Abstract
AIM
To investigate the antiangiogenic effects and safety of topically administered low-molecular-weight heparin-taurocholate 7 (LHT7) on corneal neovascularization (CoNV).
METHODS
Twenty-four Sprague-Dawley rats were randomly distributed into four groups of six rats each. The central corneas were cauterized using a silver/potassium nitrate solution. From 2d after cauterization, 12.5 mg/mL (low LHT7 group) or 25 mg/mL (high LHT7 group) LHT7 was topically administered three times daily; 12.5 mg/mL bevacizumab was topically administered as positive control (bevacizumab) group, with normal saline (NS) administered as negative control (NS group). The corneas were digitally photographed to calculate the CoNV percentage from the neovascularized corneal area at 1 and 2wk.
RESULTS
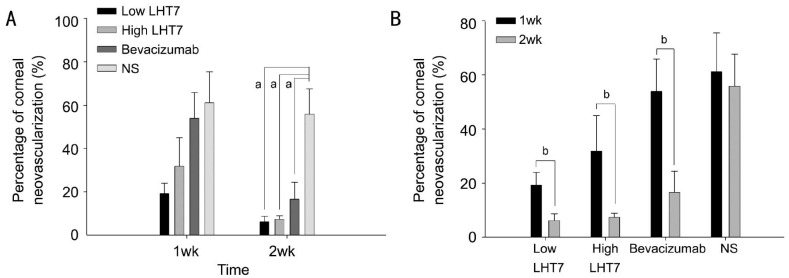
The 4 study groups did not have different CoNV percentages at 1wk after injury (P>0.05). However, the low LHT, high LHT, and bevacizumab groups had significantly lower CoNV percentages than the NS group at 2wk (all P<0.05). No significant differences in CoNV percentage were found among the low LHT, high LHT, and bevacizumab groups (all P>0.05). All groups except the NS group had lower CoNV percentages at 2wk post-injury than the levels observed at 1wk (all P<0.05).
CONCLUSION
Topically-administered LHT7 inhibited CoNV without complication after chemical cauterization in the rat.
Keywords: bevacizumab, chemical cauterization, corneal neovascularization, low-molecular-weight heparin-taurocholate 7
INTRODUCTION
In order to control corneal neovascularization (CoNV), various strategies including the medical modalities including the application of steroids, cyclosporin A, methotrexate, and non-steroidal anti-inflammatory drugs; photodynamic therapy; fine needle diathermy; argon laser photocoagulation and have been attempted[1]–[9]. The systemic or intravitreal anti-vascular endothelial growth factor (VEGF) monoclonal antibody, bevacizumab (Avastin; Roche, Basel, Switzerland) is injected to successfully treat retinopathy of prematurity, age-related macular degeneration, and macular edema caused by diabetic retinopathy and retinal vein occlusion[13]–[16]. The topical administration of bevacizumab can inhibit CoNV[10]–[12].
Heparin antagonizes blood clotting by creating a complex involving antithrombin III[17]–[18]. Heparin sulfate binding to growth factors stabilizes them and attenuates their activation by blocking their diffusion and diminishing proteolytic degradation[19]–[20]. Heparin derivatives are created to not only attenuate hemorrhagic adverse effects but also strengthen the beneficial effects of heparin, such as anti-angiogenesis. Low-molecular-weight heparin-taurocholate 7 (LHT7) is an low-molecular weight heparin (LMWH) conjugated with seven taurocholates, having a polyproline helical structure with more negative charges and facilitating stronger binding to growth factors, including VEGF, than other heparin derivatives[21]. The LHT7 molecule shows unique features that strongly attenuate VEGF165-dependent angiogenesis by antagonizing phosphorylation of the VEGF165 receptor. However, bevacizumab is a monoclonal antibody directly against VEGF165. We recently showed that the subconjunctival LHT7 administration attenuates CoNVs using rat chemical cauterization, despite complications that included corneal stromal hemorrhage[22]. This suggested that topical applications of LHT7 should be considered to overcome this adverse effect. In our present study, we attempted to investigate the effects and safety of topically administered LHT7 on CoNV using the same rat chemical cauterization. So far, the antiangiogenic effects and safety of topical LHT7 have not previously been determined.
MATERIALS AND METHODS
All animal were managed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Asan Medical Center, Seoul, Korea.
Twenty eight healthy 5- to 6-week-old Sprague-Dawley rats weighing 225 g to 275 g were included. Under the deep anesthesia induced by intraperitoneal xylazine hydrochloride (10 mg/kg) administration, the whole procedures were carried out. In addition with topical anesthesia induced by 0.5% (wt/vol) proparacaine hydrochloride (Alcaine; Alcon laboratories, Fort Worth, TX, USA) administration, a chemical injury was created 3 mm in diameter by touching an 75% (wt/vol) silver nitrate/25% (wt/vol) potassium nitrate (Arzol Chemical, Keen, NH, USA)-coated applicator stick onto the central cornea for 8s[23]. Vigorous irrigation was carried out with 10 mL of balanced salt solution (Alcon Laboratories) to remove remained silver nitrate/potassium nitrate solution. All chemical injuries were created by a single researcher to maintain the consistency of chemical injury.
Fourty-eight hours after the injury, the corneal burn injuries were scored as previously reported[20]. Only corneas with burn injury scores of equal or greater than +2 were involved in the evaluation of CoNV score[24]. Four of 28 eyes were excluded in the first week after the injury due to immoderate intraperitoneal anesthesia. Thus, 24 rats were randomly divided to one of four groups after cauterization: the low LHT7 group (n=6) received 0.02 mL of 12.5 mg/mL of LHT7 topically and the high LHT7 group (n=6) received 0.02 mL of 25 mg/mL of LHT7 topically. With regard to randomization, mice were randomized using a stratified design according to which cage they lived. Within each cage, they were randomly assigned to one of four groups. As a positive control, rats in the bevacizumab group (n=6) received 0.02 mL of 12.5 mg/mL of bevacizumab topically, whereas those in the normal saline (NS) group (n=6) received 0.02 mL of 0.9% (wt/vol)) of NS topically as a negative control. In all groups, treatment eyedrops were topically administered three times a day for 14d and started at 48h after injury induction in all groups. The bevacizumab concentration was chosen based on previous reports to facilitate inter-study comparisons[12],[25]. Topical administrations were performed using 20 µg micropipettes (Pipetman P; Gilson, Inc., Paris, France). All LHT7 and bevacizumab eyedrops were made with sterile NS. LHT7 was kindly given by Professor Youngro Byun, College of Pharmacy, Seoul National University, Seoul, Korea.
All eyes were checked using an optical microscopy at 1 and 2wk after injury under the deep anesthesia. All corneas were digitally photographed using a digital camera (32× magnification; Coolpix 4500, Nikon Imaging Japan, Tokyo, Japan). The image analysis for each cornea was carried out using the image software program (Image J; v.1.40; National Institute of Mental Health, Bethesda, MD, USA). We calculated the area of the CoNV in pixels and scored the proportion of this neovascularized area with respect to the whole cornea as the CoNV percentage[5],[26]–[29]. After these calculations for all groups, the animals were sacrificed on week 2.
Data were shown as the means±standard errors (SE). Statistical analyses were carried out to compare CoNV percentages and changes in the CoNV among four groups with the paired Wilcoxon-signed rank test, the Mann-Whitney U test, and analysis of variance (ANOVA) using the SPSS statistics program (version 13.0; SPSS, Chicago, IL, USA). The 95% confidence was obtained as P<0.05.
RESULTS
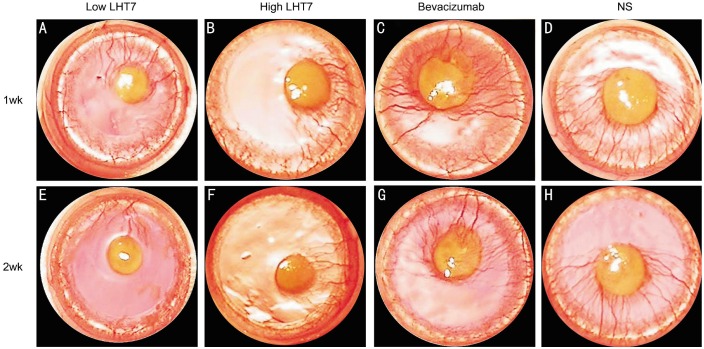
Two days after cauterization, the four study groups did not have different burn injury scores (P=0.83). The CoNV percentage levels at 1 and 2wk after chemical injury (Table 1, Figure 1). All four groups did not have significantly different percentages of CoNV at 1wk post-injury (P>0.05). However, the low LHT, high LHT and bevacizumab groups had significantly lower CoNV levels at 2wk post-injury than the NS control group (all P<0.05). No significant differences in CoNV percentage were found among the low LHT, high LHT, and bevacizumab groups (all P>0.05) (Table 1; Figure 1A). All groups except the NS group had significantly lower CoNV percentages at 2wk than the levels observed at 1wk post-injury (all P<0.05; Table 1; Figure 1B). No adverse effects such as corneal perforation or corneal stromal hemorrhage were observed in any group after cauterization (Figure 2).
Table 1. The low LHT, high LHT and bevacizumab groups had significantly lower CoNV percentages than the NS group.
| Percentage of CoNV, % | Low LHT7 group | High LHT7 group | Bevacizumab group | NS group |
| 1wk | 19.2±4.8 | 31.8±13.2 | 53.9±11.9 | 61.2±14.3 |
| 2wk | 6.2±2.5a | 7.3±1.6a | 16.5±7.8a | 55.9±11.8a |
| Difference between 1 and 2wk | -13.0±2.5b | -24.5±12.7b | -37.4±10.9b | -0.5±4.9 |
aSignificant difference among four groups (P<0.05); bSignificant difference in CoNV between 1 and 2wk (P<0.05).
Figure 1. The low LHT, high LHT and bevacizumab groups had significantly lower CoNV percentages than the NS group.
aSignificant difference among four groups (P<0.05); bSignificant difference in CoNV between 1 and 2wk (P<0.05).
Figure 2. Slit lamp microscopic photographs.
Slit lamp microscopic photographs showing CoNV in eyes treated with LHT7 (low and high LHT7 groups), bevacizumab-treated eyes (bevacizumab group), and normal saline-treated control eyes (NS Group) at 1wk (A, B, C and D) and 2wk (E, F, G and H) after chemical cauterization.
DISCUSSION
Topical bevacizumab administration has been performed previously for the treatment of CoNV in rats[24],[30]–[32] and rabbits[33]–[34]. In our current study, the bevacizumab group, receiving 12.5 mg/mL of this drug topically, was included as a positive control, whilst the NS group as a negative control. The same concentration of topical LHT7 as topical bevacizumab was determined to use in low LHT group, because our previous study showed that the anti-angiogenicity of subconjunctivally administed LHT7 is equivalent to that of subconjunctivally administered bevacizumab[1]. In high LHT group, 25 mg/mL topical LHT was used to observe dose-dependency. Our results suggest that the antiangiogenic effects of a topical application of LHT7 are marginally superior to those of bevacizumab, although this difference did not reach statistical significance (Table 1; Figure 1).
Comparing our current results with those reported previously for subconjunctival LHT7 injection[22], there was a significant difference found in the percentage of CoNV at 2wk after cauterization following topical LHT7 administration. This suggested that for LHT7, a topical treatment produces a more stable and consistent antiangiogenic effect than a subconjunctival injection. Topically administered bevacizumab has been reported to show a longer lasting antiangiogenic effect than subconjunctivally injected bevacizumab in CoNV after chemical injury in rats[12]. However, Hashemian et al[31] have reported that both subconjunctival and topical bevacizumab have equal potency in preventing CoNV in rats.
In terms of complications in our current study, we observed no adverse effects, such as corneal perforation or corneal stromal hemorrhage, in our LHT groups. Conversely, we have previously reported two cases of corneal stromal bleeding, one in each of a low and high LHT7 group that subconjunctivally received 0.02 and 0.04 mL of 25 mg/mL of LHT7, respectively[22]. Hence, the topical administration of LHT7 is proving to be less invasive and safer than the subconjunctival injection of this compound.
Our study had several limitations of note. First, the followed-up period was only 2wk after chemical cauterization, which was a relatively short. Second, we did not determine the optimal dosage for treating CoNV by topical LHT7 and further studies are needed to evaluate the critical range of LHT7 concentrations to use in a clinical application. Finally, the corneas should have been immunohistochemically stained with special antibodies including anti-CD31 antibody after whole mounts to get more accurate results[35], despite CoNVs can be clearly investigated in the rat chemical cauterization model. We conclude from our current analyses, however, that the topical application of LHT7 efficiently attenuates CoNV after chemical cauterization in the rat without producing adverse effects.
Acknowledgments
Foundations: Supported by the Student Research Grant of University of Ulsan College of Medicine, Seoul, Korea (No. 12-13); the Asan Institute for Life Sciences, Seoul, Korea (No. 2014-464).
Conflicts of Interest: Kim JY, None; Kim SY, None; Cheon MH, None; Kim ES, None; Song IS, None; Kim MJ, None; Tchah H, None.
REFERENCES
- 1.Fossarello M, Peiretti E, Zucca I, Serra A. Photodynamic therapy of corneal neovascularization with verteporfin. Cornea. 2003;22(5):485–488. doi: 10.1097/00003226-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Gohto Y, Obana A, Kaneda K, Miki T. Photodynamic effect of a new photosensitizer ATX-S10 on corneal neovascularization. Exp Eye Res. 1998;67(3):313–322. doi: 10.1006/exer.1998.0527. [DOI] [PubMed] [Google Scholar]
- 3.Joussen AM, Poulaki V, Mitsiades N, Stechschulte SU, Kirchhof B, Dartt DA, Fong GH, Rudge J, Wiegand SJ, Yancopoulos GD, Adamis AP. VEGF-dependent conjunctivalization of the corneal surface. Invest Ophthalmol Vis Sci. 2003;44(1):117–123. doi: 10.1167/iovs.01-1277. [DOI] [PubMed] [Google Scholar]
- 4.Nirankari VS, Baer JC. Corneal argon laser photocoagulation for neovascularization in penetrating keratoplasty. Ophthalmology. 1986;93(10):1304–1309. doi: 10.1016/s0161-6420(86)33581-4. [DOI] [PubMed] [Google Scholar]
- 5.Peyman GA, Kivilcim M, Morales AM, DellaCroce JT, Conway MD. Inhibition of corneal angiogenesis by ascorbic acid in the rat model. Graefes Arch Clin Exp Ophthalmol. 2007;245(10):1461–1467. doi: 10.1007/s00417-007-0542-4. [DOI] [PubMed] [Google Scholar]
- 6.Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41(8):2148–2153. [PubMed] [Google Scholar]
- 7.Mirabelli P, Peebo BB, Xeroudaki M, Koulikovska M, Lagali N. Early effects of dexamethasone and anti-VEGF therapy in an inflammatory corneal neovascularization model. Exp Eye Res. 2014;125:118–127. doi: 10.1016/j.exer.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Zhang MC, Wang Z, Zhang Y. Ultrastructural pathology of corneal neovascularization after photodynamic therapy in rabbits. Int J Ophthalmol. 2010;3(4):308–310. doi: 10.3980/j.issn.2222-3959.2010.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucak YY, Erdurmus M, Terzi EH, Kukner A, Celebi S. Inhibitory effects of topical cyclosporine A 0.05% on immune-mediated corneal neovascularization in rabbits. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2555–2561. doi: 10.1007/s00417-013-2467-4. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol. 2009;37(7):730–736. doi: 10.1111/j.1442-9071.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 11.Waisbourd M, Levinger E, Varssano D, Moisseiev E, Zayit-Soudri S, Barak A, Loewenstein A, Barequet I. High-dose topical bevacizumab for corneal neovascularization. Pharmacology. 2013;92(5–6):310–314. doi: 10.1159/000356407. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim D, Kim ES, Kim MJ, Tchah H. Topically administered bevacizumab had longer standing anti-angiogenic effect than subconjunctivally injected bevacizumab in rat corneal neovacularization. Int J Ophthalmol. 2013;6(5):588–591. doi: 10.3980/j.issn.2222-3959.2013.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26(3):352–354. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 15.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36(4):336–339. [PubMed] [Google Scholar]
- 17.Hirsh J. Heparin. N Engl J Med. 1991;324(22):1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci U S A. 1997;94(26):14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Biological implications in neovascularization. Int J Clin Lab Res. 1996;26(1):15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- 20.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E, Kim YS, Bae SM, Kim SK, Jin S, Chung SW, Lee M, Moon HT, Jeon OC, Park RW, Kim IS, Byun Y, Kim SY. Polyproline-type helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int J Cancer. 2009;124(12):2755–2765. doi: 10.1002/ijc.24239. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SY, Kim JY, Kim ES, Kim SY, Kim MJ, Tchah H. Subconjunctival injection of low-molecular-weight heparin-taurocholate 7 inhibits corneal neovascularization. Cornea. 2013;32(11):1488–1492. doi: 10.1097/ICO.0b013e3182a48009. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney JM, Waterbury LD. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Cur Eye Res. 1985;4(5):531–535. doi: 10.3109/02713688508999984. [DOI] [PubMed] [Google Scholar]
- 24.Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin) Br J Ophthalmol. 2007;91(6):804–807. doi: 10.1136/bjo.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115(6):e33–e38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Proia AD, Chandler DB, Haynes WL, Smith CF, Suvarnamani C, Erkel FH, Klintworth GK. Quantitation of corneal neovascularization using computerized image analysis. Lab Invest. 1988;58(4):473–479. [PubMed] [Google Scholar]
- 27.Erdurmus M, Yagci R, Yilmaz B, Hepsen IF, Turkmen C, Aydin B, Karadag R. Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea. 2007;26(6):715–719. doi: 10.1097/ICO.0b013e31804f5a45. [DOI] [PubMed] [Google Scholar]
- 28.Riazi-Esfahani M, Peyman GA, Aydin E, Kazi AA, Kivilcim M, Sanders DR. Prevention of corneal neovascularization: evaluation of various commercially available compounds in an experimental rat model. Cornea. 2006;25(7):801–805. doi: 10.1097/01.ico.0000220768.11778.60. [DOI] [PubMed] [Google Scholar]
- 29.Seo JW, Chung SH, Choi JS, Joo CK. Inhibition of corneal neovascularization in rats by systemic administration of sorafenib. Cornea. 2012;31(8):907–912. doi: 10.1097/ICO.0b013e31823f8b9c. [DOI] [PubMed] [Google Scholar]
- 30.Habot-Wilner Z, Barequet IS, Ivanir Y, Moisseiev J, Rosner M. The inhibitory effect of different concentrations of topical bevacizumab on corneal neovascularization. Acta Ophthalmol. 2010;88(8):862–867. doi: 10.1111/j.1755-3768.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 31.Hashemian MN, H ZM, Moghimi S, Tahvildari M, Mojazi-Amiri H. Prevention of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with its topical form in experimental rats. Ophthalmic Res. 2011;46(1):50–54. doi: 10.1159/000322061. [DOI] [PubMed] [Google Scholar]
- 32.Dursun A, Arici MK, Dursun F, Ozec AV, Toker MI, Erdogan H, Topalkara A. Comparison of the effects of bevacizumab and ranibizumab injection on corneal angiogenesis in an alkali burn induced model. Int J Ophthalmol. 2012;5(4):448–451. doi: 10.3980/j.issn.2222-3959.2012.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadar T, Amir A, Cohen L, Cohen M, Sahar R, Gutman H, Horwitz V, Dachir S. Anti-VEGF therapy (bevacizumab) for sulfur mustard-induced corneal neovascularization associated with delayed limbal stem cell deficiency in rabbits. Curr Eye Res. 2014;39(5):439–450. doi: 10.3109/02713683.2013.850098. [DOI] [PubMed] [Google Scholar]
- 34.Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S, Bartz-Schmidt KU, Szurman P, Tübingen Bevacizumab Study Group Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86(3):322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 35.Ling S, Lin H, Xiang D, Feng G, Zhang X. Clinical and experimental research of corneal lymphangiogenesis after keratoplasty. Ophthalmologica. 2008;222(5):308–316. doi: 10.1159/000144030. [DOI] [PubMed] [Google Scholar]