Abstract
Background
Parkinson disease (PD) is a debilitating movement disorder that afflicts 1–2% of the population over 50 years of age. The common hallmark for both sporadic and familial forms of PD is mitochondrial dysfunction. Mammals have at least twenty proapoptotic and antiapoptotic Bcl-2 family members, in contrast, only two Bcl-2 family genes have been identified in Drosophila melanogaster, the proapoptotic mitochondrial localized Debcl and the antiapoptotic Buffy. The expression of the human transgene α-synuclein, a gene that is strongly associated with inherited forms of PD, in dopaminergic neurons (DA) of Drosophila, results in loss of neurons and locomotor dysfunction to model PD in flies. The altered expression of Debcl in the DA neurons and neuron-rich eye and along with the expression of α-synuclein offers an opportunity to highlight the role of Debcl in mitochondrial-dependent neuronal degeneration and death.
Results
The directed overexpression of Debcl using the Ddc-Gal4 transgene in the DA of Drosophila resulted in flies with severely decreased survival and a premature age-dependent loss in climbing ability. The inhibition of Debcl resulted in enhanced survival and improved climbing ability whereas the overexpression of Debcl in the α-synuclein-induced Drosophila model of PD resulted in more severe phenotypes. In addition, the co-expression of Debcl along with Buffy partially counteracts the Debcl-induced phenotypes, to improve the lifespan and the associated loss of locomotor ability observed. In complementary experiments, the overexpression of Debcl along with the expression of α-synuclein in the eye, enhanced the eye ablation that results from the overexpression of Debcl. The co-expression of Buffy along with Debcl overexpression results in the rescue of the moderate developmental eye defects. The co-expression of Buffy along with inhibition of Debcl partially restores the eye to a roughened eye phenotype.
Discussion
The overexpression of Debcl in DA neurons produces flies with shortened lifespan and impaired locomotor ability, phenotypes that are strongly associated with models of PD in Drosophila. The co-expression of Debcl along with α-synuclein enhanced the PD-like phenotypes. The co-expression of Debcl along with Buffy suppresses these phenotypes. Complementary experiments in the Drosophila eye show similar trends during development. Taken all together these results suggest a role for Debcl in neurodegenerative disorders.
Keywords: Debcl, Buffy, Drosophila melanogaster, Alpha-synuclein, Model of Parkinson disease
Introduction
Parkinson disease (PD) is a human movement disorder that is strongly associated with the selective and profound degeneration and loss of dopaminergic (DA) neurons to result in a set of marked clinical features (Forno, 1996). The neuropathological hallmarks exhibited by PD patients include the presence of Lewy Bodies (LB) which are intracytoplasmic proteinaceous inclusions composed of α-synuclein and ubiquitin among other proteins (Forno, 1996; Leroy et al., 1998; Polymeropoulos et al., 1997). This atypical protein aggregation and accumulation is believed to lead to cellular toxicity and contribute to the pathogenesis of PD. Additional pathological mechanisms that are associated with PD include aberrant protein aggregation and mitochondrial damage (Gupta, Dawson & Dawson, 2008; Schulz, 2007; Whitworth, 2011). Familial forms of PD have highlighted the genetic basis of PD and the study of the associated gene loci in model organisms offers great understanding of the disease aetiology and pathology (Ambegaokar, Roy & Jackson, 2010; Gasser, 2009; Guo, 2012). The gene encoding α-synuclein, a small soluble protein of largely unknown function predominantly found in neural tissues, was first to be identified as responsible for inherited PD (Polymeropoulos et al., 1997). Mitochondrial dysfunction due to the accumulation of α-synuclein has been implicated as one of the mechanisms leading to PD (Chinta et al., 2010; Choubey et al., 2011; Esteves et al., 2011; Zhu et al., 2011). The association of α-synuclein with components of the mitochondria is thought to lead to oxidative stress, apoptosis, autophagy and eventually, neurodegeneration. The first Drosophila model of PD utilized a human α-synuclein transgene to induce the PD-like symptoms (Feany & Bender, 2000). This model system is very successful and widely applied, as it displays the age-dependent loss of locomotor function, the degeneration of DA neurons and LB-like inclusions, features that are present in human PD (Auluck et al., 2002; Botella et al., 2009; Büttner et al., 2014; Feany & Bender, 2000; Kong et al., 2015; Staveley, 2014; Webb et al., 2003; Zhu et al., 2016). Drosophila has available tissue specific gene enhancers such as TH-Gal4, elav-Gal4 and Ddc-Gal4, which are used to model PD in flies in combination with the powerful bipartite UAS/Gal4 (Brand & Perrimon, 1993) system. Of importance is the correlation between DA neuron loss and the age-dependent loss of locomotor function (Park, Schulz & Lee, 2007; Staveley, 2014) which validates the implication that age-dependent loss of locomotor function is as a result of DA neuron degeneration.
The Bcl-2 family of genes are crucial controllers of apoptosis in animals and are functionally composed of proapoptotic and antiapoptotic members (Adams & Cory, 1998; Cory & Adams, 2002; Fu & Fan, 2002; Siddiqui, Ahad & Ahsan, 2015). In mammals, this multigene family has about 20 members, the antiapoptotic proteins protect the mitochondria from disruption by the proapoptotic proteins (Colin et al., 2009; Cory & Adams, 2002; Martinou & Youle, 2011; Suen, Norris & Youle, 2008; Tsujimoto, 2002). The antiapoptotic members possess four Bcl-2 homology (BH) domains while the proapoptotic members have three to four BH domains. The proapoptotic proteins initiate apoptosis by the permeabilization of the outer mitochondrial membrane which results in the release of apoptogenic factors into the cytosol (Delbridge & Strasser, 2015; Doerflinger, Glab & Puthalakath, 2015; Li & Dewson, 2015; Lopez & Tait, 2015). The antiapoptotic members protect the mitochondria from permeabilization by the proapoptotic members and block the release of apoptogenic factors such as cytochrome c, apoptosis inducing factor (AIF) among others from being released from the inner mitochondrial membrane into the cytosol.
Drosophila melanogaster possesses many of the apoptotic pathway proteins that participate in the intrinsic and extrinsic cell death pathways (Kornbluth & White, 2005; Richardson & Kumar, 2002). The Bcl-2 family member homologues in Drosophila are limited to the single antiapoptotic Buffy (Quinn et al., 2003), and the sole proapoptotic death executioner Bcl-2 homologue, Debcl (Brachmann et al., 2000; Colussi et al., 2000; Igaki et al., 2000; Quinn et al., 2003; Zhang et al., 2000). Debcl has a strong similarity with the mammalian mitochondria outer membrane permeabilization protein Bok/Mtd.
The promoter region of Debcl contains four dNF-Y-binding consensus sequences which play positive roles in promoter activity and indicate that dNF-Y regulates Debcl gene expression (Ly et al., 2013). The tumour suppressor gene Retinoblastoma (Rbf1 in Drosophila) induces a Debcl-and Drp1-dependent mitochondrial cell death (Clavier et al., 2015). Rbf1 induces cell death by reducing the expression of the sole Debcl antagonist Buffy (Clavier et al., 2014). The Rbf1-induced apoptosis is dependent on Debcl-dependent mitochondrial ROS production and essentially Debcl is required downstream of Buffy for apoptosis to occur. The Debcl-induced ROS production appears to be through Glycerophosphate oxidase 1 participation to increase mitochondria ROS accumulation (Colin et al., 2015). The organic solute carrier partner 1/oxidored nitrodomain-containing protein 1 (OSCP1/NOR1), a known tumour suppressor induces apoptosis by the down-regulation of the Buffy gene and the up-regulation of the Debcl gene (Huu, Yoshida & Yamaguchi, 2015). Debcl is not required for most developmental cell death, but has been shown to play a role in embryonic cell death (Galindo et al., 2009) and stress-induced apoptosis (Sevrioukov et al., 2007). Antiapoptotic Buffy antagonizes Debcl-induced apoptosis by physical interaction (Quinn et al., 2003), probably at the mitochondria where Debcl localizes (Doumanis, Dorstyn & Kumar, 2007). The presence of a mitochondrial outer membrane (MOM)-targeting motif in Debcl indicates it possibly has a role in mitochondrial cell death pathway.
The role of the mitochondria in PD pathogenesis makes the α-synuclein-induced model of PD (Feany & Bender, 2000) a very attractive model for the investigation of the role of Bcl-2 proteins. Here, we investigate the potential enhancement or suppression of the α-synuclein-induced PD phenotypes by the inhibition and overexpression of the pro-apoptotic Bcl-2 homologue Debcl.
Materials and Methods
Drosophila media and culture
Stocks and crosses were maintained on standard cornmeal/molasses/yeast/agar media treated with propionic acid and methylparaben. Stocks were sustained on solid media for two to three weeks before being transferred onto new media to re-culture. Stocks were kept at room temperature (22 ± 2 °C) while crosses and experiments were carried out at 25 and 29 °C.
Drosophila stocks and derivative lines
UAS-debcl, UAS-Buffy (Quinn et al., 2003) were a gift from Dr. Leonie Quinn of University of Melbourne, UAS-α-synuclein (Feany & Bender, 2000) by Dr. M. Feany of Harvard Medical School and Ddc-Gal4 (Li et al., 2000) by Dr. J. Hirsch of University of Virginia. y1 v1; P(y(+t7.7) v(+t1.8) = TRiP.JF02429)attP2 was developed at Harvard Medical School (http://fgr.hms.harvard.edu/trip-vivo-rnai-approach) (Perkins et al., 2015) hereby referred to as UAS-Debcl-RNAi, GMR-Gal4 (Freeman, 1996) and UAS-lacZ were sourced from the Bloomington Drosophila Stock Center at Indiana University. The UAS-α-synuclein Ddc-Gal4/CyO; UAS-α-synuclein GMR-Gal4/CyO; UAS-Buffy Ddc-Gal4//CyO and UAS-Buffy GMR-Gal4/CyO derivative lines were generated using standard homologous recombination methods that have previously been described (Rong & Golic, 2000). Briefly, virgin females of UAS-α-synuclein or UAS-Buffy were crossed to males bearing the Gal4 transgene of interest (Ddc-Gal4 or GMR-Gal4). In the next generation, virgin female progeny were collected and mated to a balancer line (L/CyO), then individual male progeny are collected and mated to females of the balancer line. The resulting progeny was then mated and the homozygotes arising from the next generation was selected and tested by PCR. The derivative lines were used for the overexpression of either α-synuclein or Buffy along with selected transgenes 1) in Ddc-Gal4-expressing neurons that include the DA neurons or 2) in the developing eye behind the morphogenetic furrow directed by the GMR-Gal4 transgene. PCR reactions and gel electrophoresis were used for analysis of recombination events. PCR reaction was used to determine the amplification of DNA products from primers designed from the Homo sapiens synuclein, alpha (non A4 component of amyloid precursor) (SNCA), transcript variant 1 mRNA, NCBI reference sequence: NM_000345.3 using the NCBI primer design tool. The 5′ to 3′ sequence of the forward primer was GTGCCCAGTCATGACATTT, while that of the reverse primer was CCACAAAATCCACAGCACAC and were ordered from Invitrogen. The Drosophila melanogaster Buffy mRNA, NCBI reference sequence: NM_078978.2, was used to design a set of Buffy primers that would target both the endogenous and the overexpression transcripts. The 5′ to 3′ sequence of the forward primers were CACAGCGTTTATCCTGCTGA and CGGGTGGTGAGTTCCATACT, while that of the reverse primers were TCGCAGTGTGAAGATTCAGG and TTAATCCACGGAACCAGCTC, and were ordered from Eurofins MWG Operon. Gel electrophoresis was used for confirmation of recombination events via presence of the PCR product.
Ageing assay
Several single vial matings were made and a cohort of critical class male flies was collected upon eclosion. At least 200 flies were aged per genotype at a density of 20 or fewer flies per vial to avoid crowding on fresh media which was replenished every other day. Flies were observed and scored every two days for the presence of deceased adults. Flies were considered dead when they did not display movement upon agitation (Staveley, Phillips & Hilliker, 1990). Longevity data was analysed using the GraphPad Prism version 5.04 and survival curves were compared using the log-rank (Mantel-Cox) test. Significance was determined at 95%, at a P-value less than or equal to 0.05 with Bonferroni correction.
Climbing assay
A batch of male flies was collected upon eclosion and scored for their ability to climb (Todd & Staveley, 2004). Every seven days, 50 males from every genotype were assayed for their ability to climb 10 cm in 10 s in a clean climbing apparatus in 10 repetitions. Analysis was performed using GraphPad Prism version 5.04 and climbing curves were fitted using non-linear regression and compared using 95% confidence interval with a 0.05 P-value.
Scanning electron microscopy of the Drosophila eye
Several single vial crosses were made at 29 °C and adult male flies collected upon eclosion and aged for three days before being frozen at −80 °C. Whole flies were mounted on scanning electron microscope stubs, desiccated overnight and photographed with a FEI Mineral Liberation Analyzer 650F scanning electron microscope. For each cross at least 10 eye images were analysed using the National Institutes of Health (NIH) ImageJ software (Schneider, Rasband & Eliceiri, 2012) and biometric analysis performed using GraphPad Prism version 5.04. The percent area of eye disruption was calculated as previously described (M’Angale & Staveley, 2012).
Results
Debcl is similar to the human proapoptotic Bcl-2 ovarian killer (Bok)
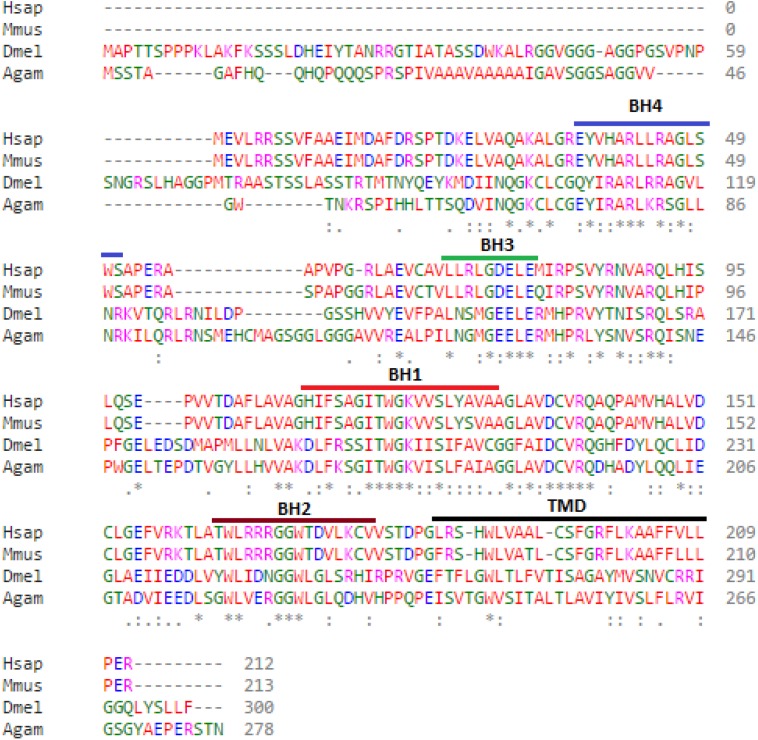
Bioinformatic analysis of the protein sequences encoded by the Debcl and Bok genes reveal 37% identity and 55% similarity. The Debcl protein consists of 300 amino acids and indicates the existence of the BH1, BH2, BH3, BH4 and TM domains, similar to the 212 amino acids human Bok (Fig. 1). An ELM resource search for functional sites (Dinkel et al., 2016) indicates the presence of a transmembrane domain (membrane anchor region), an inhibitor of apoptosis binding motif (IBM) at amino acids 1–5, a PDZ domain at amino acids 295–300, an ER retention motif at amino acids 109–115 and between amino acids 258–262, an Atg8 binding motif at amino acids 36–42, a nuclear receptor box motif at amino acids 295–300, and a ubiquitination motif of the SPOP-binding consensus at amino acids 2–6 and another one at position 74–79. There is a number of BH3-homology region binding sites in the central region of the protein as determined by an NCBI conserved domain search (Marchler-Bauer et al., 2015). Although the two proteins Bok and Debcl have been determined to be antiapoptotic, both show the presence of a BH4 domain, the homology domain that is most often associated with pro-survival proteins.
Figure 1. Debcl is related to human Bcl-2 ovarian killer (Bok).
When debcl protein is aligned with human Bok the Bcl-2 homology (BH) domains show strong conservation. Clustal Omega multiple sequence alignment (Goujon et al., 2010; Sievers et al., 2011) of Drosophila melanogaster debcl protein (Dmel is Drosophila melanogaster NP_788278.1) with the human Bok (Hsap is Homo sapiens NP_115904.1), mouse Bok (Mmus is Mus musculus NP_058058.1) and mosquito Bok (Agam is Anopheles gambiae XP_309956.4) showing the highlighted conserved BH domains and the TM helices. The domains were identified using NCBI Conserved Domain Database Search (CDD) (Marchler-Bauer et al., 2015) and ELM resource search for functional sites (Dinkel et al., 2016). “*” indicate the residues that are identical, “:” indicate the conserved substitutions, “.” indicate the semi-conserved substitutions. Colours show the chemical nature of amino acids. Red is small hydrophobic (including aromatic), Blue is acidic, Magenta is basic, and Green is basic with hydroxyl or amine groups.
Directed misexpression of Debcl in DA neurons alters lifespan and locomotor ability
The inhibition of Debcl in the DA neurons by RNA interference results in a lifespan with a median survival of 64 days that is similar to 62 days for the controls expressing the benign lacZ transgene as determined by a Log-rank (Mantel-Cox) test (Fig. 2A). The locomotor ability showed a slight improvement when nonlinear fitting of the climbing curves was performed, with significant differences at 95% confidence intervals (Fig. 2B). This suggests that the inhibition of the proapoptotic Debcl confers a small advantage for the normal functioning of DA neurons.
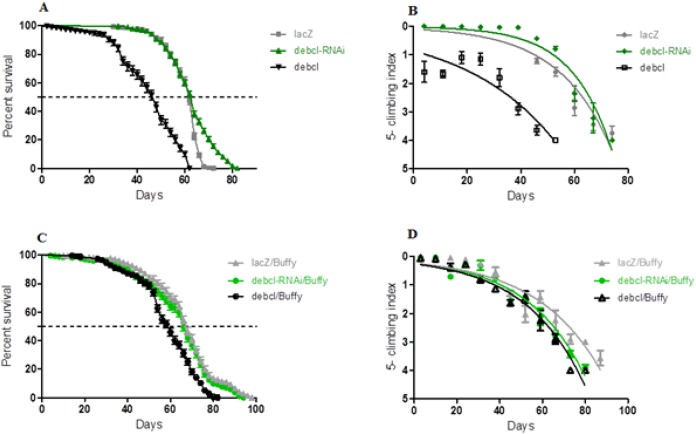
Figure 2. Debcl-induced phenotypes are rescued by the pro-survival Buffy.
(A) The directed inhibition of debcl in the DA neurons driven by Ddc-Gal4 results in a slightly increased median survival compared to the control flies overexpressing UAS-lacZ, while the overexpression of debcl results in severely reduced survival. The genotypes are UAS-lacZ/Ddc-Gal4; UAS-debcl-RNAi/Ddc-Gal4 and UAS-debcl/Ddc-Gal4. Longevity is shown as percent survival (P < 0.01, determined by log-rank and n ≥ 200). (B) The inhibition of debcl results in improved climbing ability whereas the overexpression of debcl results in a highly compromised climbing ability as determined by non-linear fitting of the climbing curves and comparing at 95% confidence intervals. The genotypes are UAS-lacZ/Ddc-Gal4; UAS-debcl-RNAi/Ddc-Gal4 and UAS-debcl/Ddc-Gal4. Error bars indicate the standard error of the mean (SEM) and n = 50. (C) The overexpression of Buffy along with the overexpression of debcl or debcl-RNAi restores lifespan and (D) significantly improves the climbing ability of these flies. The genotypes are UAS-Buffy; Ddc-Gal4/UAS-lacZ, UAS-Buffy; Ddc-Gal4/UAS-debcl-RNAi and UAS-Buffy; Ddc-Gal4/UAS-debcl. Longevity was determined by log-rank (Mantel-Cox) test and n ≥ 200 while climbing ability curves were fitted non-linearly and compared with 95% CI.
When Debcl is overexpressed in DA neurons, the survival criteria of these flies differ greatly (Fig. 2A), with Debcl-overexpressing flies having a median lifespan of 48 days compared to 62 days for the controls expressing the benign lacZ transgene as indicated by a Log-rank (Mantel-Cox) test. The overexpression of Debcl in DA neurons severely impairs climbing ability as determined by the nonlinear fitting of the curve with 95% CI (Fig. 2B). This suggests that the overexpression of Debcl in DA neurons interferes with the normal functioning of these flies and results in compromised “healthspan.”
The overexpression of the pro-survival Buffy rescues the Debcl-induced phenotypes
The overexpression of Buffy and Debcl in DA neurons results in a longer lifespan and improved locomotor ability (Fig. 2). The median lifespan of these flies was 62 days when compared to Buffy and lacZ overexpressing controls at 68 days. The median survival of Debcl-RNAi flies was 68 days as determined by a Log-rank (Mantel-Cox) test (Fig. 2C). The climbing ability of these flies was also much improved as determined by comparing the climbing indices at 95% CI (Fig. 2D). Taken together these results suggest that Buffy antagonizes the Debcl-induced phenotypes of shortened lifespan and poor climbing ability to markedly improve “healthspan.”
Altered expression of Debcl influences the α-synuclein-induced phenotypes
The inhibition of Debcl by RNAi along with the expression of α-synuclein under the direction of the Ddc-Gal4 transgene results in increased lifespan and healthier climbing ability compared to the control (Fig. 3). The Debcl-RNAi along with α-synuclein-expressing flies had a median lifespan of 67 days, while that of α-synuclein-expressing controls was 60 days as determined by a Log-rank (Mantel-Cox) test (Fig. 3A). The climbing ability of these flies was slightly improved than of the α-synuclein-expressing controls as indicated by the nonlinear fitting of the climbing curves and compared the 95% CI (Fig. 3B). These results show that the inhibition of the proapoptotic Debcl confers a significant advantage to flies under the influence of the neurotoxic effects of the human transgene α-synuclein.
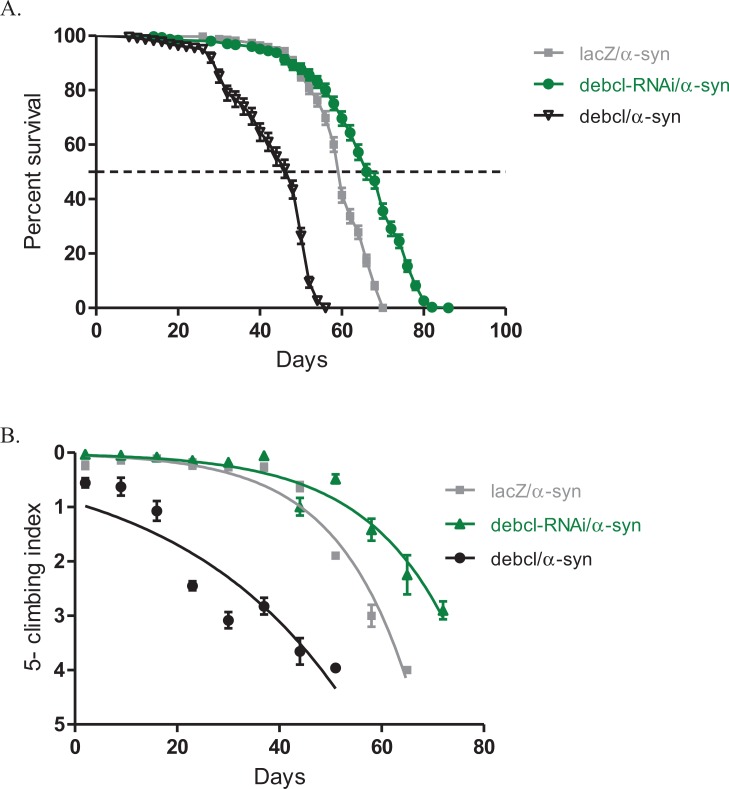
Figure 3. Overexpression of debcl enhances the α-synuclein-induced phenotypes.
(A) Directed overexpression of debcl in the DA neurons severely decreases longevity whereas its inhibition shows an improvement in lifespan. Genotypes are UAS-α-synuclein Ddc-Gal4/UAS-lacZ; UAS-α-synuclein Ddc-Gal4/UAS-Debcl-RNAi; and UAS-α-synuclein Ddc-Gal4/UAS-Debcl. Longevity is shown as percent survival (P < 0.01, determined by log-rank and n ≥ 200). (B) The co-expression of debcl in the α-synuclein model of PD enhanced the age-dependent loss in climbing ability. The directed inhibition of debcl in the DA neurons improved the climbing ability over time compared to the control. The genotypes are UAS-α-synuclein; Ddc-Gal4/UAS-lacZ, UAS-α-synuclein; Ddc-Gal4/UAS-debcl-RNAi, and UAS-α-synuclein; Ddc-Gal4/UAS-debcl. Analysis of the climbing curves and significance was determined by comparing the 95% confidence intervals. Error bars indicate the SEM and n = 50.
The overexpression of Debcl along with α-synuclein in DA neurons results in decreased median lifespan of 44 days, compared to 60 days for the control flies as determined by a Log-rank (Mantel-Cox) test (Fig. 3A). The climbing curves indicate that there was a significant reduction in the climbing ability of the flies with overexpression of Debcl (Fig. 3B) and thus, enhancing the phenotypes observed when α-synuclein is expressed in DA neurons. This suggests that the overexpression of Debcl further increases the toxic effects of the expression of α-synuclein.
Overexpression of Debcl enhances the α-synuclein-induced developmental eye defects
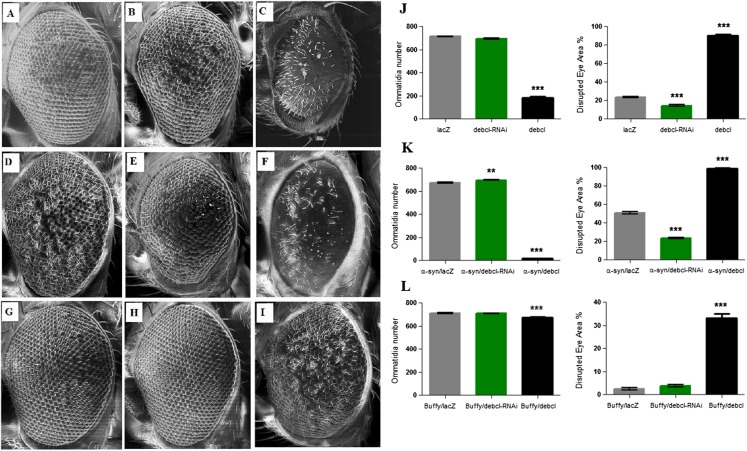
The overexpression of Debcl in the Drosophila eye results in severe ablation of the eye due to apoptosis (Colussi et al., 2000; Igaki et al., 2000) while expression of α-synuclein in the eye results in developmental defects (Fig. 4D). When Debcl is overexpressed in the eye, developmental defects resulting from Gal4 (Kramer & Staveley, 2003) (Figs. 4A and 4J), inhibition of Debcl (Figs. 4B and 4J), and overexpression of Debcl (Figs. 4C and 4J) are enhanced. Biometric analysis of the ommatidia number and the percentage of eye disruption showed significant differences in the compared genotypes to the control that express the benign lacZ transgene (Fig. 4J). The inhibition of Debcl along with α-synuclein expression (Figs. 4E and 4K) and the co-expression of Debcl and α-synuclein (Figs. 4F and 4K) result in enhanced phenotypes. The disruption of the ommatidial array due to fusion of the ommatidia and smaller eye is severely enhanced by the overexpression of Debcl together with α-synuclein (Figs. 4F and 4K). The analysis of the ommatidia number and disruption of the eye reveals significant differences, the inhibition of Debcl yields “healthier” eyes and its overexpression results in worsened phenotypes (Fig. 4K). The ommatidial disarray that results from inhibition of Debcl are completely rescued by overexpression of the pro-survival Buffy (Figs. 4H and 4L), while the ablated eye that result from Debcl overexpression is partially rescued upon Buffy overexpression, this restores the eye ablation to a mildly severe rough eye phenotype (Figs. 4I and 4L). Biometric analysis showed recouped ommatidia number and a lessened disruption of the eye, though they were still significantly different from the control (Fig. 4L). These results suggest that overexpression of Debcl along with expression of α-synuclein enhances the Debcl-induced eye ablation, while the overexpression of Debcl together with Buffy partially rescues the eye phenotype.
Figure 4. Buffy partially rescues the Debcl-induced developmental eye defects.
Scanning electron micrographs when Debcl is overexpressed or inhibited in the eye with the eye-specific GMR-Gal4 transgene; (A) GMR-Gal4/UAS-lacZ; (B) GMR-Gal4/UAS-Debcl-RNAi; (C) GMR-Gal4/UAS-Debcl; when co-expressed with α-synuclein; (D) UAS-α-synuclein; GMR-Gal4/UAS-lacZ; (E) UAS-α-synuclein; GMR-Gal4/UAS-Debcl-RNAi (F) UAS-α-synuclein; GMR-Gal4/UAS-Debcl; and when co-expressed with Buffy; (G) UAS-Buffy; GMR-Gal4/UAS-lacZ (H) UAS-Buffy; GMR-Gal4/UAS-Debcl-RNAi and (I) UAS-Buffy; GMR-Gal4/UAS-Debcl. (J) Biometric analysis showed a significant difference in the disrupted area of the eye when Debcl is inhibited in the developing eye, and a decreased number of ommatidia and high levels of disruption when Debcl is overexpressed. (K) Biometric analysis indicates a marked difference when Debcl is inhibited along with the expression of α-synuclein, with increased ommatidia number and a less disrupted ommatidial array, whereas the overexpression of Debcl along with the expression of α-synuclein results in a dramatic decrease in ommatidia number coupled with severe ommatidial disarray. (L) The biometric analysis reveals the restoration of Debcl-induced phenotypes by overexpression of Buffy. The inhibition and overexpression of Debcl along with overexpression of Buffy, results in increased ommatidia number and improved disruption of the ommatidial array, to produce “healthier” eyes as determined by a one-way ANOVA and Dunnett’s multiple comparison test (P < 0.05 and 95% CI), error bars indicate the SEM, asterisks (*) represents statistically significant result and n = 10.
Discussion
Since mitochondrial dysfunction is central to the pathology of both sporadic and familial forms of PD (Subramaniam & Chesselet, 2013), it was important to highlight the role and consequences of the altered expression of the proapoptotic mitochondrial gene Debcl in this process. The overexpression of Debcl in Drosophila and other systems, including mammalian, has been demonstrated to lead to apoptosis (Brachmann et al., 2000; Colussi et al., 2000; Galindo et al., 2009; Igaki et al., 2000; Senoo-Matsuda, Igaki & Miura, 2005; Sevrioukov et al., 2007; Zhang et al., 2000). The recapitulation of PD-like symptoms in Drosophila melanogaster, especially the age-dependent loss of climbing ability, has led to investigation of genes that could suppress these phenotypes (Auluck et al., 2002; Feany & Bender, 2000; Haywood & Staveley, 2004). Our results show that the overexpression of Debcl results in a severely shortened lifespan followed by premature loss in climbing ability; phenotypes that are reminiscent of PD-like symptoms in model organisms. Thus our work shows the intricate balance between life and death decisions in the sensitive dopamine producing neurons. It seems that excess amounts of Debcl protein are sufficient to upset the survival mechanisms and lead to degeneration and death of DA neurons. The importance of Debcl-induced apoptosis is exhibited by the strict control in its gene product by the tumour suppressors Rbf1 (Clavier et al., 2015), OSCP1/NOR1 (Huu, Yoshida & Yamaguchi, 2015), and NF-Y (Ly et al., 2013). Furthermore, it has a motif for ubiquitination, probably by the TrCP homologue slimb that targets it for destruction by the proteasome (Colin et al., 2014). The inhibition of Debcl had a converse result, with flies that had a longer lifespan and healthy climbing ability. It is possible that the suppression of Debcl tips the balance towards the survival pathways controlled by the antiapoptotic Buffy. Our results indicate that overexpression of Debcl appears to be a novel model of PD as a result of neuronal apoptosis.
The α-synuclein-induced model of PD in Drosophila shows little difference in lifespan between the control and wild type, A53T and A30P α-synuclein flies (Feany & Bender, 2000). In our study, the overexpression of Debcl in the DA neurons resulted in a marked decrease in lifespan. This is in part due to toxic effects as a result of the expression of α-synuclein, and additionally, due to Debcl-induced apoptosis. The Debcl-induced apoptosis is mediated by other factors including; the mitochondrial fission protein Drp1 (Clavier et al., 2015) that interacts with Debcl to induce mitochondrial fragmentation; Glycerophosphate oxidase-1 (Colin et al., 2015) that increases mitochondrial ROS accumulation; and possibly through the initiation of autophagy, since both α-synuclein expression (Xilouri & Stefanis, 2015) and Debcl (Hou et al., 2008) overexpression are implicated in this process. This worsening of phenotypes was also observed when Debcl was overexpressed with α-synuclein in the eye. The inhibition of Debcl in the DA neurons resulted in a marked increase in survival and improved locomotor ability. This inhibition of Debcl is sufficient to negate its apoptotic role and thus promote cell survival through the opposing antiapoptotic Buffy.
Locomotor dysfunction is one of the major symptoms of PD. The demonstration of an age-dependent loss of climbing ability is pivotal to highlighting the effects of degeneration and death of DA neurons, ultimately as a consequence of altered gene expression as opposed to cellular senescence (Rodriguez et al., 2015). The overexpression of Debcl in the DA neurons produced a climbing index significantly different from that of control flies with the loss of climbing ability in an age-dependent manner and likely due to Debcl-induced neuronal degeneration. The degree of locomotor dysfunction seemed to be similar to that observed when α-synuclein is overexpressed in DA neurons. Taken together, these results would indicate a detrimental effect in overexpression of Debcl in DA neurons that result in a novel model of PD in flies.
In contrast, the inhibition of Debcl in the same neurons results in a remarkable improvement in climbing ability when compared to the controls. The inhibition of Debcl in the DA neurons of the α-synuclein-induced PD model significantly increased lifespan and climbing ability, indicating that reduced levels of Debcl are sufficient to alter the healthspan of DA neurons. The Debcl-induced apoptosis relies on downstream effectors that either induces ROS accumulation (Colin et al., 2015) or the fragmentation of the mitochondria (Clavier et al., 2015). As the down-regulation of Buffy or up-regulation of Debcl results in apoptosis (Huu, Yoshida & Yamaguchi, 2015), the cellular advantage of Debcl inhibition may be indirect through the de-repression of the Buffy gene product that confers survival advantages. The directed expression of Buffy along with Debcl results in an improved “healthspan” compared to the Debcl-induced phenotypes and corroborate other studies that show the overexpression of the pro-survival Buffy confers survival advantages through increased survival and improved climbing ability under conditions of stress (M’Angale & Staveley, 2016). Our study suggests that the overexpression of Buffy is similar to an up-regulation that ultimately blocks Debcl-induced apoptosis, similar to results obtained when its regulation by Rbf1 or dE2F2 is altered to repress it transcriptionally (Clavier et al., 2014; Clavier et al., 2015). This suppression of Buffy is sufficient to induce Debcl-dependent apoptosis, in addition to the promotion of Debcl activity by dNF-Y (Ly et al., 2013). The co-overexpression of Debcl and Buffy in the eye resulted in a partial rescue of the Debcl-induced phenotypes. Therefore, overexpression of the pro-survival Buffy suppresses the Debcl-dependent phenotypes.
Conclusions
Directed inhibition of Debcl results in improved survivorship and extended climbing ability whereas the directed expression of Debcl results in reduced lifespan and impaired locomotor function. These phenotypes are rescued upon co-expression with the pro-survival Buffy. The overexpression of Debcl enhances the effects of α-synuclein expression. Buffy counteracts Debcl-induced phenotypes, and represents a potential target to enhance neuronal survival in response to the detrimental effects of Debcl-induced apoptosis.
Supplemental Information
Funding Statement
PGM was partially funded by the Department of Biology Teaching Assistantships and a School of Graduate Studies Fellowship from Memorial University of Newfoundland. BES was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
P. Githure M’Angale conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Brian E. Staveley conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Data Deposition
The following information was supplied regarding data availability:
The raw data has been supplied as Supplemental Dataset Files.
References
- Adams & Cory (1998).Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Ambegaokar, Roy & Jackson (2010).Ambegaokar SS, Roy B, Jackson GR. Neurodegenerative models in Drosophila: polyglutamine disorders, Parkinson disease, and amyotrophic lateral sclerosis. Neurobiology of Disease. 2010;40(1):29–39. doi: 10.1016/j.nbd.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck et al. (2002).Auluck PK, Chan HYE, Trojanowski JQ, Lee VMY, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- Botella et al. (2009).Botella JA, Bayersdorfer F, Gmeiner F, Schneuwly S. Modelling Parkinson’s disease in Drosophila. NeuroMolecular Medicine. 2009;11(4):268–280. doi: 10.1007/s12017-009-8098-6. [DOI] [PubMed] [Google Scholar]
- Brachmann et al. (2000).Brachmann CB, Jassim OW, Wachsmuth BD, Cagan RL. The Drosophila bcl-2 family member dBorg-1 functions in the apoptotic response to UV-irradiation. Current Biology. 2000;10(9):547–550. doi: 10.1016/S0960-9822(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Brand & Perrimon (1993).Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Büttner et al. (2014).Büttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, Carmona-Gutierrez D, Eisenberg T, Michael E, Kroemer G, Tavernarakis N, Sigrist SJ, Madeo F. Spermidine protects against α-synuclein neurotoxicity. Cell Cycle. 2014;13(24):3903–3908. doi: 10.4161/15384101.2014.973309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta et al. (2010).Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neuroscience Letters. 2010;486(3):235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey et al. (2011).Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, Zharkovsky A, Kaasik A. Mutant A53T α-synuclein induces neuronal death by increasing mitochondrial autophagy. Journal of Biological Chemistry. 2011;286(12):10814–10824. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavier et al. (2014).Clavier A, Baillet A, Rincheval-Arnold A, Coléno-Costes A, Lasbleiz C, Mignotte B, Guénal I. The pro-apoptotic activity of Drosophila Rbf1 involves dE2F2-dependent downregulation of diap1 and buffy mRNA. Cell Death and Disease. 2014;5(9):e2461. doi: 10.1038/cddis.2014.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavier et al. (2015).Clavier A, Ruby V, Rincheval-Arnold A, Mignotte B, Guénal I. The Drosophila retinoblastoma protein, Rbf1, induces a Debcl- and Drp1-dependent mitochondrial apoptosis. Journal of Cell Science. 2015;128(17):3239–3249. doi: 10.1242/jcs.169896. [DOI] [PubMed] [Google Scholar]
- Colin et al. (2014).Colin J, Garibal J, Clavier A, Rincheval-Arnold A, Gaumer S, Mignotte B, Guénal I. The drosophila Bcl-2 family protein Debcl is targeted to the proteasome by the beta-TrCP homologue slimb. Apoptosis. 2014;19(10):1444–1456. doi: 10.1007/s10495-014-1034-8. [DOI] [PubMed] [Google Scholar]
- Colin et al. (2015).Colin J, Garibal J, Clavier A, Szuplewski S, Risler Y, Milet C, Gaumer S, Guénal I, Mignotte B. Screening of suppressors of bax-induced cell death identifies glycerophosphate oxidase-1 as a mediator of debcl-induced apoptosis in Drosophila. Genes Cancer. 2015;6(5–6):241–253. doi: 10.18632/genesandcancer.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin et al. (2009).Colin J, Gaumer S, Guenal I, Mignotte B. Mitochondria, Bcl-2 family proteins and apoptosomes: of worms, flies and men. Frontiers in Bioscience. 2009;14:4127–4137. doi: 10.2741/3517. [DOI] [PubMed] [Google Scholar]
- Colussi et al. (2000).Colussi PA, Quinn LM, Huang DCS, Coombe M, Read SH, Richardson H, Kumar S. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. Journal of Cell Biology. 2000;148(4):703–714. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory & Adams (2002).Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Reviews: Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Delbridge & Strasser (2015).Delbridge ARD, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death and Differentiation. 2015;22(7):1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel et al. (2016).Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, Dahl SL, Damerell V, Diebel S, Kalman S, Klein S, Knudsen AC, Mäder C, Merrill S, Staudt A, Thiel V, Welti L, Davey NE, Diella F, Gibson TJ. ELM 2016—data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Research. 2016;44(D1):D294–D300. doi: 10.1093/nar/gkv1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger, Glab & Puthalakath (2015).Doerflinger M, Glab JA, Puthalakath H. BH3-only proteins: a 20-year stock-take. FEBS Journal. 2015;282(6):1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- Doumanis, Dorstyn & Kumar (2007).Doumanis J, Dorstyn L, Kumar S. Molecular determinants of the subcellular localization of the Drosophila Bcl-2 homologues DEBCL and BUFFY. Cell Death and Differentiation. 2007;14(5):907–915. doi: 10.1038/sj.cdd.4402082. [DOI] [PubMed] [Google Scholar]
- Esteves et al. (2011).Esteves AR, Arduíno DM, Silva DFF, Oliveira CR, Cardoso SM. Mitochondrial dysfunction: the road to α-synuclein oligomerization in PD. Parkinson’s Disease. 2011;2011:693761. doi: 10.4061/2011/693761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany & Bender (2000).Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Forno (1996).Forno LS. Neuropathology of Parkinson’s disease. Journal of Neuropathology & Experimental Neurology. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Freeman (1996).Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87(4):651–660. doi: 10.1016/S0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Fu & Fan (2002).Fu YF, Fan TJ. Bcl-2 family proteins and apoptosis. Acta Biochimica et Biophysica Sinica. 2002;34(4):389–394. [PubMed] [Google Scholar]
- Galindo et al. (2009).Galindo KA, Lu W-J, Park JH, Abrams JM. The Bax/Bak ortholog in Drosophila, Debcl, exerts limited control over programmed cell death. Development. 2009;136(2):275–283. doi: 10.1242/dev.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser (2009).Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Reviews in Molecular Medicine. 2009;11:e2461. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- Goujon et al. (2010).Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL–EBI. Nucleic Acids Research. 2010;38(Suppl 2):W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo (2012).Guo M. Drosophila as a model to study mitochondrial dysfunction in Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine. 2012;2(11):e2461. doi: 10.1101/cshperspect.a009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, Dawson & Dawson (2008).Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Annals of Neurology. 2008;64(S2):S3–S15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood & Staveley (2004).Haywood AFM, Staveley BE. Parkin counteracts symptoms in a Drosophila model of Parkinson’s disease. BMC Neuroscience. 2004;5(1):14. doi: 10.1186/1471-2202-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou et al. (2008).Hou YCC, Chittaranjan S, Barbosa SG, McCall K, Gorski SM. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. Journal of Cell Biology. 2008;182(6):1127–1139. doi: 10.1083/jcb.200712091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu, Yoshida & Yamaguchi (2015).Huu NT, Yoshida H, Yamaguchi M. Tumor suppressor gene OSCP1/NOR1 regulates apoptosis, proliferation, differentiation, and ROS generation during eye development of Drosophila melanogaster. FEBS Journal. 2015;282(24):4727–4746. doi: 10.1111/febs.13528. [DOI] [PubMed] [Google Scholar]
- Igaki et al. (2000).Igaki T, Kanuka H, Inohara N, Sawamoto K, Núñez G, Okano H, Miura M. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(2):662–667. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong et al. (2015).Kong Y, Liang X, Liu L, Zhang D, Wan C, Gan Z, Yuan L. High throughput sequencing identifies microRNAs mediating α-synuclein toxicity by targeting neuroactive-ligand receptor interaction pathway in early stage of Drosophila Parkinson’s disease model. PLoS ONE. 2015;10(9):e2461. doi: 10.1371/journal.pone.0137432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth & White (2005).Kornbluth S, White K. Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm) Journal of Cell Science. 2005;118(9):1779–1787. doi: 10.1242/jcs.02377. [DOI] [PubMed] [Google Scholar]
- Kramer & Staveley (2003).Kramer JM, Staveley BE. GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genetics and Molecular Research. 2003;2(1):43–47. [PubMed] [Google Scholar]
- Leroy et al. (1998).Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Li et al. (2000).Li H, Chaney S, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Current Biology. 2000;10(4):211–214. doi: 10.1016/S0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Li & Dewson (2015).Li MX, Dewson G. Mitochondria and apoptosis: emerging concepts. F1000 Prime Reports. 2015;7:42. doi: 10.12703/P7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez & Tait (2015).Lopez J, Tait SWG. Mitochondrial apoptosis: killing cancer using the enemy within. British Journal of Cancer. 2015;112(6):957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly et al. (2013).Ly LL, Suyari O, Yoshioka Y, Tue NT, Yoshida H, Yamaguchi M. dNF-YB plays dual roles in cell death and cell differentiation during Drosophila eye development. Gene. 2013;520(2):106–118. doi: 10.1016/j.gene.2013.02.036. [DOI] [PubMed] [Google Scholar]
- M’Angale & Staveley (2012).M’Angale PG, Staveley BE. Effects of α-synuclein expression in the developing Drosophila eye. Drosophila Information Services. 2012;95:85–89. [Google Scholar]
- M’Angale & Staveley (2016).M’Angale PG, Staveley BE. The Bcl-2 homologue Buffy rescues α-synuclein-induced Parkinson disease-like phenotypes in Drosophila. BMC Neuroscience. 2016;17(1):24. doi: 10.1186/s12868-016-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer et al. (2015).Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH. CDD: NCBI’s conserved domain database. Nucleic Acids Research. 2015;43(D1):D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou & Youle (2011).Martinou J-C, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Developmental Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Schulz & Lee (2007).Park SS, Schulz EM, Lee D. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by α-synuclein. European Journal of Neuroscience. 2007;26(11):3104–3112. doi: 10.1111/j.1460-9568.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- Perkins et al. (2015).Perkins LA, Holderbaum L, Tao R, Hu Y, Sopko R, McCall K, Yang-Zhou D, Flockhart I, Binari R, Shim H-S, Miller A, Housden A, Foos M, Randkelv S, Kelley C, Namgyal P, Villalta C, Liu L-P, Jiang X, Huan-Huan Q, Wang X, Fujiyama A, Toyoda A, Ayers K, Blum A, Czech B, Neumuller R, Yan D, Cavallaro A, Hibbard K, Hall D, Cooley L, Hannon GJ, Lehmann R, Parks A, Mohr SE, Ueda R, Kondo S, Ni J-Q, Perrimon N. The transgenic RNAi project at harvard medical school: resources and validation. Genetics. 2015;201(3):843–852. doi: 10.1534/genetics.115.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos et al. (1997).Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Quinn et al. (2003).Quinn L, Coombe M, Mills K, Daish T, Colussi P, Kumar S, Richardson H. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO Journal. 2003;22(14):3568–3579. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson & Kumar (2002).Richardson H, Kumar S. Death to flies: Drosophila as a model system to study programmed cell death. Journal of Immunological Methods. 2002;265(1–2):21–38. doi: 10.1016/S0022-1759(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez et al. (2015).Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. Parkinson’s disease as a result of aging. Aging Cell. 2015;14(3):293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong & Golic (2000).Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288(5473):2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Schneider, Rasband & Eliceiri (2012).Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz (2007).Schulz JB. Mechanisms of neurodegeneration in idiopathic Parkinson’s disease. Parkinsonism & Related Disorders. 2007;13(Suppl 3):S306–S308. doi: 10.1016/S1353-8020(08)70021-X. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda, Igaki & Miura (2005).Senoo-Matsuda N, Igaki T, Miura M. Bax-like protein Drob-1 protects neurons from expanded polyglutamine-induced toxicity in Drosophila. EMBO Journal. 2005;24(14):2700–2713. doi: 10.1038/sj.emboj.7600721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukov et al. (2007).Sevrioukov EA, Burr J, Huang EW, Assi HH, Monserrate JP, Purves DC, Wu JN, Song EJ, Brachmann CB. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis. 2007;45(4):184–193. doi: 10.1002/dvg.20279. [DOI] [PubMed] [Google Scholar]
- Siddiqui, Ahad & Ahsan (2015).Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Archives of Toxicology. 2015;89(3):289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- Sievers et al. (2011).Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7(1):539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley (2014).Staveley BE. Drosophila Models of Parkinson disease. In: LeDoux MS, editor. Movement Disorders: Genetics and Models. Second edition. London: Elsevier Science; 2014. pp. 345–354. [Google Scholar]
- Staveley, Phillips & Hilliker (1990).Staveley BE, Phillips JP, Hilliker AJ. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990;33(6):867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- Subramaniam & Chesselet (2013).Subramaniam SR, Chesselet M-F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Progress in Neurobiology. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen, Norris & Youle (2008).Suen D-F, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes & Development. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd & Staveley (2004).Todd AM, Staveley BE. Novel assay and analysis for measuring climbing ability in Drosophila. Drosophila Information Services. 2004;87:101–107. [Google Scholar]
- Tsujimoto (2002).Tsujimoto Y. Bcl-2 family of proteins: life-or-death switch in mitochondria. Bioscience Reports. 2002;22(1):47–58. doi: 10.1023/A:1016061006256. [DOI] [PubMed] [Google Scholar]
- Webb et al. (2003).Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. α-Synuclein is degraded by both autophagy and the proteasome. Journal of Biological Chemistry. 2003;278(27):25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Whitworth (2011).Whitworth AJ. Drosophila models of Parkinson’s disease. Advances in Genetics. 2011;73:1–50. doi: 10.1016/B978-0-12-380860-8.00001-X. [DOI] [PubMed] [Google Scholar]
- Xilouri & Stefanis (2015).Xilouri M, Stefanis L. Chaperone mediated autophagy to the rescue: a new-fangled target for the treatment of neurodegenerative diseases. Molecular and Cellular Neuroscience. 2015;66:29–36. doi: 10.1016/j.mcn.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2000).Zhang H, Huang Q, Ke N, Matsuyama S, Hammock B, Godzik A, Reed JC. Drosophila pro-apoptotic Bcl-2/Bax homologue reveals evolutionary conservation of cell death mechanisms. Journal of Biological Chemistry. 2000;275:27303–27306. doi: 10.1074/jbc.M002846200. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2011).Zhu Y, Duan C, Lü L, Gao H, Zhao C, Yu S, Uéda K, Chan P, Yang H. α-Synuclein overexpression impairs mitochondrial function by associating with adenylate translocator. International Journal of Biochemistry & Cell Biology. 2011;43(5):732–741. doi: 10.1016/j.biocel.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2016).Zhu Z-J, Wu K-C, Yung W-H, Qian Z-M, Ke Y. Differential interaction between iron and mutant α-synuclein causes distinctive Parkinsonian phenotypes in Drosophila. Biochimica et Biophysica Acta (BBA)–Molecular Basis of Disease. 2016;1862(4):518–525. doi: 10.1016/j.bbadis.2016.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.