Abstract
AIM
To explore the effects of lectin-like ox-LDL receptor (LOX-1) on innate immunity against Aspergillus fumigatus (A. fumigatus ) in mice cornea.
METHODS
The mRNA levels of LOX-1 were tested in normal and A. fumigatus infected corneas of C57BL/6 and BALB/c mice. The expression of LOX-1, pro-inflammatory cytokines TNF-α, CXCL1 and IL-6, anti-inflammatory cytokines IL-10, and matrix metalloproteinase 9 (MMP9) were tested with treatment with LOX-1 neutralizing antibody or control IgG in A. fumigatus infected corneas of C57BL/6. Macrophages and neutrophils were extracted from susceptible C57BL/6 mice, and pretreated with LOX-1 neutralizing antibody or IgG, then stimulated with A. fumigatus. The mRNA levels of LOX-1, TNF-α, CXCL1, IL-6, IL-10 and MMP9 were evaluated by polymerase chain reaction.
RESULTS
The expression of LOX-1 was significantly increased in C57BL/6 mice corneas after A. fumigatus infection compared with BABL/c mice. After treatment with LOX-1 neutralizing antibody, the expression of LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 in C57BL/6 corneas were significantly decreased compared with treatment with control IgG; the expression of LOX-1, CXCL1, IL-6 and IL-10 were significantly decreased in macrophages, while TNF-α and MMP9 expressions had no change; LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 expressions were significantly decreased in neutrophils.
CONCLUSION
The expression of LOX-1 can affect the expression of pro-inflammatory and anti-inflammatory cytokines in fungal infected corneas, macrophages and neutrophils of C57BL/6. LOX-1 inhibition rebalances the inflammatory response of fungal keratitis in mice.
Keywords: lectin-like ox-LDL receptor, fungal keratitis, macrophages, neutrophils, mice
INTRODUCTION
Fungal keratitis (FK) is caused by a variety of pathogenic fungi[1], and Aspergillus fumigatus (A. fumigatus) is the most common pathogenic fungi[2]–[3]. Recently the incidence of FK has been increased because of eye trauma, long-term usage of antibiotic or corticosteroid, extended usage of contact lens[2]. As a serious infectious corneal disease, FK induced higher blindness rate than other corneal diseases. Innate immune system is the first line to defense against fungal infection, and participates in the defense through pattern-recognition receptors (PRRs). PRRs mainly include C-type lectin receptors (CLRs), Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs)[4].
Lectin-like ox-LDL receptor (LOX-1) is one of type II membrane protein which belongs to the C-type lectin family, and includes a short N-terminal cytoplasmic domain, a trans-membrane domain and a C-terminal extra-cellular domain. In 1997, Sawamara et al[5] found that LOX-1 is the key receptor of ox-LDL in endothelial cells. LOX-1 is also expressed in monocytes, platelets, smooth muscle cells, and macrophages[6]. LOX-1 can be induced by lots of pathological stimulus. According to the study of Honjo et al[7], LOX-1 inhibition can reduce the inflammatory response, and LOX-1 plays an important role in inflammation. LOX-1 can trigger the oxidative stress response (ROS) which associated with many of the inflammatory signals activation, such as MAPK and nuclear factor-kappa B (NF-κB)[8]. There's no study showed the role of LOX-1 in fungal infected corneas of C57BL/6 mice and in fungal infected macrophages and neutrophils. Therefore, this study explored the role of LOX-1 in inflammatory response of A. fumigatus keratitis and A. fumigatus stimulated macrophages and neutrophils.
MATERIALS AND METHODS
Mice and Reagents
This study was supported by Qingdao University and the experiment conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eight-week-old female C57BL/6 mice was purchased from the Chang Zhou Cavens Laboratory Animal Ltd. A. fumigatus strains (NO3.0772) were purchased from China General Microbiological Culture Collection Center; Sabouroud medium was purchased from Babio biotech (Jinan, Shandong Province, China); Delbeccon's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (San Diego, California, USA); thioglycollate medium was purchased from Hope Bio-Technology Co., Ltd. (Qingdao, Shandong Province, China); sodium thioglycollate was purchased from Xiya Chemical Industry Co., Ltd. (Shanghai, China); goat anti-mouse LOX-1 neutralizing antibody and goat IgG were purchased from R&D Systems (Minneapolis, MN, USA); RNAiso Plus, PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time), primers and SYBR® Premix Ex TaqTM were purchased from TAKARA (Dalian, Liaoning Province, China).
Corneal Infection
Mice were anesthetized by chloral hydrate and magnified 40× using the stereoscopic microscope, and diameter 2 mm scratch was made on central corneal epithelium. The corneal surface was covered by A. fumigatus, and contact lens was placed. Eyelids were sutured. Mice were examined at 1 and 3d post infection (p.i.) to ensure corneas were infected.
Macrophages and Neutrophils Extraction
Thioglycollate medium (3 g) or sodium thioglycollate (3 g) was dissolved by 100 mL distilled water, and liquid was stored at 4°C after high pressure sterilization. For macrophages extraction, 1 mL 3% thioglycollate medium was given by intraperitoneal injection (i.p.) into C57BL/6 mice, and on 7d p.i. mice were sacrificed by cervical dislocation. For neutrophils extraction, 1 mL 3% sodium thioglycollate was given i.p. into C57BL/6 mice, and after 3h, mice were sacrificed. Abdominal skin of sacrificial mice was wiped by 75% alcohol and opened along the middle line. Total 5 mL medium with syringe was injected into mice abdominal cavity. Macrophages and neutrophils were extracted by peritoneal lavage. Cell suspensions were seeded in 12-well culture plates.
Cells Culture and Stimulation
Macrophages and neutrophils were extracted from susceptible C57BL/6 mice. Cells were seeded in growth medium containing DMEM and 5% FBS. Cells suspensions (1×106/mL) were seeded in 12-well culture plates, then cultured in a humidified incubator containing 5% CO2 at 37°C. Macrophages and neutrophils were stimulated by A. fumigatus.
LOX-1 Neutralization
Goat anti-mouse LOX-1 neutralizing antibody (5 µg/5 µL) or control goat IgG (5 µg/5 µL) was given to the left eyes of C57BL/6 mice by subconjunctival injection the day before infection. Then corneas were infected with A. fumigatus 1d p.i. Macrophages and neutrophils were pretreated with LOX-1 neutralizing antibody (10 µg/mL) or IgG (10 µg/mL) for 2h, then macrophages were incubated with A. fumigatus for 12h stimulation and neutrophils were incubated with A. fumigatus for 8h stimulation.
RNA Isolation and Real-time Reverse Transcription-polymerase Chain Reaction Assay
Normal and infected corneas were removed at 0, 1 and 3d p.i.. Macrophages were collected at 0, 4, 8, 12, 16, 20h stimulation, and neutrophils were collected at 0, 4, 8, 12, 16h stimulation. After treatment with LOX-1 neutralizing antibody or IgG, corneas were removed at 1d p.i., macrophages were collected at 12h stimulation, and neutrophils were collected at 8h stimulation. RNA was separated from suspension by RNAiso plus reagent, and then quantified using spectrophotometry rapidly. RNA (1 µg) was used for first-strand cDNA synthesis according to the reverse transcription system. Then cDNA (2 µL) was used for polymerase chain reaction (PCR) in 20 µL reaction volume according to the manufacturer's instructions. cDNA was diluted with diethylpyrocarbonate (DEPC)-treated water according to 1:25 proportion. LOX-1, TNF-α, IL-10, CXCL1, IL-6 and MMP9 mRNA levels were tested by real-time reverse transcription (RT)-PCR. Quantification of mRNA expression was analyzed by threshold cycle (CT) method compared with normalized by β-actin. In Table 1, primer pairs sequences used for PCR are shown.
Table 1. Nucleotide sequences of mouse primers for real-time RT-PCR.
| Gene | GenBank No. | Primer sequence (5′-3′) |
| β-actin | NM_007393.3 | F: GAT TAC TGC TCT GGC TCC TAG C |
| R: GAC TCA TCG TAC TCC TGC TTG C | ||
| LOX-1 | NM_138648.2 | F: AGG TCC TTG TCC ACA AGA CTG G |
| R: ACG CCC CTG GTC TTA AAG AAT TG | ||
| TNF-α | NM_013693.2 | F: ACC CTC ACA CTC AGA TCA TCT T |
| R: GGT TGT CTT TGA GAT CCA TGC | ||
| IL-6 | NM_031168.1 | F: CAC AAG TCC GGA GAG GAG AC |
| R: CAG AAT TGC CAT TGC ACA AC | ||
| CXCL1 | NM_008176.3 | F: TGC ACC CAA ACC GAA GTC |
| R: GTC AGA AGC CAG CGT TCA CC | ||
| MMP9 | NM_0113599.2 | F: CTC TAC AGA GTC TTT GAG TCC GGC AG |
| R: TCA GGA ACT TCC AGT ACC AAC CGT C | ||
| IL-10 | NM_010548.2 | F: TGC TAA CCG ACT CCT TAA TGC AGG AC |
| R: CCT TGA TTT CTG GGC CAT GCT TCT C |
Statistical Analysis
The data was expressed as the mean±standard error of the mean (SEM). Data analysis of cells stimulation at multiple time points was performed by One-way ANOVA test using Graphpad Prism 3.0 software. t-test was used to analyze the data of LOX-1 expression in corneas of C57BL/6 and BALB/c mice and the data of corneas and cells treatment with LOX-1 neutralizing antibody or control IgG. Values were considered to be significant at P<0.05.
RESULTS
LOX-1 Expression in Cornea of C57BL/6 and BALB/c Mice
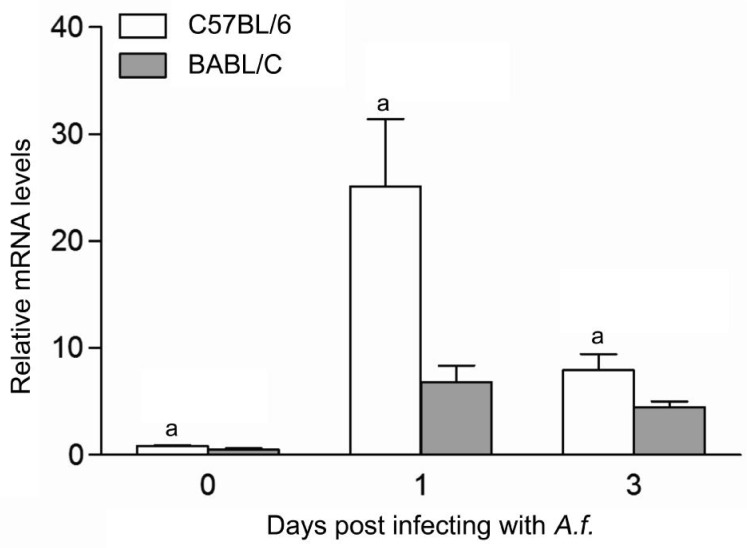
To investigate the expression of LOX-1 in corneas of C57BL/6 and BALB/c mice after A. fumigatus corneal infection for 0, 1, 3d p.i., mRNA levels of LOX-1 was tested in normal and infected corneas of C57BL/6 and BALB/c mice by real-time RT-PCR. Results indicated that the mRNA levels of LOX-1 (Figure 1, P<0.05) was significantly higher in corneas of C57BL/6 mice than BALB/c mice at 0, 1, 3d p.i.
Figure 1. A. fumigatus induced mRNA expression of LOX-1 in corneas of C57BL/6 and BALB/c mice.
After infected with A. fumigatus for 0, 1, 3d, the mRNA levels of LOX-1 were significantly increased in corneas of C57BL/6 mice than BALB/c mice at 0, 1, 3d p.i. aP<0.05.
C57BL/6 Mice Treatment with LOX-1 Neutralizing Antibody Up-regulate LOX-1 Expression in Corneas of C57BL/6 than BALB/c
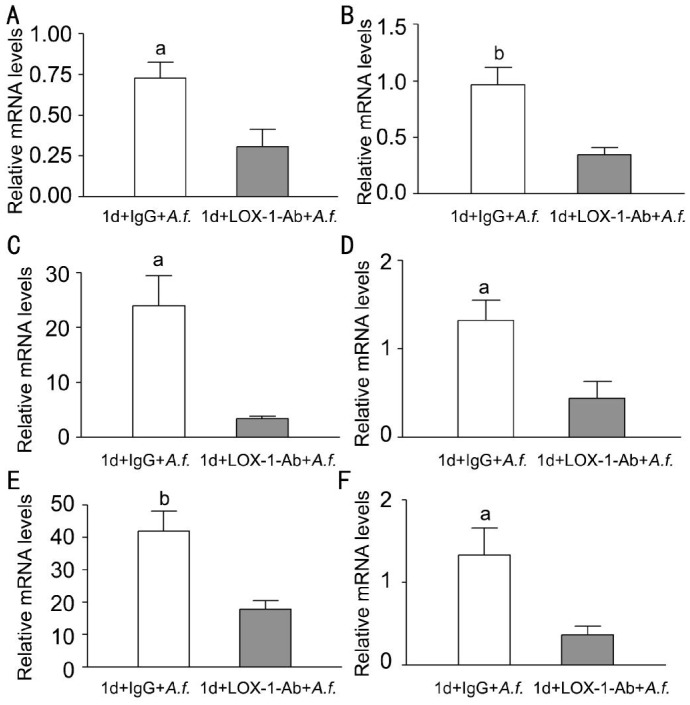
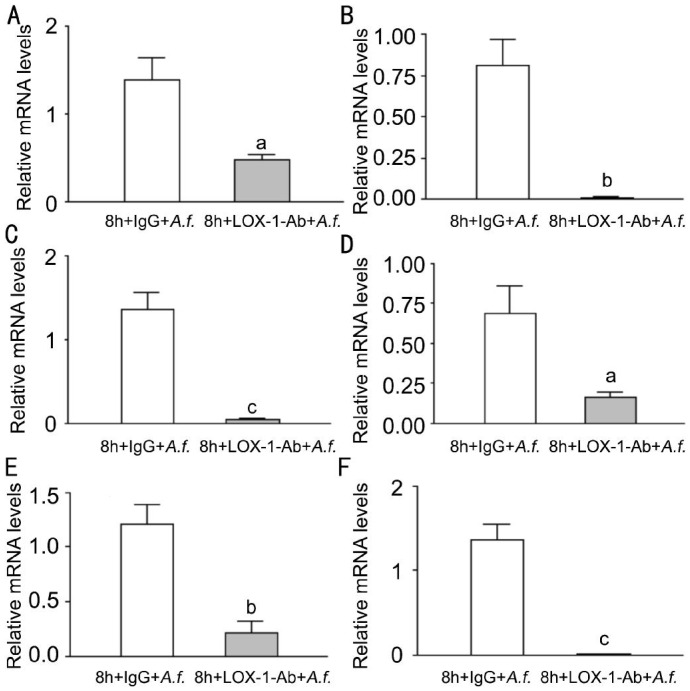
To investigate whether LOX-1 can modulate the corneal infection in corneas of C57BL/6 mice, C57BL/6 corneas were pretreated with LOX-1 neutralizing antibody or IgG before infection, and then infected with A. fumigatus at 1d p.i. The mRNA levels of LOX-1 (Figure 2A, P<0.05); pro-inflammatory cytokines TNF-α (Figure 2B, P<0.01), CXCL1 (Figure 2C, P<0.05) and IL-6 (Figure 2D, P<0.05); MMP9 (Figure 2E, P<0.01); Anti-inflammatory cytokines IL-10 (Figure 2F, P<0.05) were significantly decreased after LOX-1 neutralizing antibody treatment compared with IgG treatment.
Figure 2. Effects of LOX-1 neutralizing antibody treatment on LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 in C57BL/6 mice corneas.
Corneas of C57BL/6 mice treatment with LOX-1 neutralizing antibody compared with IgG at 1d p.i. The mRNA expression of LOX-1 (A), TNF-α (B), CXCL1 (C), IL-6 (D), MMP9 (E), IL-10 (F)were significantly decreased. aP<0.05, bP<0.01.
Effects of LOX-1 on Macrophages of C57BL/6 Mice
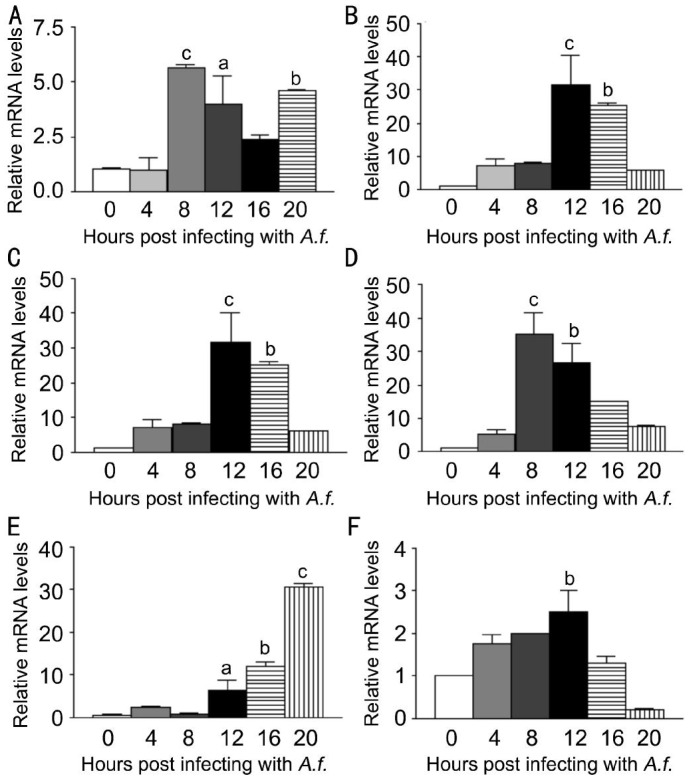
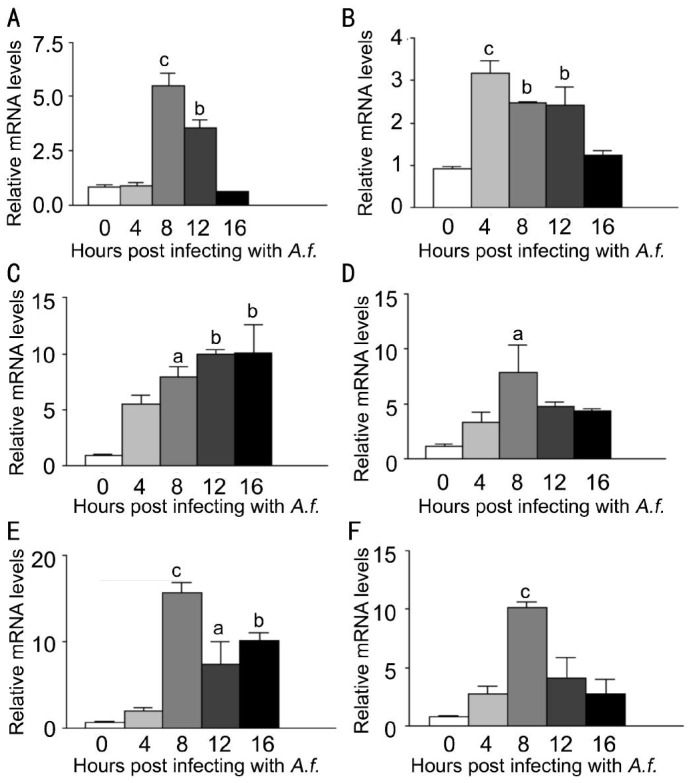
To investigate the expression of LOX-1 and other cytokines after A. fumigatus stimulation of macrophages, macrophages were stimulated with A. fumigatus for 0, 4, 8, 12, 16, 20h.Compared with untreated macrophages, LOX-1 mRNA levels (Figure 3A, P<0.001, P<0.05, P<0.01) were significantly increased at 8, 12, 20h after stimulation. TNF-α mRNA levels (Figure 3B, P<0.001, P<0.01) and CXCL1 mRNA levels (Figure 3C, P<0.001, P<0.01) were significantly increased at 12, 16h, while levels of IL-6 (Figure 3D, P<0.001, P<0.01) were significantly increased at 8, 12h. MMP9 mRNA levels (Figure 3E, P<0.05, P<0.001, P<0.001) were significantly increased at 12, 16, 20h, while IL-10 mRNA levels (Figure 3F, P<0.01) were significantly increased at 12h. To investigate the effect of LOX-1 on macrophages, macrophages were pretreated with LOX-1 neutralizing antibody or IgG for 2h, followed by stimulation of A. fumigatus for 12h. Data indicated that mRNA levels of LOX-1 (Figure 4A, P<0.05) were significantly decreased in antibody treatment compared with IgG treatment. Levels of pro-inflammatory cytokines CXCL1 (Figure 4C, P<0.05) and IL-6 (Figure 4D, P<0.05) mRNA levels were significantly decreased, while TNF-α (Figure 4B) mRNA levels showed no difference. MMP9 mRNA levels (Figure 4E, P<0.05) were significantly increased, while anti-inflammatory cytokines IL-10 mRNA levels (Figure 4F, P<0.05) were significantly decreased.
Figure 3. LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 mRNA expression induced by A. fumigatus stimulation in macrophages extracted from C57BL/6 mice.
Macrophages were infected with A. fumigatus and mRNA expressions were evaluated at 0, 4, 8, 12, 16 and 20h. A: Compared with normal macrophages, the mRNA expression of LOX-1 was significantly increased in the infected cells at 8, 12, 20h; B: The mRNA expression of TNF-α was significantly increased in the infected cells at 12, 16h; C: The mRNA expression of CXCL1 was significantly increased in the infected cells at 12, 16h; D: The mRNA expression of IL-6 was significantly increased in the infected cells at 8, 12h; E: The mRNA expression of MMP9 was significantly increased in the infected cells at 12, 16 and 20h; F: The mRNA expression of IL-10 was significantly increased in the infected cells at 12h. aP<0.05, bP<0.01, cP<0.001.
Figure 4. Effects of LOX-1 neutralizing antibody treatment on LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 in macrophages of C57BL/6.
Macrophages treated with LOX-1 neutralizing antibody compared with IgG at 12h. A: The mRNA expression of LOX-1 was significantly decreased; B: The mRNA expression of TNF-α showed no difference; C: The mRNA expression of CXCL1 was significantly decreased; D: The mRNA expression of IL-6 was significantly decreased; E: The mRNA expression of MMP9 was significantly increased; F: The mRNA expression of IL-10 was significantly decreased. aP<0.05.
Effects of LOX-1 on Neutrophils of C57BL/6 Mice
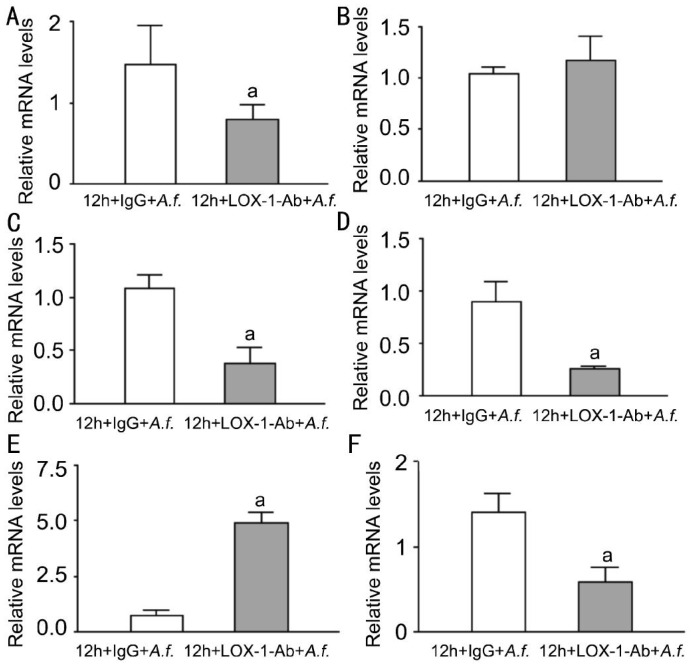
To investigate the expression of LOX-1 and other cytokines after A. fumigatus stimulation of neutrophils, neutrophils were stimulated with A. fumigatus for 0, 4, 8, 12, 16h. The mRNA levels of LOX-1 and other cytokines were tested by PCR. Compared with normal neutrophils, LOX-1 mRNA levels (Figure 5A, P<0.001, P<0.01) were significantly increased at 8, 12h. TNF-α mRNA levels (Figure 5B, P<0.001, P<0.01, P<0.01) were significantly increased at 4, 8, 12h, while CXCL1 mRNA levels (Figure 5C, P<0.05, P<0.01, P<0.01) were significantly increased at 8, 12, 16h. Levels of IL-6 (Figure 5D, P<0.05) were significantly increased at 8h. MMP9 mRNA levels (Figure 5E, P<0.001 P<0.05, P<0.01) were significantly increased at 8, 12, 16h, while IL-10 mRNA levels (Figure 5F, P<0.001) were significantly increased at 8h. To investigate the effect of LOX-1 on neutrophils, neutrophils were pretreated with LOX-1 neutralizing antibody or IgG for 2h, followed by A. fumigatus stimulation for 8h. The mRNA levels of LOX-1 (Figure 6A, P<0.05) were significantly decreased in LOX-1 neutralizing antibody treatment compared with IgG treatment. The levels of pro-inflammatory cytokines TNF-α (Figure 6B, P<0.01), CXCL1 (Figure 6C, P<0.001) and IL-6 (Figure 6D, P<0.05) were significantly decreased. MMP9 mRNA levels (Figure 6E, P<0.01) were significantly decreased. Anti-inflammatory cytokines IL-10 mRNA levels (Figure 6F, P<0.001) were significantly decreased.
Figure 5. LOX-1, TNF-α, CXCL1, IL-6, MMP9 and IL-10 mRNA expression induced by A. fumigatus stimulation in neutrophils extracted from C57BL/6 mice.
Neutrophils were infected with A. fumigatus, and mRNA expression was evaluated at 0, 4, 8, 12 and 16h. A: Compared with normal neutrophils, the mRNA expression of LOX-1 was significantly increased in the infected cells at 8, 12h; B: The mRNA expression of TNF-α was significantly increased in the infected cells at 4, 8 and 12h; C: The mRNA expression of CXCL1 was significantly increased in the infected cells at 8, 12 and 16h; D: The mRNA expression of IL-6 was significantly increased in the infected cells at 8h; E: The mRNA expression of MMP9 was significantly increased in the infected cells at 8, 12 and 16h; F: The mRNA expression of IL-10 was significantly increased in the infected cells at 8h. aP<0.05, bP<0.01, cP<0.001.
Figure 6. Effects of LOX-1 neutralizing antibody treatment on LOX-1 TNF-α, CXCL1, IL-6, MMP9 and IL-10 in neutrophils of C57BL/6.
LOX-1 neutralizing antibody treatment compared with IgG at 8h. A: The mRNA expression of LOX-1 was significantly decreased; B, C, D: The mRNA expression of TNF-α, CXCL1 and IL-6 were significantly decreased; E: The mRNA expression of MMP9 was significantly decreased; F: The mRNA expression of IL-10 was significantly decreased. aP<0.05, bP<0.01, cP<0.001.
DISCUSSION
FK, a serious and infectious corneal ulcer disease, is common in many developing countries[9]–[11]. As the first line, innate immune response plays an important role in defense against fungus. The research of FK innate immune mechanism has a great significance to elucidate the pathogenesis, and provides the direction and basis for clinical treatment.
LOX-1 belongs to the C-type lectin family, and is expressed in endothelial cells, monocytes, platelets, smooth muscle cells, and macrophages. There was study showed that in LOX-1 knockout mice with endotoxemia, the lung injury was significantly reduced, and NF-κB activation was reduced in lungs[12]. LOX-1 expression can affect downstream inflammatory pathways[13]–[15]. After LOX-1 bound by pathogen-associated molecular patterns (PAMPs), ROS is triggered which leads to cell apoptosis by oxidative stress reaction and plays an important role in inflammatory pathways, such as NF-κB. The inflammatory pathways NF-κB promotes the expression of pro-inflammatory cytokines, such as TNF-α, CXCL1 and IL-6. In addition to inflammatory pathways, LOX-1 activation induces pro-angiogenic proteins, such as MMP-9[16]–[19].
In this study, LOX-1 expression was significantly unregulated in mice corneas after A. fumigatus infected and significantly higher in C57BL/6 than BALB/c, explaining that LOX-1 possibly participated in the inflammatory infection. Study of Zou et al[20] had showed that the corneal transparency of C57BL/6 mice was lower than BALB/c mice after corneas infection, and C57BL/6 corneas contained fewer pathogens than BALB/c corneas. To explore the role of LOX-1 in inflammatory response of FK, LOX-1 neutralizing antibody was used to treat C57BL/6 corneas. The data showed that the expression of LOX-1 was inhibited and significantly decreased after treatment with neutralizing antibody compared with control IgG. It has been known that LOX-1 activation can induce the pro-inflammatory pathways NF-κB which promotes the expression of pro-inflammatory cytokines. Data analysis showed that the expression of pro-inflammatory cytokines TNF-α, CXCL1 and IL-6 were also significantly decreased. The result is similar to Wu et al[21] whose study proved the inflammatory response and lung injury caused by sepsis induction decreased in LOX-1 knockout mice. It explained that LOX-1 inhibition can reduce pro-inflammatory cytokines expression in inflammatory response. The expression of inflammatory indicator MMP9 was also significantly decreased. The study of Li et al[22] showed that stronger LOX-1 signals increased the expression of MMP9 in atherogenic mice. IL-10 is an anti-inflammatory cytokines which can down-regulated inflammation and antagonize inflammatory mediators. IL-10 expression was significantly decreased after treatment with LOX-1 neutralizing antibody compared with control IgG.
In vitro, macrophages and neutrophils were extracted from C57BL/6 mice. To explore the effects of LOX-1 on macrophages and neutrophils, cells were pretreated with LOX-1 neutralizing antibody or IgG for 2h. Then cells were stimulated for 8h (macrophages) and 12h (neutrophils) which based on the result of cells stimulation at multiple time points. The data showed that LOX-1, CXCL1, IL-6, and IL-10 expressions were significantly decreased in macrophages; LOX-1 and inflammatory cytokines expressions were significantly decreased in neutrophils. These results were consistent with in vivo. The study of Lee et al[23] showed that pro-inflammatory cytokines expression IL-6 was significantly increased in human umbilical vein endothelial cells treatment with LOX-1 key ligand ox-LDL compared with control cells. And in research of González-Chavarría et al[24] has explained that ox-LDL increases the expression of MMP9 in prostate cancer cells. It explained that inflammatory cytokines expressions reduced after LOX-1 inhibition.
In conclusion, LOX-1 participates in the innate immune system against fungal infections in mice, and LOX-1 inhibition can reduce the inflammatory response.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81170825); Key Project of Natural Science Foundation of Shandong Province (No.ZR2012HZ001); Specialized Research Fund for the Doctoral Program of Higher Education (No.20123706110003); Youth Project of Natural Science Foundation of Shandong Province (No.ZR2013HQ007).
Conflicts of Interest: He K, None; Yue LH, None; Zhao GQ, None; Li C, None; Lin J, None; Jiang N, None; Wang Q, None; Xu Q, None; Peng XD, None; Hu LT, None; Zhang J, None.
REFERENCES
- 1.Nielsen E, Heegaard S, Prause JU, Ivarsen A, Mortensen KL, Hjortdal J. Fungal keratitis-improving diagnostics by confocal microscopy. Case Rep Ophthalmol. 2013;4(3):303–310. doi: 10.1159/000357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 4.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136(1):11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Signal. 2012;10(1):33. doi: 10.1186/1478-811X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100(3):1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 9.Kredics L, Narendran V, Shobana CS, Vágvölgyi C, Manikandan P, Indo-Hungarian Fungal Keratitis Working Group Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses. 2015;58(4):243–260. doi: 10.1111/myc.12306. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37(8):763–771. doi: 10.1111/j.1442-9071.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 11.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14(2):61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Liu MC, Cheng L, Liang M, Ji HL, Fu J. Blockade of LOX-1 prevents endotoxin-induced acute lung inflammation and injury in mice. J Innate Immun. 2009;1(4):358–365. doi: 10.1159/000161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20(4):1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 14.Kuge Y, Kume N, Ishino S, Takai N, Ogawa Y, Mukai T, Minami M, Shiomi M, Saji H. Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull. 2008;31(8):1475–1482. doi: 10.1248/bpb.31.1475. [DOI] [PubMed] [Google Scholar]
- 15.Ishino S, Mukai T, Kuge Y, Kume N, Ogawa M, Takai N, Kamihashi J, Shiomi M, Minami M, Kita T, Saji H. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med. 2008;49(10):1677–1685. doi: 10.2967/jnumed.107.049536. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto K, Ishibashi T, Sawamura T, Inoue N, Kamioka M, et al. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc Res. 2009;84(1):127–136. doi: 10.1093/cvr/cvp177. [DOI] [PubMed] [Google Scholar]
- 17.Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, Pasini A, Cominacini M, Evangelista S, Cominacini L. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediators Inflamm. 2008;2008:367590. doi: 10.1155/2008/367590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol. 2007;27(11):2435–2442. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 19.Hu C, Dandapat A, Mehta JL. Angiotensin II induces capillary formation from endothelial cells via the LOX-1 dependent redox-sensitive pathway. Hypertension. 2007;50(5):952–957. doi: 10.1161/HYPERTENSIONAHA.107.096446. [DOI] [PubMed] [Google Scholar]
- 20.Zou Y, Zhang H, Li H, Chen H, Song W, Wang Y. Strain-dependent production of interleukin-17/interferon-γ and matrix remodeling-associated genes in experimental Candida albicans keratitis. Mol Vis. 2012;18:1215–1225. [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Sawamura T, Kurdowska AK, Ji HL, Idell S, Fu J. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun. 2011;79(7):2865–2870. doi: 10.1128/IAI.01317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, Mehta JL, Beller GA, Glover DK, Meyer CH. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging. 2010;3(4):464–472. doi: 10.1161/CIRCIMAGING.109.896654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010;52(5):1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 24.González-Chavarría I, Cerro RP, Parra NP, Sandoval FA, Zuñiga FA, Omazábal VA, Lamperti LI, Jiménez SP, Fernandez EA, Gutiérrez NA, Rodriguez FS, Onate SA, Sánchez O, Vera JC, Toledo JR. Lectin-like oxidized LDL receptor-1 is an enhancer of tumor angiogenesis in human prostate cancer cells. PLoS One. 2014;9(8):e106219. doi: 10.1371/journal.pone.0106219. [DOI] [PMC free article] [PubMed] [Google Scholar]