Abstract
AIM--To investigate the use of the polymerase chain reaction (PCR) in the routine laboratory for the detection of Mycobacterium tuberculosis in clinical samples. METHODS--Samples were divided and processed separately for the detection of M tuberculosis by microscopy, culture and PCR. After DNA extraction, PCR was performed with primers specific for the insertion element IS6110 and the product was analysed by agarose gel electrophoresis, Southern blotting or dot blotting and hybridisation with a digoxigenin labelled internal probe. Each sample was tested for inhibitors of Taq polymerase with the aid of an internal control. Multiple negative and positive controls were used to monitor each step of the procedure. RESULTS--The data from two laboratories, using the same operating procedures, were combined. Of 1957 specimens, 79 (4%) were culture and PCR positive, while 1839 (93.9%) were negative in both tests. Thirty specimens (1.5%) were PCR positive only and nine (0.5%) were culture positive but PCR negative. CONCLUSION--Using culture and clinical history as the gold standard, sensitivity and specificity for PCR were 92.1% and 99.8%, respectively. With elaborate precautions, PCR is a suitable and reliable method for the detection of M tuberculosis in clinical samples in a routine microbiology laboratory.
Full text
PDF

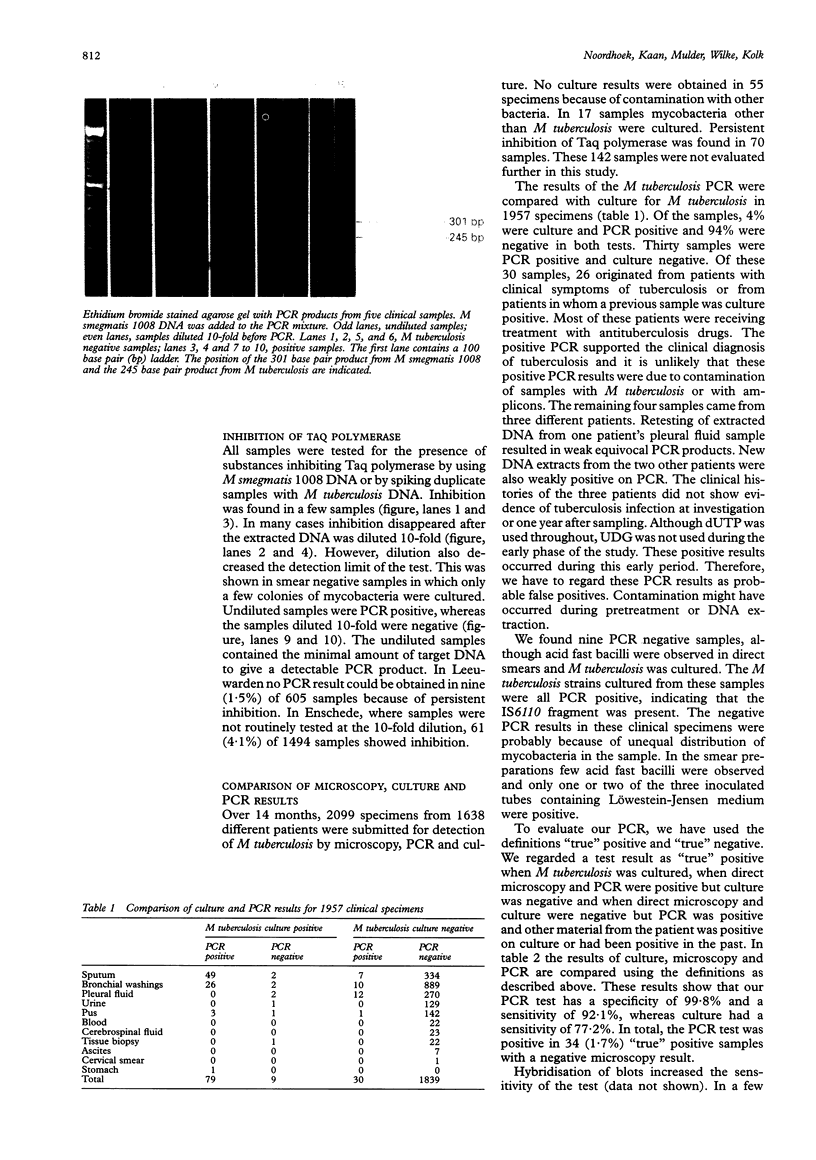


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Hirano K., Wada M., Kazumi Y., Takahashi M., Fukasawa Y., Yoshimura T., Miyagi C., Goto S. Detection of Mycobacterium tuberculosis in clinical specimens by polymerase chain reaction and Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test. J Clin Microbiol. 1993 Dec;31(12):3270–3274. doi: 10.1128/jcm.31.12.3270-3274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. B., Thybo S., Godfrey-Faussett P., Stoker N. G. Polymerase chain reaction for detection of Mycobacterium tuberculosis in sputum. Eur J Clin Microbiol Infect Dis. 1993 Dec;12(12):922–927. doi: 10.1007/BF01992166. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Bodmer T., Gurtner A., Schopfer K., Matter L. Screening of respiratory tract specimens for the presence of Mycobacterium tuberculosis by using the Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test. J Clin Microbiol. 1994 Jun;32(6):1483–1487. doi: 10.1128/jcm.32.6.1483-1487.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noel A., Aznar C., Chureau C., Nguyen S., Pierre C., Bartoli M., Bonete R., Pialoux G., Gicquel B., Garrigue G. Diagnosis of tuberculosis by DNA amplification in clinical practice evaluation. Lancet. 1991 Aug 10;338(8763):364–366. doi: 10.1016/0140-6736(91)90492-8. [DOI] [PubMed] [Google Scholar]
- Clarridge J. E., 3rd, Shawar R. M., Shinnick T. M., Plikaytis B. B. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol. 1993 Aug;31(8):2049–2056. doi: 10.1128/jcm.31.8.2049-2056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Chan S. L., Allen B. W., Mitchison D. A., Lowrie D. B. Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis patients in Hong Kong before, during and after short-course chemotherapy. Tuber Lung Dis. 1993 Feb;74(1):47–51. doi: 10.1016/0962-8479(93)90068-9. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Sifford M. D., Cave M. D., Bates J. H., Crawford J. T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991 Nov;144(5):1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- Forbes B. A., Hicks K. E. Direct detection of Mycobacterium tuberculosis in respiratory specimens in a clinical laboratory by polymerase chain reaction. J Clin Microbiol. 1993 Jul;31(7):1688–1694. doi: 10.1128/jcm.31.7.1688-1694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas V., Alden M. J., Curry J. I., Kamisango K., Knott C. A., Lankford R., Wolfe J. M., Moore D. F. Detection and identification of Mycobacterium tuberculosis directly from sputum sediments by amplification of rRNA. J Clin Microbiol. 1993 Sep;31(9):2410–2416. doi: 10.1128/jcm.31.9.2410-2416.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Noordhoek G. T., de Leeuw O., Kuijper S., van Embden J. D. Mycobacterium smegmatis strain for detection of Mycobacterium tuberculosis by PCR used as internal control for inhibition of amplification and for quantification of bacteria. J Clin Microbiol. 1994 May;32(5):1354–1356. doi: 10.1128/jcm.32.5.1354-1356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Schuitema A. R., Kuijper S., van Leeuwen J., Hermans P. W., van Embden J. D., Hartskeerl R. A. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and a nonradioactive detection system. J Clin Microbiol. 1992 Oct;30(10):2567–2575. doi: 10.1128/jcm.30.10.2567-2575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox L. F., Rhienthong D., Miranda A. M., Udomsantisuk N., Ellis K., van Leeuwen J., van Heusden S., Kuijper S., Kolk A. H. A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J Clin Microbiol. 1994 Mar;32(3):672–678. doi: 10.1128/jcm.32.3.672-678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Longo M. C., Berninger M. S., Hartley J. L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990 Sep 1;93(1):125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- Miller N., Hernandez S. G., Cleary T. J. Evaluation of Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test and PCR for direct detection of Mycobacterium tuberculosis in clinical specimens. J Clin Microbiol. 1994 Feb;32(2):393–397. doi: 10.1128/jcm.32.2.393-397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Koga H., Kohno S., Kaku M. Nested polymerase chain reaction for detection of Mycobacterium tuberculosis in clinical samples. J Clin Microbiol. 1993 Aug;31(8):2228–2232. doi: 10.1128/jcm.31.8.2228-2232.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte F. S., Metchock B., McGowan J. E., Jr, Edwards A., Okwumabua O., Thurmond C., Mitchell P. S., Plikaytis B., Shinnick T. Direct detection of Mycobacterium tuberculosis in sputum by polymerase chain reaction and DNA hybridization. J Clin Microbiol. 1993 Jul;31(7):1777–1782. doi: 10.1128/jcm.31.7.1777-1782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordhoek G. T., Kolk A. H., Bjune G., Catty D., Dale J. W., Fine P. E., Godfrey-Faussett P., Cho S. N., Shinnick T., Svenson S. B. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis: a blind comparison study among seven laboratories. J Clin Microbiol. 1994 Feb;32(2):277–284. doi: 10.1128/jcm.32.2.277-284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordhoek G. T., Wolters E. C., de Jonge M. E., van Embden J. D. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol. 1991 Sep;29(9):1976–1984. doi: 10.1128/jcm.29.9.1976-1984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordhoek G. T., van Embden J. D., Kolk A. H. Questionable reliability of the polymerase chain reaction in the detection of Mycobacterium tuberculosis. N Engl J Med. 1993 Dec 30;329(27):2036–2036. doi: 10.1056/NEJM199312303292713. [DOI] [PubMed] [Google Scholar]
- Pfyffer G. E., Kissling P., Wirth R., Weber R. Direct detection of Mycobacterium tuberculosis complex in respiratory specimens by a target-amplified test system. J Clin Microbiol. 1994 Apr;32(4):918–923. doi: 10.1128/jcm.32.4.918-923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini H., Skurnik M., Liippo K., Tala E., Viljanen M. K. Detection and identification of mycobacteria by amplification of a segment of the gene coding for the 32-kilodalton protein. J Clin Microbiol. 1992 Aug;30(8):2025–2028. doi: 10.1128/jcm.30.8.2025-2028.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. K., Ross B. C., Jackson K. M., Dwyer B. Characterization of Mycobacterium tuberculosis strains from Vietnamese patients by Southern blot hybridization. J Clin Microbiol. 1993 Jun;31(6):1615–1618. doi: 10.1128/jcm.31.6.1615-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soolingen D., de Haas P. E., Hermans P. W., Groenen P. M., van Embden J. D. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993 Aug;31(8):1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




