Abstract
AIMS--To generate new monoclonal antibodies directed against melanoma associated antigens using a new melanoma cell line, KAL. METHODS--The melanoma cell line was established in culture from a lymph node metastasis of malignant melanoma. Normal Balb/c mice were immunised with KAL cells. Splenocytes were used for fusion experiments using standard techniques. Hybridoma supernatants were tested for antibody binding activity using an indirect immunoperoxidase method on frozen sections from KAL tumour cells xenografted onto nude mice and human tonsils. KBA.62 was selected because of its reactivity with melanocytic proliferations on both frozen and paraffin wax sections. RESULTS--On immunoblotting, KBA.62 reacted with three bands of 140, 135 and 128 kD and two weak bands of 88 and 73 kD. In normal human tissues basal melanocytes in the epidermis did not react with this antibody and only occasional labelling of endothelial cells was noted. Of the human tumours, KBA.62 reacted strongly and uniformly with the majority of benign (21/21) and malignant (75/86) melanocytic proliferations. Staining was localised predominantly to the cell membrane with little or no cytoplasmic reactivity. Negative staining was observed in the majority of human non-melanocytic neoplasms, the exceptions being some carcinomas (11/89), particularly the well differentiated squamous cell type. This, however, was not thought to present a diagnostic problem. CONCLUSIONS--KBA.62 appears to be potentially useful in ascertaining the immunomorphological diagnosis of malignant melanoma in routinely processed paraffin wax sections.
Full text
PDF


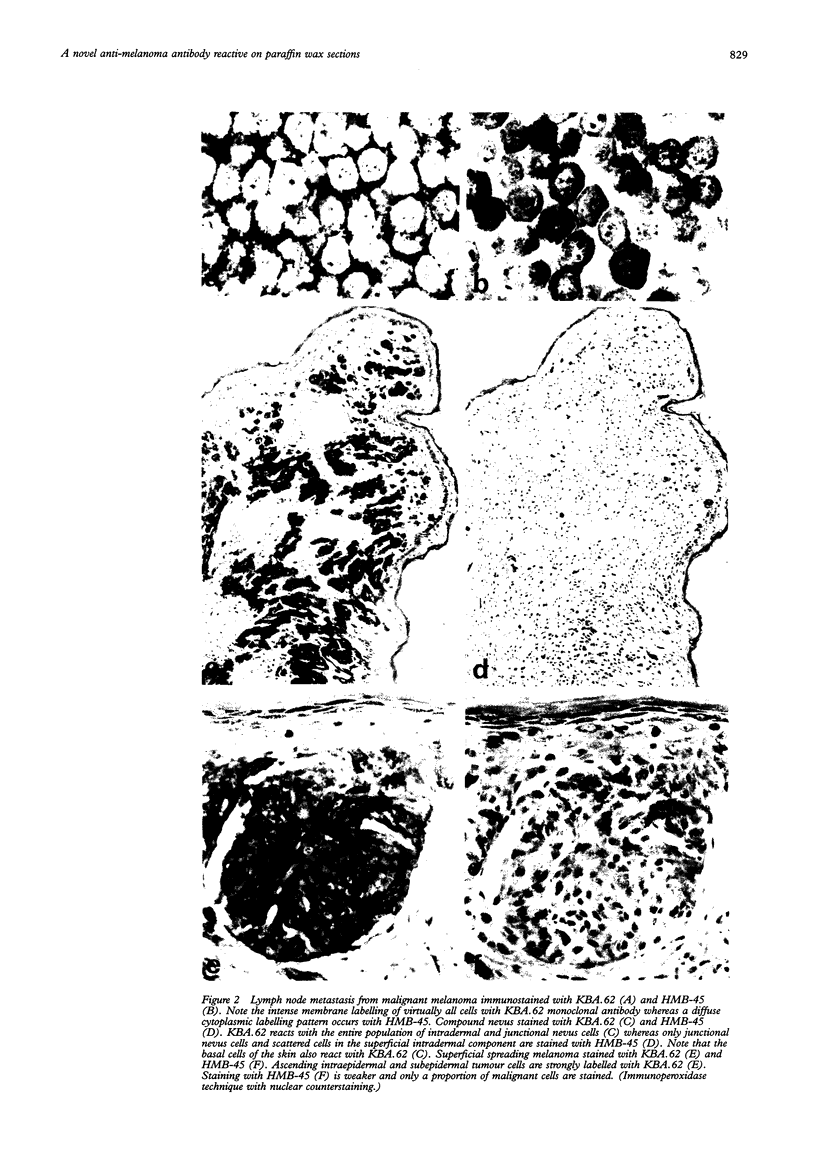


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., de Boer A. J., van 't Hullenaar R., Denijn M., Ruiter D. J., Vogel A. M., Figdor C. G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993 Dec;143(6):1579–1585. [PMC free article] [PubMed] [Google Scholar]
- Al Saati T., Blancher A., Calvas P., Neulat-Duga I., Delsol G. Production of monoclonal antibodies using spleen cells from nude mice bearing human tumors. Ann Pathol. 1987;7(1):1–8. [PubMed] [Google Scholar]
- Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986 Aug;55(2):244–248. [PubMed] [Google Scholar]
- Delsol G., Blancher A., al Saati T., Ralfkiaer E., Lauritzen A., Bruigères L., Brousset P., Rigal-Huguet F., Mazerolles C., Robert A. Antibody BNH9 detects red blood cell-related antigens on anaplastic large cell (CD30+) lymphomas. Br J Cancer. 1991 Aug;64(2):321–326. doi: 10.1038/bjc.1991.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclamado R. M., Gown A. M., Vogel A. M. Unique proteins defined by monoclonal antibodies specific for human melanoma. Some potential clinical applications. Am J Surg. 1986 Oct;152(4):376–385. doi: 10.1016/0002-9610(86)90308-9. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Pulford K. A., Vanstapel M. J., Roach B., Mortimer P., Woolston R. E., Taylor-Papadimitriou J., Lane E. B., Mason D. Y. An immunohistological study of benign and malignant skin tumours: epithelial aspects. Histopathology. 1984 Mar;8(2):209–227. doi: 10.1111/j.1365-2559.1984.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Ralfkiaer E., Skinner J., Brown D., Heryet A., Pulford K. A., Hou-Jensen K., Mason D. Y. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumours. J Clin Pathol. 1985 Dec;38(12):1353–1357. doi: 10.1136/jcp.38.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Herrera G. A., Hancock C., Allen B. C. Specificity of antibody HMB-45. Arch Pathol Lab Med. 1992 Sep;116(9):900–901. [PubMed] [Google Scholar]
- Herrera G. A., Turbat-Herrera E. A., Lott R. L. S-100 protein expression by primary and metastatic adenocarcinomas. Am J Clin Pathol. 1988 Feb;89(2):168–176. doi: 10.1093/ajcp/89.2.168. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurent G., Delsol G., Reyes F., Abbal M., Mihaesco E. Detection of J chain in lymphomas and related disorders. Clin Exp Immunol. 1981 Jun;44(3):620–628. [PMC free article] [PubMed] [Google Scholar]
- Lauritzen A. F., Hou-Jensen K., Ralfkiaer E. P53 protein expression in Hodgkin's disease. APMIS. 1993 Sep;101(9):689–694. doi: 10.1111/j.1699-0463.1993.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Mackie R. M., Campbell I., Turbitt M. L. Use of NK1 C3 monoclonal antibody in the assessment of benign and malignant melanocytic lesions. J Clin Pathol. 1984 Apr;37(4):367–372. doi: 10.1136/jcp.37.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Gatter K. C. The role of immunocytochemistry in diagnostic pathology. J Clin Pathol. 1987 Sep;40(9):1042–1054. doi: 10.1136/jcp.40.9.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Watanabe S., Sato Y., Kameya T., Hirota T., Shimosato Y. An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. Am J Surg Pathol. 1982 Dec;6(8):715–727. doi: 10.1097/00000478-198212000-00003. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Watanabe S., Sato Y., Kameya T., Shimosato Y., Ishihara K. Immunohistochemical demonstration of S100 protein in malignant melanoma and pigmented nevus, and its diagnostic application. Cancer. 1982 Sep 1;50(5):912–918. doi: 10.1002/1097-0142(19820901)50:5<912::aid-cncr2820500519>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Palazzo J., Duray P. H. Typical, dysplastic, congenital, and Spitz nevi: a comparative immunohistochemical study. Hum Pathol. 1989 Apr;20(4):341–346. doi: 10.1016/0046-8177(89)90043-9. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. S., Tron V. A. Analysis of HMB-45 immunoreactivity in common and cellular blue nevi. J Cutan Pathol. 1991 Aug;18(4):261–263. doi: 10.1111/j.1600-0560.1991.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Yates A. J., Banerjee S. S., Bishop P. W., Graham K. E. HMB-45 in non-melanocytic tumours. Histopathology. 1993 Nov;23(5):477–478. doi: 10.1111/j.1365-2559.1993.tb00499.x. [DOI] [PubMed] [Google Scholar]
- al Saati T., Caspar S., Brousset P., Chittal S., Caverivière P., Hounieu H., Dastugue N., Idoipe J. B., Icart J., Mazerolles C. Production of anti-B monoclonal antibodies (DBB.42, DBA.44, DNA.7, and DND.53) reactive on paraffin-embedded tissues with a new B-lymphoma cell line grafted into athymic nude mice. Blood. 1989 Nov 15;74(7):2476–2485. [PubMed] [Google Scholar]
- al Saati T., Clamens S., Cohen-Knafo E., Faye J. C., Prats H., Coindre J. M., Wafflart J., Caverivière P., Bayard F., Delsol G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer. 1993 Oct 21;55(4):651–654. doi: 10.1002/ijc.2910550423. [DOI] [PubMed] [Google Scholar]
- van Duinen S. G., Ruiter D. J., Hageman P., Vennegoor C., Dickersin G. R., Scheffer E., Rümke P. Immunohistochemical and histochemical tools in the diagnosis of amelanotic melanoma. Cancer. 1984 Apr 1;53(7):1566–1573. doi: 10.1002/1097-0142(19840401)53:7<1566::aid-cncr2820530724>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]