 1.43).
Conclusion
This meta-analysis demonstrated that PCOS no does increase the risk of breast cancer. Further prospective cohort studies are needed to provide convincing evidence in order to PCOS can increase or not effect on the risk of the breast cancer.
Keywords: Breast neoplasms, Case-control studies, Cohort studies, Meta-analysis, Polycystic ovary syndrome
Introduction
The polycystic ovary syndrome (PCOS) is the most common endocrine disturbance [1], affecting 5% to 8% of women of reproductive age [2]. PCOS is defined as a multi-system disorder [3]. Obesity, hypertension, diabetes mellitus type 2, dyslipidemia and cancer commonly are coexisted in women with PCOS [4]. Women with PCOS are more likely to suffer complications of pregnancy than women with normal ovaries and these include increased risk of miscarriage, gestational diabetes and pre-eclampsia [5]. A meta-analysis in 2009 has reported the significantly association between women with PCOS and endometrial cancer (odds ratio [OR], 2.70; 95% confidence interval [CI], 1.00 to 7.29) [6].
Breast cancer is the second most frequent type of cancer in the world and the most common among women in the developed and the developing countries [7,8]. The relationship between PCOS and breast cancer is complicated by the fact that PCOS is associated with factors that both increase (later age at first pregnancy) and reduce (later age at menarche, anovulatory cycles) the risk of breast cancer. Also, obesity is a major risk factor for breast cancer and is often linked with PCOS [9].
Several epidemiological studies investigated the association between PCOS and the breast cancer, but the results are inconsistent [10,11,12,13]. In a meta-analysis of three comparative studies, Barry et al. [3] showed that the risk of breast cancer was not significantly increased in women with PCOS. They searched Embase and Medline databases. The present meta-analysis conducted to estimate the overall effect PCOS on the breast cancer in women of reproductive age based on the current evidence.
Materials and methods
1. Criteria for including studies
The outcome of interest was breast cancer. The exposure of interest was PCOS. Epidemiological studies, including Cohort, case-control, and cross-sectional studies were included so as to address the relationship between PCOS and breast cancer, irrespective of age, race, publication date and language.
2. Search methods
The key words used were "carcinoma or cancer or tumor or malignancy" and "polycystic ovar or polycystic ovary syndrome" and "breast". Major electronic databases including PubMed, Web of Science, and Scopus were searched until June 2015. In order to find additional references, the reference lists of the included studies were screened. In addition, we contacted the authors of the studies for more potentially eligible studies. Furthermore, the conference databases were searched.
3. Data collection and analysis
Two authors independently made the decision on which studies met the inclusion criteria for the objective of this meta-analysis. Any disagreement was resolved after a discussion by the authors. Two authors extracted the data from the included studies. The variables extracted for analysis contained the first author's name, the year and the country where the study was conducted, study design, age mean/range (year), sample size, the effect measure and its 95% CI.
We assessed the risk of bias among the included studies using Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) considering the following key elements: limitation, consistency, directness, precision, reporting bias, strength, gradient, and confounding [14].
4. Heterogeneity and publication bias
The statistical heterogeneity was explored applying the Q-test. The I2 statistic was used for assessing inconsistency in the results [15]. The potential for publication bias was examined by the Egger's [16] and Begg's [17] tests and was visualized using a funnel plot. The OR and its associated 95% CI were mentioned as a measure of the association between PCOS and the breast cancer risk. Wherever reported, we employed the full adjusted forms of OR controlled for at least one or more of the potential confounding factors such as age, income, oral contraceptive, Body mass index, family history of breast carcinoma and menopause status. Data were analyzed and the results were employed for using of a random-effects model [18]. Since the results and the number of the included studies were respectively homogenous and limited, no subgroup analysis was performed. All statistical analyses were performed at a significance level of 0.05 using the Stata ver. 11 (StataCorp, College Station, TX, USA).
Results
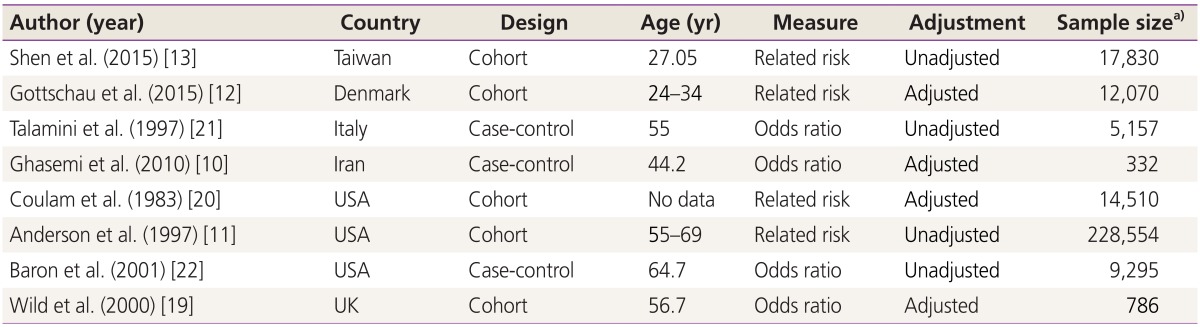
We identified 970 references, 725 references through searching electronic database and 245 references after checking reference lists. No reference was found through searching conference databases. Four hundred and seventeen duplicates and 525 irrelevant references were excluded after reading titles and abstracts, while 20 were excluded after reviewing full texts (Fig. 1). Eight studies remained for the final meta-analysis, which included 45,470 participants and 243,064 person-year: five cohort [11,12,13,19,20], and three case-control studies [10,21,22] (Table 1).
Fig. 1. Flow of information through the different phases of the meta-analysis.
Table 1. Summary of studies results.
a)Year or person year.
The relationship between PCOS and breast cancer is shown in Fig. 2. Based on OR estimates obtained from case-control studies and related risk from cohort studies, there was no significantly association between PCOS and the breast cancer (0.87; 95% CI, 0.44 to 1.31) and (1.18; 95% CI, 0.93 to 1.43), respectively.
Fig. 2. Forest plot of the association between polycystic ovary syndrome and breast cancer.
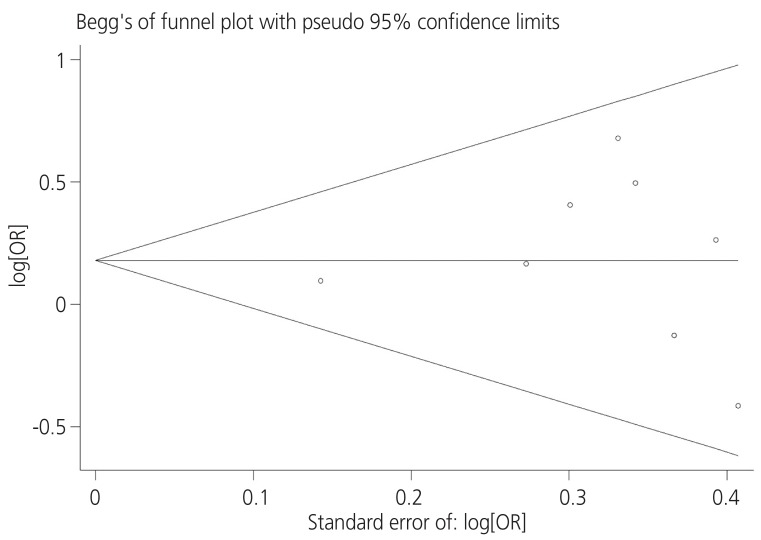
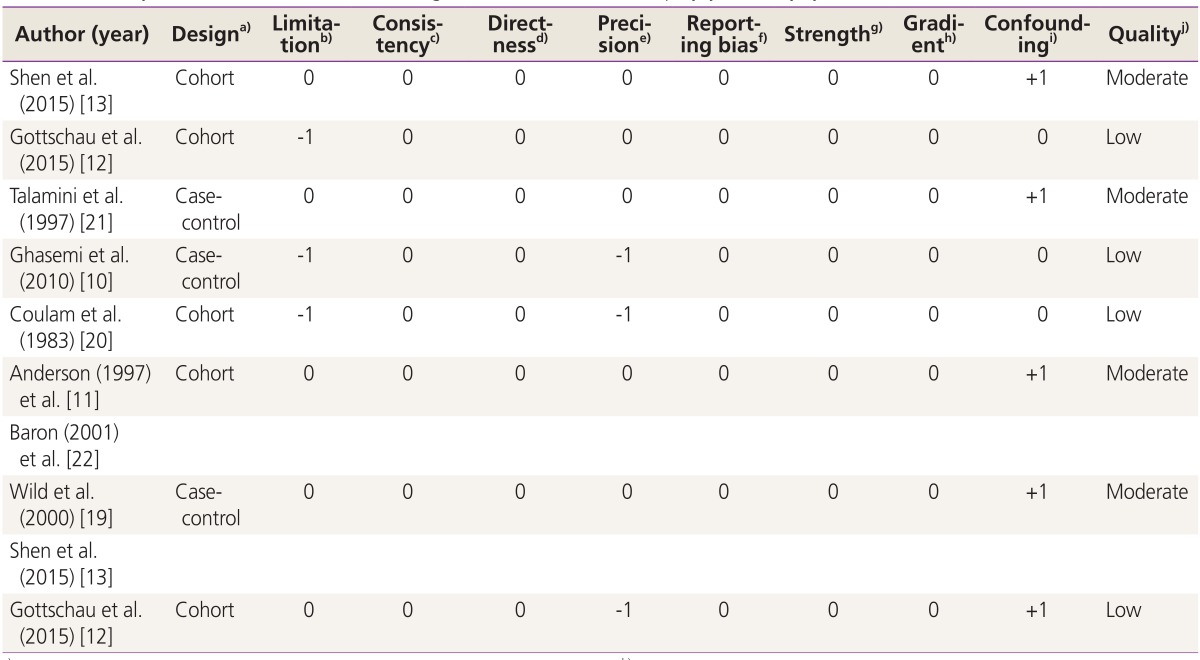
The I2 statistics and Cochran homogeneity test revealed no evidence of heterogeneity among the included studies addressing the association between PCOS and breast cancer in case-control studies (5.2%, P=0.348) and in cohort studies (0%, P=0.721). Publication bias was employed using Begg's and Egger's tests and was visualized employing a funnel plot. The Egger regression test and Begg's test revealed no evidence of publication bias among the included studies that addressed addressing the association between PCOS and the breast cancer risk (P=0.716 and P=0.621), respectively. No evidence of publication bias was observed in the funnel plots (Fig. 3). Table 2 indicates the quality of the studies assessed using GRADE. As is seen, four studies were moderate in quality while four the other studies had low qualities [10,11,12,13,19,20,21,22].
Fig. 3. Funnel plot of included studies addressing the association between polycystic ovary syndrome and breast cancer. OR, odds ratio.
Table 2. Quality assessment of studies addressing the association between polycystic ovary syndrome and breast cancer risk.
a)Randomized trial, cohort study, case-control study, cross-sectional study; b) (a) Incomparability of the groups, (b) different measurement methods, (c) unmatched or unadjusted, (d) short follow-up (in cohort studies), 1 limitation=-1, ≥2 limitation=-2; c)Inverse point estimate compared to overall estimate | no overlap overestimate=-1; d)Interested intervention/exposure, interested outcome, interested population; some (-1) or major (-2) uncertainty about directness; e)If sample size <2,000 and confidence interval includes 1.0=-1, otherwise=0; f)Possibility of selective outcome reporting=-1, otherwise=0; g)RR >2 | <0.5=+1, RR >5 | <0.2)=+2, otherwise=0; h)≥2 levels of exposure=+1, 1 level of exposure=0; i) Adjusted/matched=+1, otherwise=0; j)High: if having no negative score with all positive scores, moderate: if having no negative score with at least one positive score, low: if otherwise.
Discussion
This is the first meta-analysis of PCOS and breast cancer that included cohort studies and the meta-analysis results indicated that PCOS not was associated with an increased risk of the breast cancer. Obesity increases the risk of breast, endometrial and ovarian cancers [20,23,24]. In view of the reproductive characteristics and the high prevalence of obesity among women with PCOS [25], we would have expected the association with risk the breast cancer. However, this meta-analysis indicated that PCOS not associated with an increased risk of the breast cancer. A meta-analysis was conducted by Barry et al. in 2014 [3]. They searched Medline and Embase databases until 2013 and retrieved three case-control studies. It was shown that the risk of the breast cancer not increased with PCOS. The overall effect of PCOS on the breast cancer risk was 0.95 (95% CI, 0.64 to 1.39). This meta-analysis searched Medline and Embase databases and did not included cohort studies. Chittenden et al. in 2009 [6] conducted another meta-analysis in order to assess the effect of PCOS on the breast cancer risk. Three case-control studies indexed in Medline and Embase until 2009 were retrieved. They reported that the breast cancer risk not increased with PCOS 0.88 (95% CI, 0.44 to 1.77). However, this meta-analysis was limited to case-control studies. The Begg and Egger tests for publication bias were statistically significant neither for cohort nor for case-control studies. This determinates that the sensitivity of the search strategy was good enough to find the eligible studies.
Certain limitations to our finding should, however, be considered, the most important of which was the limited number of eligible studies. Second, we attempted to use an adjusted form of OR estimate, while some studies did not report adjusted forms of effect measure, an issue which may result in information bias. Third, we could not assess the effect of confounding variables such as hormone therapy, menopausal status and family history of breast cancer, an issue which may lead to selection bias. Finally, we found 'ONE' study that seemed potentially eligible to be included in this meta-analysis, but we could not access the full text of this study. This issue may raise the possibility of selection bias.
This meta-analysis demonstrated that PCOS no does increase the risk of the breast cancer. Further prospective cohort studies are needed to provide convincing evidence in order to PCOS can increase or not effect on the risk of the breast cancer.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update. 2001;7:522–525. doi: 10.1093/humupd/7.6.522. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 5.Wild SH, Bryden JR, Lee RJ, Bishop JL, Finlayson AR, Byrne CD, et al. Cancer, cardiovascular disease and diabetes mortality among women with a history of endometrial cancer. Br J Cancer. 2007;96:1747–1749. doi: 10.1038/sj.bjc.6603761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for management of breast cancer. Cairo: World Health Organization; 2006. [Google Scholar]
- 8.Tehranian N, Shobeiri F, Pour FH, Hagizadeh E. Risk factors for breast cancer in Iranian women aged less than 40 years. Asian Pac J Cancer Prev. 2010;11:1723–1725. [PubMed] [Google Scholar]
- 9.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Ghasemi N, Mortazavizadeh MR, Gerdekoohi AK. Frequency of poly cystic ovary syndrome in patients with premenopausal breast cancer. Iran J Reprod Med. 2010;8:86–89. [Google Scholar]
- 11.Anderson KE, Sellers TA, Chen PL, Rich SS, Hong CP, Folsom AR. Association of Stein-Leventhal syndrome with the incidence of postmenopausal breast carcinoma in a large prospective study of women in Iowa. Cancer. 1997;79:494–499. [PubMed] [Google Scholar]
- 12.Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136:99–103. doi: 10.1016/j.ygyno.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Shen CC, Yang AC, Hung JH, Hu LY, Tsai SJ. A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist. 2015;20:45–49. doi: 10.1634/theoncologist.2014-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schunemann H, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations [Internet] [Place unknown]: The GRADE Working Group; 2013. [cited 2016 Aug 5]. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 20.Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61:403–407. [PubMed] [Google Scholar]
- 21.Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, La Vecchia C. Selected medical conditions and risk of breast cancer. Br J Cancer. 1997;75:1699–1703. doi: 10.1038/bjc.1997.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron JA, Weiderpass E, Newcomb PA, Stampfer M, Titus-Ernstoff L, Egan KM, et al. Metabolic disorders and breast cancer risk (United States) Cancer Causes Control. 2001;12:875–880. doi: 10.1023/a:1013796112348. [DOI] [PubMed] [Google Scholar]
- 23.Poorolajal J, Jenabi E, Masoumi SZ. Body mass index effects on risk of ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:7665–7671. doi: 10.7314/apjcp.2014.15.18.7665. [DOI] [PubMed] [Google Scholar]
- 24.Jenabi E, Poorolajal J. The effect of body mass index on endometrial cancer: a meta-analysis. Public Health. 2015;129:872–880. doi: 10.1016/j.puhe.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Polycystic ovary syndrome and gynecological cancers: is there a link? Gynecol Endocrinol. 2005;20:200–208. doi: 10.1080/09513590400021201. [DOI] [PubMed] [Google Scholar]