Abstract
Aim. The incidence of diabetic osteoporosis (DOP) is increasing due to lack of effective management over the past few decades. This review aims to summarize traditional Chinese medicine (TCM) suitability in the pathogenesis and clinical and preclinical management of DOP. Methods. Literature sources used were from Medline (Pubmed), CNKI (China Knowledge Resource Integrated Database), and CSTJ (China Science and Technology Journal Database) online databases. For the consultation, keywords such as diabetic osteoporosis (DOP), TCM, clinical study, animal experiment, toxicity, and research progress were used in various combinations. Around 100 research papers and reviews were visited. Results. Liver-spleen-kidney insufficiency may result in development of DOP. 18 clinical trials are identified to use TCM compound prescriptions for management of patients with DOP. TCM herbs and their active ingredients are effective in preventing the development of DOP in streptozotocin (STZ) and alloxan as well as STZ combined with ovariectomy insulted rats. Among them, most frequently used TCM herbs in clinical trials are Radix Astragali, Radix et Rhizoma Salviae Miltiorrhizae, Radix Rehmanniae Preparata, and Herba Epimedii. Some of TCM herbs also exhibit toxicities in clinical and preclinical research. Conclusions. TCM herbs may act as the novel sources of anti-DOP drugs by improving bone and glucolipid metabolisms. However, the pathogenesis of DOP and the material base of TCM herbs still merit further study.
1. Introduction
Diabetic osteoporosis (DOP) is a chronic bone metabolic disease induced by diabetes mellitus (DM), and its pathogenesis involves an increase in osteoclast activity, a decrease in osteoblast activity, aggravation of bone microcirculation, an increase in adipogenic differentiation of mesenchymal multipotential stem cells (MMSCs), and an increase in advanced glycation end products (AGEs) [1, 2]. With the increasing incidence of DM in the world, the number of patients with DOP increased accordingly [3]. Clinical studies have shown that about 1/2 to 2/3 of diabetic patients suffered from decreasing bone strength and/or increasing incidence of fractures, of whom nearly 1/3 were diagnosed as osteoporosis [4]. In recent years, traditional Chinese medicine (TCM) has attracted increasing attention in prevention and treatment of DOP. This review highlights the pathogenetical, clinical, and experimental advances in management of DOP in TCM and also provides a strong scientific evidence for understanding and improving the treatment of DOP.
2. DOP Pathogenesis in TCM
DOP belongs to the secondary osteoporosis, whose concept was first proposed in 1948 [2, 46]. In TCM, there is no corresponding term to describe DOP. However, the clinical performances are similar to the TCM symptoms of “atrophic debility of bones,” “Gubi,” “dryness of bone,” or “pain in waist and lower extremities” [47]. According to TCM theories and clinical symptoms of DOP, the etiology of DOP is mainly attributed to the insufficiency of the spleen, liver, and kidney. In traditional Chinese physiology, the spleen (as is well known, all the organs in TCM bear the least resemblance to its western counterpart) is assumed to be responsible for assimilation of nutrients and maintenance of physical strength. The spleen governs blood, digestion, transformation, and transportation in the body [48]. So the spleen has the ability of transforming food into Qi and blood and transporting them throughout the body. Proper functioning of the organ is essential to maintain muscle mass and strengthen limbs. Also, in traditional Chinese physiology, the liver (Gan) stores blood, ensures the smooth flow of Qi, and controls the action and nutrition of tendon [49]. And the kidney (Shen) stores essence and controls the growth and development of bone [50, 51]. Meanwhile, Qi (Qi is the term used in TCM to describe the body's vital life energy [52]) deficiency and blood stasis also contributed to the development of DOP.
Xiao-Ke is an official terminology for diabetes in TCM [53]. TCM believes that Xiao-Ke mainly resulted from the dysfunctions of kidney, spleen, and liver [54]. As kidney controls bone growth and development via neuroendocrine-immune network and osteoclast regulatory pathway osteoprotegerin- (OPG-) receptor activator of nuclear factor kappa-B ligand- (RANKL-) RANK [51, 55], the deficiency of the kidney caused by Xiao-Ke further results in bone malnutrition. The liver dredges and smooths the route of something and produces blood by promoting circulation of blood and metabolism of body fluid and assisting the spleen and the stomach to digest food (http://old.tcmwiki.com/wiki/the-liver). The loss of liver Yin in the development of Xiao-Ke causes blood deficiency, which leads to blood stasis, tendon, and vessels undernutrition [56]. The spleen has the function to transport and transform nutrients by digesting food, absorbing nutrients of food and water, and then transporting them to the heart and the lung (http://old.tcmwiki.com/wiki/the-spleen). If the essence transportation and transformation fails in spleen, dampness and circulation disorders of Qi and blood will occur to the body [57]. Further, the bone is loss of nutrition which may cause insufficiency of bone marrow and bone pain [58]. The codysfunctions of liver, spleen, and kidney lead to Qi deficiency and blood stasis, which further contributes to insufficiency of blood and essence [57, 59]. All the abnormalities lead to bone lesions and occurrence of DOP. The views on the pathogenesis of DOP have been also discussed in the ancient Chinese medicine works, such as “Inner Canon of Huangdi,” “Clinic Guideline of Medical Records,” and “Xue Zheng Lun.” Therefore, prevention and treatment of DOP should focus on regulation and tonification of the liver, spleen, and kidney. The proper function of liver, spleen, and kidney promotes Qi to disperse stagnation and reinforce kidney to replenish Yin, which beneficially contributes to improving bone metabolism.
In recent years, great achievements have been made in prevention and treatment of DOP in TCM. The results have demonstrated that TCM herbs not only had the abilities of increasing the bone quality by enhancing the mechanical strength of bone but also exhibited the effect on improving the primary disease by regulating glycaemia metabolism. Next, we review the clinical and preclinical research advances of using TCM in the treatment of DOP.
3. Clinical Advances of DOP in TCM
As mentioned previously, the clinical guidelines for TCM treatment of DOP are tonifying liver, spleen, and kidney as well as promoting Qi flow and removing blood stasis. We summarized the TCM compound prescriptions used in the clinical trials of DOP as shown in Table 1. Till now, there are around 18 TCM prescriptions that have been used in managing patients with DOP, in which clinical studies have been performed by different investigators with various prescriptions. Zhang et al. [5] found that Tanggukang treatment reduced serum malondialdehyde (MDA) and blood glucose and increased the activity of superoxide dismutase (SOD) that contributed to controlling the glycaemia and suppressing osteoporosis in patients with DOP. In addition, both Tanggukang [6] and Qianggubao granule treatments also had significant effects on patients with DOP evidenced by improving calcium and phosphorus metabolism and hydroxyproline level as well. In addition, Ji et al. [7] found that Bushen Huoxue decoction treatment not only controlled glycaemia and lipidemia but also obviously improved bone mineral density (BMD) in patients with DOP. Shang et al. [8, 9] found that Bushen Tongluo decoction had the ability of improving BMD and reducing the risk of bone fractures in patients with DOP. Gong and Li [10] found that Jiawei Qing'e pill treatment improved DOP patients in blood glycosylated hemoglobin (HbA1C), calcium and phosphorus level, and fasting urine Ca/creatine ratio which beneficially contributed to the balance of bone metabolism. Yushanjiang and Halida [11] showed that the Xihongkang treatment prevented the development of DOP by tonifying Qi, nourishing Yin, and promoting blood circulation. Deng [12] demonstrated that Yishen Zhuanggu decoction treatment increased serum bone gla-protein (BGP) and 1,25-(OH)2D3 levels and BMD value in patients with DOP.
Table 1.
Clinical used TCM prescriptions for treatment of patients with DOP.
| Compound prescription name | Treatment aim and biomarker examination | Results (# of patients) | References |
|---|---|---|---|
| Tanggukang decoction 7a |
Fasting blood glucose (FBG), glycosylated hemoglobin (HbA1C), bone mineral density (BMD), superoxide dismutase (SOD), malondialdehyde (MDA), bone gla-protein (BGP), Ca and P in serum, and urine | FBG and HbA1C levels decreased; BMD, BGP, and serum SOD levels increased; serum MDA decreased; urine Ca and P decreased. Control treatment using oyster shell calcium chewable tablet |
[5, 6] |
|
| |||
| Bushen Huoxue decoction 11b |
Significant effect: BMD increased; clinical symptoms and pains disappeared. Effective: clinical symptoms alleviated; BMD remained the same. Ineffective: BMD and clinical symptoms remained the same |
Markedly improved (24) Moderately improved (4) Ineffective (2) Overall efficacy: 93.3% Control treatment: Qianggu capsule |
[7] |
|
| |||
| Bushen Tongluo decoction 9c |
Broadband ultrasound attenuation (BUA), sound of speed (SOS), Ca and P in serum, alkaline phosphatase (ALP), FBG, and HbA1C | BUA and SOS significantly increased; FBG and HbA1C levels reduced. Control treatment using caltrate D |
[8, 9] |
|
| |||
|
Jiawei Qing'e Pill 9d |
Ca, P/creatinine (Cr) ratios in blood and urine | Markedly improved (25) Moderately improved (11) Ineffective (4) Overall efficacy: 90% Serum Ca decreased by 0.48 mM/L; serum P increased by 0.44 mM/L; urine Ca/Cr ratio decreased by 0.29. Control treatment using alfacalcidol and caltrate D |
[10] |
|
| |||
|
Xihongkang 7e |
Significant effect: clinical symptoms disappeared; FBG < 7.2 mM; two-hour postprandial blood glucose (2 hPG) < 8.3 mM; HbA1C < 6% or HbA1C decreased by ⩾30%; BMD increased by ⩾20%. Effective: clinical symptoms alleviated; FBG < 8.3 mM/L; 2 hPG < 10.0 mM/L; HbA1C < 8% or HbA1C decreased by ⩾10%; BMD increased by ⩾10%. Ineffective: no significant improvement in symptoms; HbA1C and BMD remained the same |
Markedly improved (56) Moderately improved (68) Ineffective (16) Overall efficacy: 88.6% Control treatment using alfacalcidol, glipizide, and caltrate D |
[11] |
|
| |||
| Yishen Zhuanggu compound 9f |
Significant effect: pain disappeared; BMD increased by 0.05 g/cm2. Effective: pain significantly alleviated; BMD remained the same or decreased by <0.05 g/cm2. Ineffective: no improvement in symptoms |
Markedly improved (14) Moderately improved (17) Ineffective (1) Overall efficacy: 96.8% Control treatment using alfacalcidol and caltrate D |
[12] |
|
| |||
|
Huqian pill 8g |
Significant effect: symptom and physical sign disappeared; 23 mM/d ⩽ urine P ⩽ 48 mM/d; 2.5 mM/d ⩽ urine Ca ⩽ 7.5 mM/d; 16.7 mM/d ⩽ urine Mg2+ ⩽ 100 mM/d; 0.96 mM/L ⩽ serum P ⩽ 1.45 mM/L; 0.8 mM ⩽ serum Mg2+ ⩽ 1.2 mM. X-ray appeared normal. Effective: clinical symptoms alleviated; urine Ca remained 6.5 ± 2.5 mmol/d; urine P remained 38 ± 15 mmol/d; urine Mg2+ remained 80 ± 40 mmol/d; X-ray without obviously abnormal. Ineffective: no improvement in symptoms and signs as well as other biomarkers |
Markedly improved (23) Moderately improved (28) Ineffective (5) Overall efficacy: 91.07% No controls |
[13] |
|
| |||
|
Gusong decoction 13h |
Significant effect: symptoms and physical signs score decreased by ⩾70%; BMD increased by ⩾2%; significant improvement in bone metabolism index; FPG and 2 hPG returned to the normal or decreased by ⩾40%; HbA1C ⩽ 6.2% or decreased by ⩾30%. Effective: symptoms and physical signs score decreased between 30% and 70%; BMD increased between 1% and 2%; bone metabolism index improved; FPG and 2 hPG decreased by ⩾20%; HbA1C decreased between 10% and 30%. Ineffective: no significant change in clinical symptoms; symptoms and physical signs score decreased by <30%; BMD increased by <1%; no improvement in bone metabolism index, FPG and 2 hPG, and HbA1C |
Markedly improved (15) Moderately improved (22) Ineffective (3) Overall efficacy: 92.5% Control treatment using vitamin D3 calcium carbonate tablets and alendronate sodium tablets |
[14] |
|
| |||
| Gusong decoction 4i |
Significant effect: lumbar vertebra pain and other clinical symptoms disappeared or total scores decreased by more than 2/3. Effective: lumbar vertebra pain and other clinical symptoms markedly alleviated or total scores decreased by more than 1/3. Ineffective: no improvement in clinical symptoms; total scores did not reach the effective value |
Markedly improved (21) Moderately improved (14) Ineffective (3) Overall efficacy: 92.11% Control treatment using glipizide and alendronate sodium tablets |
[15] |
|
| |||
| Tangmaikang granules combined with alendronate sodium tablets 9j |
Significant effect: lumbar vertebra pain disappeared; significant increase in BMD. Effective: lumbar vertebra pain alleviated with no significant increase in BMD. Ineffective: lumbar vertebra pain still existed; BMD remained the same |
Markedly improved (24) Moderately improved (21) Ineffective (3) Overall efficacy: 93.75% Control treatment using alendronate sodium tablets |
[16] |
|
| |||
| Bushen Huoxue decoction 0k |
Significant effect: back pain disappeared; symptoms and physical signs score decreased by 2/3; significant improvement in bone metabolism index; BMD increased by more than 0.06 g/cm2. Effective: back pain obviously alleviated; symptoms and physical signs scores decreased by 1/3; no change or aggravation was found in the biomarkers and BMD. Ineffective: no improvement in all the aspects |
Markedly improved (3) Moderately improved (26) Ineffective (1) Overall efficacy: 96.7% Control treatment using caltrate D |
[17] |
|
| |||
| Jiawei Gutongxian capsule 14l |
Cure: lumbar vertebra pain disappeared; X-ray appeared normal. Significant effect: lumbar vertebra pain obviously alleviated; significant improvement in X-ray. Effective: lumbar vertebra pain alleviated; improvement in X-ray. Ineffective: lumbar vertebra pain and X-ray remained the same |
Cure (1) Markedly improved (8) Moderately improved (27) Ineffective (4) Overall efficacy: 90.0% Control treatment using osteoform capsule |
[18] |
|
| |||
| Qianggubao capsule 8m |
Back pain; BMD | Markedly improved (24) Moderately improved (13) Ineffective (3) Overall efficacy: 92.5% |
[19] |
|
| |||
| Jiangu formula 12n |
Clinical symptomatic scales | Markedly improved (18) Moderately improved (28) Ineffective (4) Overall efficacy: 92.0% Control treatment using osteoform capsule |
[20] |
|
| |||
| Jiangtang Bushen formula 10o |
BMD, serum C-reactive protein (CRP) | BMD and serum CRP significantly increased. Control treatment using caltrate D |
[21] |
|
| |||
| Huolisu oral liquid 5p |
Significant effect: clinical symptoms markedly improved; BMD significantly increased. Effective: clinical symptoms alleviated; BMD slightly increased. Ineffective: clinical symptoms and BMD remained the same |
Markedly improved (12) Moderately improved (86) Ineffective (14) Overall efficacy: 79.1% Control treatment using salmon calcitonin |
[22] |
|
| |||
| Migu decoction 7q |
Visual analogue scale (VAS) pain score, BMD | VAS pain score significantly improved; BMD significantly increased. Control treatment using calcium carbonate |
[23] |
|
| |||
| Xianling Gubao 6r |
Clinical symptom scales and BMD | Clinical symptom scales and BMD significantly improved. Control treatment using caltrate D |
[24] |
aRadix Rehmanniae Preparata, Fructus Corni, Rhizoma Dioscoreae, Herba Cynomorii, Carapax et Plastrum Testudinis, Rhizoma Chuanxiong, and Radix et Rhizoma Salviae Miltiorrhizae (dosage information is not available).
bRadix Astragali 30 g, Herba Epimedii 12 g, Rhizoma Polygonati 12 g, Colla Cornus Cervi (melting in boiled water) 15 g, Rhizoma Dioscoreae 30 g, Semen Astragali Complanati 15 g, Radix Puerariae Lobatae 30 g, Radix Polygoni Multiflori 15 g, Radix et Rhizoma Salviae Miltiorrhizae 30 g, Radix et Rhizoma Rhei 10 g, and Sanguis Draconis 10 g.
cRadix Astragali, Colla Cervi Cornus, Radix Rehmanniae, Fructus Psoraleae, Herba Epimedii, Fructus Ligustri Lucidi, Radix Notoginseng, Radix et Rhizoma Salviae Miltiorrhizae, and Pheretima (dosage information is not available).
dCortex Eucommiae 12 g, Fructus Psoraleae 12 g, Radix Astragali 30 g, Radix Notoginseng 15 g, Rhizoma Dioscoreae 15 g, Colla Cornus Cervi (melting in boiled water) 6 g, Radix et Rhizoma Salviae Miltiorrhizae 12 g, Os Draconis 30 g, and Concha Ostreae 30 g.
eRadix Astragali, Radix et Rhizoma Rhei, Fructus Ligustri Lucidi, Fructus Lycii, Hirudo, Flos Carthami, and Radix et Rhizoma Rhodiolae Crenulatae (dosage information is not available).
fRadix Rehmanniae Preparata 15 g, Fructus Corni 10 g, Rhizoma Chuanxiong 15 g, Rhizoma Drynariae 15 g, Fructus Psoraleae 15 g, Herba Epimedii 12 g, Radix et Rhizoma Salviae Miltiorrhizae 15 g, Radix Cyathulae 15 g, and Fructus Lycii 20 g.
gCortex Phellodendri Chinensis 10 g, Radix Rehmanniae Preparata 15 g, Carapax et Plastrum Testudinis 15 g, Radix Paeoniae Alba 12 g, Rhizoma Anemarrhenae 9 g, Pericarpium Citri Reticulatae 10 g, Herba Cynomorii 10 g, and Rhizoma Zingiberis 4 g.
hRadix Astragali, Radix Codonopsis, Radix Polygoni Multiflori, Radix Angelicae Sinensis, Rhizoma Atractylodis Macrocephalae, Poria, Carapax et Plastrum Testudinis, Carapax Trionycis, Herba Taxilli, Herba Epimedii, Radix Dipsaci, Cortex Eucommiae, and Fructus Aurantii (dosage information is not available).
iRhizoma Drynariae 20 g, Herba Epimedii 20 g, Rhizoma Atractylodis Macrocephalae 15 g, and Cortex Eucommiae 15 g.
jRadix Astragali, Radix Rehmanniae, Radix Paeoniae Rubra, Radix et Rhizoma Salviae Miltiorrhizae, Radix Cyathulae, Radix Ophiopogonis, Rhizoma Polygonati, Herba Epimedii, and Radix Puerariae Lobatae (dosage information is not available).
kPrescription constituents and dosage information are not available.
lRadix Rehmanniae Preparata, Radix Morindae Officinalis, Herba Cistanches, Rhizoma Drynariae, Pyritum, Rhizoma Curculiginis, Herba Epimedii, Radix Angelicae Sinensis, Rhizoma Anemarrhenae, Radix Notoginseng, Carapax et Plastrum Testudinis, Placenta Hominis, Radix Astragali, and Endothelium Corneum Gigeriae Galli (dosage information is not available).
mRadix Astragali, Fructus Corni, Herba Dendrobii, Rhizoma Dioscoreae, Rhizoma Drynariae, Colla Cornus Cervi (melting in boiled water), Radix et Rhizoma Salviae Miltiorrhizae, and Concha Ostreae (dosage information is not available).
nHerba Epimedii 10 g, Herba Cistanches 10 g, Cortex Eucommiae 18 g, Radix Dipsaci 12 g, Radix Polygoni Multiflori 10 g, Radix Rehmanniae Preparata 12 g, Rhizoma Dioscoreae 15 g, Fructus Corni 10 g, Fructus Lycii 12 g, Radix Angelicae Sinensis 10 g, Radix Cyathulae 18 g, and Poria 12 g.
oRadix Astragali 30 g, Radix Paeoniae Rubra 20 g, Radix et Rhizoma Salviae Miltiorrhizae 20 g, Cortex Mori 10 g, Radix Trichosanthis 20 g, Fructus Lycii 15 g, Radix Scrophulariae 20 g, Radix Rehmanniae 20 g, Semen Cuscutae 15 g, and Rhizoma Drynariae 15 g (modification according to symptoms. If diagnosed as Qi deficiency, Radix Pseudostellariae (30 g) is added to the above prescription and the dosage of Radix Astragali is modified from 30 g to 50 g. If diagnosed as Yin deficiency, Herba Dendrobii (15 g) is added to the above prescription. If diagnosed as blood deficiency, Radix Paeoniae Alba (20 g) is added to the above prescription. If diagnosed as blood stasis, Rhizoma Chuanxiong (20 g) is added to the above prescription. If diagnosed as phlegm-heat, Fructus Trichosanthis (15 g) and Caulis Bambusae in Taenia (10 g) are added to the above prescription).
pHerba Epimedii, Radix et Rhizoma Salviae Miltiorrhizae, Radix Astragali, Radix Ophiopogonis, and Fructus Lycii (dosage information is not available).
qRadix Rehmanniae Preparata 15 g, Herba Epimedii 10 g, Fructus Psoraleae 15 g, Radix Notoginseng 15 g, Rhizoma Drynariae 15 g, Semen Cuscutae 15 g, and Rhizoma Dioscoreae 12 g.
rHerba Epimedii, Radix Dipsaci, Fructus Psoraleae, Radix Rehmanniae, Radix et Rhizoma Salviae Miltiorrhizae, and Rhizoma Anemarrhenae (dosage information is not available).
Furthermore, treatments with TCM formulas, such as Huqian pills [13], Gusong decoction [14, 15], Tangmaikang granules [16], Bushen Huoxue decoction [17], Jiawei Gutongxian capsule [18], Qianggubao granule [19], Jiangu decoction [20], Jiangtang Bushen formulation [21], Huolisu oral liquid [22], Migu decoction [23], and Xianling Gubao [24], have also been demonstrated to improve BMD, decrease serum C-reactive protein (CRP) level, and attenuate lumbar pain evidenced by decreasing visual analogue scale pain score in vertebra and back in the patients with DOP.
Most of prescriptions used in management of DOP consist of 4 to 14 herbs. Eight of most appearing Chinese herbs in the clinical studies are compiled in Table 2. All of these herbs possess the functions of invigorating Qi, activating blood circulation, and replenishing vital essence to tonify kidney [60]. The applications also conform to the TCM pathogenesis of DOP. The prescriptions are helpful for the investigators to screen the active ingredients against DOP from these herbs in the future.
Table 2.
Medicinal herbs most frequently used in clinical trials of DOP.
| Name of herb | Frequency |
|---|---|
| Radix Astragali | 11 |
| Herba Epimedii | 10 |
| Radix et Rhizoma Salviae Miltiorrhizae | 10 |
| Radix Rehmanniae Preparata | 6 |
| Rhizoma Drynariae | 6 |
| Fructus Lycii | 5 |
| Cortex Eucommiae | 4 |
| Rhizoma Dioscoreae | 4 |
Based on the listed prescriptions from Table 1, it is found that TCM treatments improved DOP through multichannel and different levels in the clinical trials. And the most commonly used herbs are Radix Astragali, Radix et Rhizoma Salviae Miltiorrhizae, Herba Epimedii, and Radix Rehmanniae Preparata. These four herbs have also been well evidenced to play a beneficial role in the treatment of diabetes by inhibiting AGEs formation [61], improving mitochondrial function and biogenesis, interfering with cell cycle [62], increasing insulin sensitivity and glycogen synthesis [63–65], and improving 5′ adenosine monophosphate-activated protein kinase signaling [66, 67] as well as regulating tissue regeneration and angiogenesis to promote diabetic foot ulcer healing [68]. Meanwhile, these four herbs were also demonstrated to exhibit antiosteoporotic effect by inhibiting osteoclastogenesis and promoting osteoblastogenesis through regulating mitogen-activated protein kinase [69], Wnt/β-catenin, bone morphogenetic proteins/SMAD, and OPG/RANKL/cathepsin K/nuclear factor kappa-B (NF-κB) signaling [26, 70, 71].
However, the current clinical studies are limited to small scale of samples and regional hospitals. Meanwhile, the clinical trials do not fully conform to the rule of randomized, double-blind, and parallel group which markedly impacted the correct evaluation of clinical research results. In addition, clinical observation indexes are limited more in BMD than in bone strength and fracture incidence. The stability and repeatability of the compound preparations used in clinical trials also remain open and should be studied further. Therefore, the credibility of the results needs to be further improved. So it is not conducive to developing standardized diagnosis method and treatment program as well as the development of new drugs.
4. Preclinical Advances of DOP in TCM
4.1. The Applications of Single Chinese Herbs in the Treatment of DOP in Animal Experiments
Single herbs or their extracts have been extensively studied in the DOP animal models (Table 3). In an experiment performed by Zhang et al., the water fraction of Fructus Ligustri Lucidi (FLL) ethanol extract (WF-EE, 574 mg/kg, i.g.) has been demonstrated to improve the trabecular bone deteriorations and inhibit hypercalciuria and increase serum parathyroid hormone (PTH) and fibroblast growth factor-23 in streptozotocin (STZ, 40 mg/kg) induced DBA/2J mice [25]. The underlying mechanism of WF-EE treatment may be attributed to increase of gene expressions of transient receptor potential vanilloid (TRPV) and calcium binding protein 9 K (CaBP-9K) in duodenum in DOP mice. Further, using alloxan (200 mg/kg) induced diabetic rats, Radix et Rhizoma Salviae Miltiorrhizae treatment (5 g/kg for 8 weeks) [26, 27] significantly improved serum calcium, alkaline phosphatase (ALP), BGP, and tartrate-resistant acid phosphatase levels and increased BMD value in alveolar bone osteoporosis. In addition, treatment with tetramethylpyrazine (100 mg/kg for 15 weeks, the active ingredients isolated from Rhizoma Chuanxiong) [28] markedly increased bone hydroxyproline and collagen levels in streptozotocin (STZ, 60 mg/kg) induced diabetic rats. Treatment with the alcohol extracts of Eucommia ulmoides Oliv. leaves [29] (6 g/kg) improved serum estradiol and increased BMD values in STZ (50 mg/kg) induced diabetic and ovariectomized (OVX) rats. Fructus Ligustri Lucidi [72], Rhizoma Chuanxiong [73], the leaves of Eucommia ulmoides Oliv. [74], and Radix et Rhizoma Salviae Miltiorrhizae [75] have been demonstrated with the function of tonifying kidney and promoting blood circulation.
Table 3.
Single herb or herbal extracts used in the treatment of DOP animals.
| TCM name | Active constituents | Animal model | Administration route, duration, and dosage | Reference |
|---|---|---|---|---|
| Fructus Ligustri Lucidi | Water fraction of Fructus Ligustri Lucidi ethanol extract | STZ (40 mg/Kg) | Intragastric administration (i.g.) (574 mg/kg) for 6 weeks | [25] |
| Radix et Rhizoma Salvia miltiorrhiza | — | Alloxan (200 mg/kg) | i.g. (5 g/kg) for 8 weeks | [26, 27] |
| Rhizoma Chuanxiong | Tetramethylpyrazine | STZ (60 mg/Kg) | i.g. (100 mg/kg) for 15 weeks | [28] |
| Cortex Eucommiae | Ethanol extracts of Eucommia ulmoides Oliv. leaves | STZ combined with OVX | i.g. (6 g/kg) for 8 weeks | [29] |
| Radix Puerariae | Puerarin | STZ (65 mg/Kg) | i.g. (100 mg/kg) for 6 weeks | [30, 31] |
| Aralia echinocaulis Hand-Mazz | The flavonesof Aralia echinocaulis Hand-Mazz | STZ (30 mg/kg) | i.g. (20 mg/kg) for 6 weeks | [32] |
| — | Quercetin | STZ | i.g. (5, 30 and, 50 mg/kg) for 8 weeks | [33] |
| Vitis vinifera L. seeds | — | STZ (60 mg/kg) | i.g (0.028 mg/kg every other day) for 16 weeks | [34] |
| Nigella sativa L. | Thymoquinone | STZ (50 mg/kg) | i.g. (2 mL/kg) for 4 weeks | [35–38] |
Note: “—” denotes that the content was not clearly stated in the cited reference.
Treatment with puerarin, the active substance isolated from Radix Puerariae with the function of improving blood circulation [76], significantly increased ALP level (40 μM for 48 hours, i.p.) [77], decreased expression of caspase-3 in osteoblasts (80 mg/kg/day for 6 weeks; i.p.) [30], and reduced blood glucose and improved empty lacunar and BMD (100 mg/kg for 6 weeks, i.p. [31]) in STZ (65 mg/kg) induced diabetic rats. The results suggest that puerarin promotes osteoblast proliferation and inhibits osteoclast activation.
Aralia echinocaulis Hand-Mazz has been documented to distribute to kidney channel based on TCM theories [78]. The flavonoids isolated from the Aralia echinocaulis Hand-Mazz treatment (20 mg/kg) [32] significantly improved the bone metabolism evidenced by increasing BMD value, bone strength, and bone mineral content in STZ (30 mg/kg) insulted male rats. In addition, the aqueous extract of Aralia echinocaulis Hand-Mazz (3.6 g/kg for 14 days) was evidenced to promote the expressions of fibroblast growth factor receptor 2, Fms-like tyrosine kinase, and fetal liver kinase which contributed to promoting angiogenesis and osteoblastogenesis in fracture healing model rats [78].
Treatment with quercetin increased serum osteocalcin, ALP, and urinary deoxypyridinoline (quercetin at 5 mg/kg showed little effect, while quercetin at 30 mg/kg and 50 mg/kg exhibited good effects for 8 weeks) in STZ induced diabetic osteopenia in rats [33]. The beneficial effect of quercetin also reflected on partially reversing the decreased biomechanical quality and improving microarchitecture of the femurs in the diabetic rats. The underlying mechanism may be due to its antioxidant capability. In addition, quercetin was also demonstrated to have a protective role in reducing β-cell damage and decreasing glycaemia in diabetic rats [79]. Furthermore, quercetin is widely embraced in TCM herbs and possessed the function of improving blood circulation [80, 81].
The combination treatments with polyphenols extracted from the seeds of Vitis vinifera L. (0.028 mg/kg, every two days for 16 weeks) [34] and the fruits of Sambucus nigra L. (0.04 g/kg, every two days for 16 weeks) [35, 36] statistically improved BMD in STZ induced diabetic rats. Further, the combination treatments with Nigella sativa L. and parathormone [36, 37] have also been demonstrated to improve the bone strength in diabetic rats.
Taken together, the herbs used in the treatment of DOP animals usually have the functions of nourishing Yin and tonifying kidney, promoting blood circulation, which have been demonstrated to possess the ability of improving the bone metabolism in DOP [76, 82]. The most accepted pharmacologically active ingredients of these herbs are flavonoids, polyphenols, alkaloids, polysaccharides, and so forth.
4.2. The Advances of TCM Compounds in the Treatment of DOP in Animal Experiments
As is well known, TCM compounds are composed of 2 or more Chinese drugs, which may play a synergistic action during the treatment of diseases. An experiment performed by Du et al. [39] demonstrated that Bushen Zhuanggu capsule (16 and 32 g/kg) treatment not only decreased blood glucose but also increased bone calcium and phosphorus as well as bone minerals in alloxan (120 mg/kg) induced diabetic rats. Jiang et al. [40] also found that Shenxiaokang concentrated pill (modified from Bawei Shenqi pill) treatment increased the thickness of bone epiphysis and improved trabecular nesh structure in STZ (25 mg/kg) induced diabetic male rats. One formula named Bushen Jianpi Huoxue decoction [41] was also evidenced to significantly reduce blood glucose, phosphorus, and ALP level and improve insulin resistance as well as increase BMD value in high fat diet and STZ (30 mg/kg) induced rats. In addition, Shuanghuang Yigu formula treatment [42] significantly reduced blood glucose and urine desoxypyirdoxine levels and increased blood BGP level in ovariectomized rat with STZ (50 mg/kg) injection.
Emerging formulas have been demonstrated to play a beneficial role in management of DOP animals. Tangshukang [43], one formula invented by Guan et al., was demonstrated to improve calcium and phosphorus metabolism, increase BGP level, and reduce bone ALP level in STZ (60 mg/kg) induced diabetic osteoporosis rats. The underlying mechanism may be attributed to improve mRNA levels of vitamin D receptor and CBP9K in the small intestine and increase mRNA level of transforming growth factor- (TGF-) β1 in the bone [83].
Furthermore, Bushen Zhuanggu capsule [44] treatment improved blood glucose metabolism through regulating insulin secretion, promoting bone TGF-β1 expression and inhibiting caspase-3 expression in osteoblast, and modulating the metabolic disorder of calcium-regulating hormones such as PTH, calcitonin, and vitamin D3 in alloxan (i.p. of 120 mg/kg, twice every other day) induced diabetic rats.
In addition, Qianggubao treatment [45] reduced the expressions of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and carboxyterminal cross-linked telopeptide of type I collagen (ICTP) and increased IGF-1 and carboxyterminal propeptide of type I procollagen (PICP) production in alloxan (i.p. of 120 mg/kg, twice every other day) induced diabetic osteoporosis rats. Further, Qianggubao [84] treatment also inhibited the formation of AGEs which was unfavorable for bone formation [85].
Most of formulas used in management of DOP not only have the function of improving bone metabolism but also afford effects on regulating glucose metabolism. However, as shown in Table 4, the ingredients of the formulas are not constant and fixable. And the commonly used TCM herbs also exhibit the effects of tonifying kidney, promoting blood circulation and removing blood stasis, which is also consistent with the main pathogenesis of DOP in TCM.
Table 4.
TCM prescriptions in the treatment of DOP animals.
| TCM compound prescription name | Animal model | Administration route, duration, and dosage | Reference |
|---|---|---|---|
| Bushen Zhuanggu capsule 2a |
Alloxan (120 mg/kg, twice every other day) | i.g. (8, 16 and 32 g/kg) for 8 weeks | [39] |
| Shenxiaokang concentrated pill 9b |
STZ (25 mg/kg), once a day for 5 days | i.g. (1.58 g/200 g) for 7 weeks | [40] |
| Bushen Jianpi Huoxue decoction 8c |
high fat diet for 4 weeks, i.p. of STZ (30 mg/kg) | i.g. (15 g/kg) for 12 weeks | [41] |
| Shuanghuang Yigu formula 9d |
i.p. of STZ (50 mg/kg) combined with OVX | i.g. (11.5 g/kg) for 10 weeks | [42] |
| Tangshukang capsule 0e |
i.p. of STZ (60 mg/kg) | i.g. (15 g/kg) for 12 weeks | [43] |
| Bushen Zhuanggu capsule 0f |
i.p. of alloxan (120 mg/kg, twice every other day) | i.g (8, 16 and 32 g/kg) for 7 weeks | [44] |
| Qianggubao 10g |
i.p. of alloxan (120 mg/kg, twice every other day) | i.g (1 mL/100 g) for 12 weeks | [45] |
aRadix Rehmanniae Preparata and Carapax et Plastrum Testudinis (dosage information is not available).
bRadix Rehmanniae Preparata, Rhizoma Dioscoreae, Fructus Corni, Poria, Rhizoma Alismatis, Pericarpium Citri Reticulatae, Radix Astragali, and Hirudo (dosage information is not available).
cRadix Rehmanniae Preparata, Cortex Eucommiae, Radix Astragali, Fructus Lycii, Colla Cornus Cervi (melting in boiled water), Radix et Rhizoma Salviae Miltiorrhizae, Rhizoma Anemarrhenae, and Radix Cyathulae (dosage information is not available).
d Os Draconis, Radix Rehmanniae Preparata, Radix Astragali, Cortex Eucommiae, Radix Dipsaci, Rhizoma Drynariae, Fructus Lycii, Fructus Corni, Poria, Radix Angelicae Sinensis, Radix Cyathulae, Herba Artemisiae Anomalae (dosage information is not available).
eTCM names and dosage information are not available.
fTCM names and dosage information are not available.
gRadix Astragali, Herba Dendrobii, Radix et Rhizoma Salviae Miltiorrhizae, Cortex Eucommiae, Fructus Psoraleae, Fructus Corni, Rhizoma Dioscoreae, Rhizoma Drynariae, Colla CornusCervi (melting in boiled water), and Concha Ostreae (dosage information is not available).
As is mentioned in Table 4, three types of DOP models are employed to evaluate the effect of TCM in the treatment of DOP, including STZ, alloxan, and STZ combined with OVX induced animal models. In addition, pigs are also used as the experimental DOP models [86].
5. The Toxicity of the Herbs
Toxicity is becoming a rising concern in the application of TCM in the clinical trials. Contrary to most of the modern toxicity data derived from animal experiments, the toxicities of TCM herbs in Chinese literature have been documented through clinical experiences. Before properly reviewing and studying the toxicities of the species by modern medical, pharmacological, and/or pharmaceutical sciences, the researchers must bear in mind that the isolated chemicals and the extracts from the herbs are not identical to the original herbs or formulas, while the traditional properties and indications of TCM herbs and formulas are the validated source of interpreting and extrapolating assessments of the toxicities. For knowing how to well understand the toxicities of TCM herbs, we suggest that the interested readers consult Dr. Leung's review [87].
In the above-mentioned species, several of them were evidenced to have toxicity issues for medicinal use. Nigella sativa L. extracts were demonstrated to possess the ability of lowering blood glucose in the healthy subjects. Therefore, caution must be excised when Nigella sativa L. extracts were applied to treat pregnant women, children, and diabetic patients [88]. For children, Nigella sativa L. at doses below 80 mg/kg was considered as safe dosages as there were no observed side effects being reported [89]. For detailed toxicity information of Nigella sativa L., we suggest that the readers consult Shuid et al.'s review [35].
It has been revealed that Pueraria tuberosa Linn. (Fabaceae) methanol extract of tubers showed LD50 at 227.5 mg in acute study in rats. For subchronic study, repeated challenges (5–100 mg/100 g, for 30 days) dose-dependently increased hepatic enzymes in blood, inflammatory cell infiltration, and hepatocellular necrosis. Kinetic study (single dose at 227.5 mg/100 g) revealed a decrease in GSH and an increase in free-radical generation [90, 91]. Puerarin, the major component of Radix Puerariae, has been demonstrated to cause hepatotoxicity as well as pathological changes in jejunum and spleen by high dose administration of puerarin freeze dried powder (100 and 200 mg/kg for 13 weeks, i.v.) to SD rats [92]. Interested readers are encouraged to consult Zhang et al.'s review [93].
The extracts of Radix Astragali, which consist of Astragalus polysaccharide and Astragalus membranaceus saponins, were evidenced safe without any distinct toxicity and side effects in the subchronic toxicity study. The safety dosage range is 2.85–19.95 g/kg for beagle dogs and 5.7–39.9 g/kg for rats [94]. And so far, no clinical toxicity data were reported.
The major tanshinones (cryptotanshinone, tanshinone I, tanshinone IIA, and dihydrotanshinone) isolated from Salvia miltiorrhiza Bunge were observed to induce HepG2 cell apoptosis in vitro [95]. Flow cytometry results demonstrated that tanshinone IIA (12.5 and 25 μM) treatment increased fragmented DNA in HepG2 cells. Cryptotanshinone, tanshinone I, and dihydrotanshinone elevated GSH/GSSG ratio at low concentrations (1.56 and 3.13 μM) and decreased GSH/GSSG ratio at high concentrations (6.25–25 μM), which indicates that these compounds may disturb redox balance in HepG2 cells. Another experiment performed by Xu et al. claimed that liposoluble components of Salvia miltiorrhiza Bunge (IC50 = 2.181 μg/mL) and tanshinone IIA (IC50 = 6.176 μg/mL) inhibited proliferation of rat retinal Müller cell in vitro by CCK-8 examination at 48 h [96]. But the authors did not show the cell viabilities at various time points which are useful to evaluate the drug toxicity.
Cortex Eucommiae is also widely used in the treatment of DOP. In chronic toxicity experiments in rats, the seeds and barks of Eucommia ulmoides Oliv. were found to affect the utilization rate of food, the routine blood and liver function, and the organ coefficient of the liver, spleen, testis, and ovary. But the investigators did not find abnormalities in histological examination. They also did not discover abnormalities in acute toxicity, bone marrow of mice micronuclear test, and sperm deformity experiments [97, 98].
6. Conclusions and Remarks
The clinical studies and basic research achievements support that TCM offers a new strategy in prevention and treatment of DOP (Scheme 1). TCM herbs and compounds have effects on resolving DOP through invigorating kidney Qi, promoting blood circulation, and removing blood stasis. This is consistent with TCM belief that liver-spleen-kidney insufficiency is closely associated with the induction of DOP. The most commonly used TCM herbs for treatment of DOP are Radix Astragali, Radix et Rhizoma Salviae Miltiorrhizae, Radix Rehmanniae Preparata, and Herba Epimedii. The investigators also demonstrate that these herbs have the beneficial effects on diabetes and osteoporosis. Three types of DOP models (STZ, alloxan, and STZ plus OVX induced animal disease models) are employed to evaluate the effect of TCM on DOP animals. These results clearly support that TCM herbs treatment not only improves bone metabolism but also prevents the development of diabetes. It is noticeable that some of TCM herbs and extracts may have the toxicities over the thresholds. Great care must be taken when TCM herbs are employed to treat DOP.
Scheme 1.
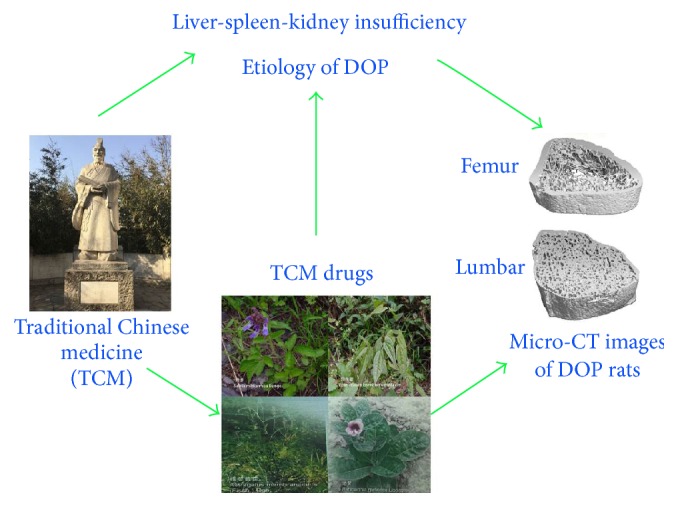
Liver-spleen-kidney insufficiency is closely associated with the induction of DOP. The most commonly used TCM herbs for treatment of DOP are Radix Astragali, Radix et Rhizoma Salviae Miltiorrhizae, Radix Rehmanniae Preparata, and Herba Epimedii. These herbs are evidenced to improve the management of DOP in clinical and preclinical study.
However, the basic research on single herbs and TCM compounds in the treatment of DOP is still very weak, and there is still a long way to find the mechanisms and active ingredients in TCM herbs. The researchers also need to pay attention that the pathogenesis of DOP is a multifaceted chronic metabolic disease, and a comprehensive study with multidisciplinary technology and comprehensive usage of various approaches should be employed to explore pathogenesis and to prevent the development of DOP. The further understanding of these concerns will contribute to providing a more scientific basis for management of DOP.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (NSFC81274041 and NSFC81273995), International Cooperation projects of MOE (2011DFA30920), and Key Drug Development Program of MOST (20122X09103201-005) as well as the 111 project of MOE (B07007).
Disclosure
These funding agencies have no roles in study design; in data collection, analysis, and interpretation; in the writing of the report; and in the decision to submit the paper for publication.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Rufeng Ma, Ruyuan Zhu, and Lili Wang equally contributed to this work.
References
- 1.Epstein S., Defeudis G., Manfrini S., et al. Diabetes and disordered bone metabolism (diabetic osteodystrophy): time for recognition. Osteoporosis International. 1931;27(6):1931–1951. doi: 10.1007/s00198-015-3454-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W.-L., Meng H.-Z., Yang M.-W. Regulation of DMT1 on bone microstructure in type 2 diabetes. International Journal of Medical Sciences. 2015;12(5):441–449. doi: 10.7150/ijms.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World Journal of Diabetes. 2013;4(4):101–113. doi: 10.4239/wjd.v4.i4.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piscitelli P., Neglia C., Vigilanza A., Colao A. Diabetes and bone: biological and environmental factors. Current Opinion in Endocrinology, Diabetes and Obesity. 2015;22(6):439–445. doi: 10.1097/med.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Yu W.-H., Wang X.-L. Effects of Tanggukang on serum free radical metabolism in patients with diabetic osteoporosis. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;12(7):1251–1254. [Google Scholar]
- 6.Yu W. H., Zhang Y. Effect of Tanggukang Decoction on the metabolism of calcium and phosphourous in the patients of diabetes with osteoporosis. Zhongguo Zhongyi Jizheng. 2009;18(1):45–46. [Google Scholar]
- 7.Ji B., Guan J., Chen X., et al. Clinical research of self-made kidney-tonifying and blood circulation-promoting Chinese medicine in patients with diabetic osteoporosis. Guide of China Medicine. 2012;10(17):3–4. [Google Scholar]
- 8.Shang X., Xu S., Zhou Q., et al. Clinical observations on the effect of Bushen Tongluo capsule for treatment of diabetic osteoporosis. China Pharmacist. 2010;13(9):1329–1330. [Google Scholar]
- 9.Shang X., Xie P., Guo X., Zhang T. The influence of Bushen Tongluo Compound on bone mineral density and balance ability of patients with diabetic osteoporosis. Captial Medicine. 2010;17(16):60–61. [Google Scholar]
- 10.Gong Z. D., Li C. J. Clinical research of modified Qing'e pill on diabetic osteoporosis. Zhongguo Zhongyi Jizheng. 2012;21(9):1391–1402. [Google Scholar]
- 11.Yushanjiang A., Halida M. Clinical study on effect of Xihongkang for the treatment diabetic osteoporosis. Chinese Journal of Basic Medicine in Traditional Chinese. 2012;18(9):1001–1002. [Google Scholar]
- 12.Deng M. Clinical studies on the effect of Yishen Zhuanggu Compound for the treatment of type 2 diabetes induced osteoporosis. Guiyang Journal of TCM College. 2013;35(1):58–59. [Google Scholar]
- 13.Zhang L. Clinical observation on 56 cases of patients with diabetic osteoporosis in using Huqian Pill. Hebei Journal of TCM. 2013;25(3):p. 180. [Google Scholar]
- 14.Liu R. Observe the Safety and Efficacy of TCM Gu-Song Decoction to Diabetic Osteoporosis (DOP) Patients. Chengdu, China: Chengdu University of Chinese Medicine; 2013. [Google Scholar]
- 15.Hao C. Clinical Study on the effect of Gusong decoction on patient with dibetic osteoporosis. Asia Pacific Traditional Medicine. 2015;11(10):117–118. [Google Scholar]
- 16.Gao M. Clinical observation of tangmaikang granules combined with alendronate sodium tablets in the treatment of bone metabolism in elderly patients with type 2 diabetes. China Pharmacy. 2012;23(28):2658–2660. [Google Scholar]
- 17.Feng Z., Yue X., Li F., Wei Y., Li Z. Clinical study of the effect of Bushen Huoxue principle on bone metabolism of patients with daibetic osteoporosis. Proceedings of the 14th TCM Diabtes Conference; 2012; Zhengzhou, China. Diabetes Sub-society of China Chinese Medicine Assocation; [Google Scholar]
- 18.Jia W., Wu Z., Dong Q. Clinical observation of the effect of Jiawei Gutongxian Capasule on the treatment of patients with diabetic osteoporosis. Hebei Journal of TCM. 2006;28(11):822–823. [Google Scholar]
- 19.Su Y., Qian S., Dong Z., Guo J., Zhang A. Clinical observation on QIANGGUBAO for treating 40 cases of diabetic osteoporosis. Journal of Fujian College of TCM. 2001;11(3):23–25. [Google Scholar]
- 20.Zhao L. 50 cases of clinical trials in the treatment of diabetic bone metabolism by self-made Jiangu decoction. Hei Long Jiang Journal of TCM. 2000;29(1):p. 46. [Google Scholar]
- 21.Huang L. Clinical study of the effect of Jiangtang Bushen formulation on bone mineral density and serum C reactive protein on patients with diabetic osteoporosis. Fujian Journal of TCM. 2009;40(6):15–16. [Google Scholar]
- 22.Shen H., Huang J., Wang Y. Clinical observation of the effect of combination of huolisu oral liqiud and salmon calcitonin on the treatment of old patients with diabetic osteoporosis. Chinese Journal of Information on TCM. 2009;15(1):78–79. [Google Scholar]
- 23.Yan F., Zhang Y., Wang Z. The effect of Migu decoction on the treatment of patients with type 2 diabetes osteoporosis. Hebei Medicinal Journal. 2002;34(21):3340–3341. [Google Scholar]
- 24.You Y., Zhuang R., Zhao H., Xie Y. Clinical therapeutical effect of xianling gubao on patients with diabetic osteoporosis. Journal of Pharmaceutical Research. 2014;33(4):113–115. [Google Scholar]
- 25.Zhang Y., Diao T.-Y., Wang L., Che C.-T., Wong M.-S. Protective effects of water fraction of Fructus Ligustri Lucidi extract against hypercalciuria and trabecular bone deterioration in experimentally type 1 diabetic mice. Journal of Ethnopharmacology. 2014;158:239–245. doi: 10.1016/j.jep.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y., Li Y., Xue L., et al. Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. Journal of Ethnopharmacology. 2014;155(3):1401–1416. doi: 10.1016/j.jep.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 27.Miao B., Wang J., Zhu Y., Yue C., Chen M. Experimental study on effect of Salvia miltiorrhiza on alveolar bone metabolism and variation in bone mass in diabetic rats. China Journal of Chinese Materia Medica. 2012;37(11):1659–1662. doi: 10.4268/cjcmm20121133. [DOI] [PubMed] [Google Scholar]
- 28.Chen X., Lin Z., Chen H., Chen X. The protection of tetramethylpyrazine on bone in diabetic osteoporosis rat. Journal of Putian University. 2008;15(5):46–49. [Google Scholar]
- 29.Zhang L., Ge H., Zhao L. Effects of eucommia leaves lavoids on osteoporosis in castrated rat with diabetes melli. Chinese Journal of Gerontology. 2003;23(6):370–372. [Google Scholar]
- 30.Liang J., Chen H., Pan W., Xu C. Puerarin inhibits caspase-3 expression in osteoblasts of diabetic rats. Molecular Medicine Reports. 2012;5(6):1419–1422. doi: 10.3892/mmr.2012.854. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Pan H., Liang Y. Experiment study on protection of Puerarin in diabetic osteoporosis. Chinese Archives of Traditional Chinese Medicine. 2012;30(4):848–850. [Google Scholar]
- 32.Pei L. The effect of flavonoids of Aralia echinocaulis Hand-Mazz on bone metabolism in the diabteic rats. Proceedings of the in 4th International Chinese Medicine and Diabetes Conferences; 2009; Beijing, China. p. p. 7. [Google Scholar]
- 33.Luo Z., Liang W., Luo Z., et al. Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. European Journal of Pharmacology. 2011;670(1):317–324. doi: 10.1016/j.ejphar.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Pohaci C., Groza M., Mareş L., Ciocoiu M., Bǎdescu M. Beneficial dexa-related effects of natural polyphenols on experimentally-induced diabetes mellitus complications. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi's. 2009;113(3):838–844. [PubMed] [Google Scholar]
- 35.Shuid A. N., Mohamed N., Mohamed I. N., et al. Nigella sativa: a potential antiosteoporotic agent. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/696230.696230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altan M. F. Effects of Nigella sativa and human parathyroid hormone on bone mass and strength in diabetic rats. Biological Trace Element Research. 2007;116(3):321–328. doi: 10.1007/bf02698016. [DOI] [PubMed] [Google Scholar]
- 37.Altan M. F., Kanter M., Donmez S., Kartal M. E., Buyukbas S. Combination therapy of Nigella sativa and human parathyroid hormone on bone mass, biomechanical behavior and structure in streptozotocin-induced diabetic rats. Acta Histochemica. 2007;109(4):304–314. doi: 10.1016/j.acthis.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Badescu L., Badulescu O., Badescu M., Ciocoiu M. Mechanism by Sambucus nigra extract improves bone mineral density in experimental diabetes. Evidence-based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/848269.848269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du L., Yin L., Chen M., Liu Y. Effects of Bushen Zhuanggu capsule on bone mineral metabolism and bone density of rats with diabetes osteoporosis. Chinese Journal of Traditional Medical Science and Technology. 2010;17(4):293–295. [Google Scholar]
- 40.Jiang D., Wang B., Chen Y., Liu C., Liu Z. Research on the effects of Shen-xiaokang on the morphology of osteoporosis in Daibetic Rats. Liao Ning Zhongyi Zazhi. 2006;33(10):1252–1254. [Google Scholar]
- 41.Xu J., Liang N., Chen Z. Research on the effect of bushen jianpi huoxue decoction on bone metabolism in rats due to type 2 diabetes with osteoporosis. Shanxi Journal of TCM. 2015;31(5):48–50. [Google Scholar]
- 42.Li S., Xiong M., Chen C., et al. Experimental studies on preventive effect of Shuanghuang Yigu prescription on menopausal diabetses osteoporosis. Traditional Chinese Drug Research and Clinical Pharmacology. 2003;14(5):296–298. [Google Scholar]
- 43.Guan Z., Dong H., Dong Q. Experimental study on the effect of Tangshukang on the bone metabolism, BMD and bone biomechanics in diabetic osteoporosis rats. Information on Traditional Chinese Medicine. 2010;27(6):80–82. [Google Scholar]
- 44.Jiang Y. Experimental Study on the Effect of Bushen Zhuanggu Capsule on Bone Resorption-Formation Couple Links in Diabetic Osteoporosis Rats. Chengdu, China: Chengdu University of Chinese Medicine; 2002. [Google Scholar]
- 45.Su Y., Xing H., Zheng L., Lin J., Chen X. Experiment on the effect of QIANGGUBAO on metabolism of bone collagen in the diabetic osteoporosis rats. Chinese Journal of Osteoporosis. 2006;12(3):289–291. [Google Scholar]
- 46.Takamoto I., Kadowaki T. Diabetes and osteoporosis. Clinical Calcium. 2004;14(2):255–261. [PubMed] [Google Scholar]
- 47.Guo Y. B., Wang L. L., Ma R. F., et al. Etiology, pathology and prospects of TCM in osteoporosis treatment. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2015;17(4):768–772. [Google Scholar]
- 48.Preliminary exploration of the TCM theory ‘spleen governs digestion and transportation’. Journal of Traditional Chinese Medicine. 1982;2(4):285–288. [PubMed] [Google Scholar]
- 49.Chen W.-H., Liu X.-X., Tong P.-J., Zhan H.-S. Diagnosis and management of knee osteoarthritis: Chinese medicine expert consensus (2015) Chinese Journal of Integrative Medicine. 2016;22(2):150–153. doi: 10.1007/s11655-015-2432-7. [DOI] [PubMed] [Google Scholar]
- 50.Tian Y. G., Li Y., Li J. S., et al. Effects of therapies for regulating and reinforcing lung and kidney on osteoporosis in rats with chronic obstructive pulmonary disease. Journal of Traditional Chinese Medicine. 2015;35(2):175–183. doi: 10.1016/s0254-6272(15)30025-x. [DOI] [PubMed] [Google Scholar]
- 51.Ju D., Liu M., Zhao H., Wang J. Mechanisms of ‘kidney governing bones’ theory in traditional Chinese medicine. Frontiers of Medicine in China. 2014;8(3):389–393. doi: 10.1007/s11684-014-0362-y. [DOI] [PubMed] [Google Scholar]
- 52.Hsu C.-H., Lee C.-J., Chien T.-J., et al. The relationship between qi deficiency, cancer-related fatigue and quality of life in cancer patients. Journal of Traditional and Complementary Medicine. 2012;2(2):129–135. doi: 10.1016/s2225-4110(16)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z.-Z., Liu W., Zhang F., Li Z., Cheng Y.-Y. Deciphering the therapeutic mechanisms of Xiao-Ke-An in treatment of type 2 diabetes in mice by a Fangjiomics approach. Acta Pharmacologica Sinica. 2015;36(6):699–707. doi: 10.1038/aps.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao D. D., Gao S. H., Mu Q. Q., Qin P. J., Mo F. F. Theoretical study on treating type 2 diabetes by regulating the liver, spleen and kidney. Chinese Medicine. 2014;55(3):205–208. [Google Scholar]
- 55.Zhou D.-A., Deng Y.-N., Liu L., Li J.-J. Effect of kidney-reinforcing and marrow-beneficial traditional Chinese medicine-intervened serum on the proliferation and osteogenic differentiation of bone marrow stromal cells. Experimental and Therapeutic Medicine. 2015;9(1):191–196. doi: 10.3892/etm.2014.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su K., Zhu F., Guo L., Zhu Y., Li W., Xiong X. Retrospective study on Professor Zhongying Zhou's experience in Traditional Chinese Medicine treatment on diabetic nephropathy. Journal of Traditional Chinese Medicine. 2013;33(2):262–267. doi: 10.1016/S0254-6272(13)60137-5. [DOI] [PubMed] [Google Scholar]
- 57.Liu X., Liu L., Chen P., et al. Clinical trials of traditional Chinese medicine in the treatment of diabetic nephropathy—a systematic review based on a subgroup analysis. Journal of Ethnopharmacology. 2014;151(2):810–819. doi: 10.1016/j.jep.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 58.Wang L., Li Y., Guo Y., et al. Herba Epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Current Pharmaceutical Design. 2016;22(3):328–349. doi: 10.2174/1381612822666151112145907. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Zhang Q.-M., Wang Y.-G., Yu D.-L., Zhang W. The TCM pattern of the six-zang and six-fu organs can be simplified into the pattern of five-zang and one-fu organs. Journal of Traditional Chinese Medicine. 2011;31(2):147–151. doi: 10.1016/S0254-6272(11)60030-7. [DOI] [PubMed] [Google Scholar]
- 60.Chen J. K. Chinese Medical Herbology and Pharmacology. Industry, Calif, USA: Art of Medicine Press; 2010. [Google Scholar]
- 61.Nakashima K., Miyashita H., Yoshimitsu H., Fujiwara Y., Nagai R., Ikeda T. Two new prenylflavonoids from Epimedii Herba and their inhibitory effects on advanced glycation end-products. Journal of Natural Medicines. 2016;70(2):290–295. doi: 10.1007/s11418-015-0962-0. [DOI] [PubMed] [Google Scholar]
- 62.Yuan W., Zhang Y., Ge Y., Yan M., Kuang R., Zheng X. Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro . Planta Medica. 2008;74(10):1259–1264. doi: 10.1055/s-2008-1081290. [DOI] [PubMed] [Google Scholar]
- 63.Raoufi S., Baluchnejadmojarad T., Roghani M., Ghazanfari T., Khojasteh F., Mansouri M. Antidiabetic potential of salvianolic acid B in multiple low-dose streptozotocin-induced diabetes. Pharmaceutical Biology. 2015;53(12):1803–1809. doi: 10.3109/13880209.2015.1008148. [DOI] [PubMed] [Google Scholar]
- 64.Huang M., Wang P., Xu S., et al. Biological activities of salvianolic acid B from Salvia miltiorrhiza on type 2 diabetes induced by high-fat diet and streptozotocin. Pharmaceutical Biology. 2015;53(7):1058–1065. doi: 10.3109/13880209.2014.959611. [DOI] [PubMed] [Google Scholar]
- 65.Li X., Xu Z., Jiang Z., et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochimica et Biophysica Sinica. 2013;46(9):738–748. doi: 10.1093/abbs/gmu065. [DOI] [PubMed] [Google Scholar]
- 66.Carai M. A., Colombo G., Loi B., et al. Hypoglycemic effects of a standardized extract of Salvia miltiorrhiza roots in rats. Pharmacognosy Magazine. 2015;11(44, supplement 4):S545–S549. doi: 10.4103/0973-1296.172959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiang G., Yang X., Shi L., et al. Antidiabetic effect of salvianolic acid a on diabetic animal models via AMPK activation and mitochondrial regulation. Cellular Physiology and Biochemistry. 2015;36(1):395–408. doi: 10.1159/000430258. [DOI] [PubMed] [Google Scholar]
- 68.Lau T. W., Lam F. F. Y., Lau K. M., et al. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. Journal of Ethnopharmacology. 2009;123(1):155–162. doi: 10.1016/j.jep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Kwak H. B., Yang D., Ha H., et al. Tanshinone IIA inhibits osteoclast differentiation through down-regulation of c-Fos and NFATc1. Experimental and Molecular Medicine. 2006;38(3):256–264. doi: 10.1038/emm.2006.31. [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Li Y., Guo Y., et al. Herba epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Current Pharmaceutical Design. 2015;22(3):328–349. doi: 10.2174/1381612822666151112145907. [DOI] [PubMed] [Google Scholar]
- 71.Bian Q., Huang J.-H., Liang Q.-Q., et al. The osteogenetic effect of astragaloside IV with centrifugating pressure on the OCT-1 cells. Pharmazie. 2011;66(1):63–68. doi: 10.1691/ph.2011.0219. [DOI] [PubMed] [Google Scholar]
- 72.Li Q., Fan Y.-S., Gao Z.-Q., Fan K., Liu Z.-J. Effect of Fructus Ligustri Lucidi on osteoblastic like cell-line MC3T3-E1. Journal of Ethnopharmacology. 2015;170:88–95. doi: 10.1016/j.jep.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Wang L., Zhang J., Hong Y., Feng Y., Chen M., Wang Y. Phytochemical and pharmacological review of da Chuanxiong formula: a famous herb pair composed of chuanxiong rhizoma and gastrodiae rhizoma for headache. Evidence-based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/425369.425369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y., Zhang H., Wang F., Yang D., Ding K., Fan J. The ethanol extract of Eucommia ulmoides Oliv. leaves inhibits disaccharidase and glucose transport in Caco-2 cells. Journal of Ethnopharmacology. 2015;163:99–105. doi: 10.1016/j.jep.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Su C.-Y., Ming Q.-L., Rahman K., Han T., Qin L.-P. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chinese Journal of Natural Medicines. 2015;13(3):163–182. doi: 10.1016/s1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 76.Xie W., Zhao Y., Du L. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. Journal of Ethnopharmacology. 2012;140(2):345–367. doi: 10.1016/j.jep.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Pan H., Liang Y. Experimental study on the effect of puerarin injection in high glucose treated osteoblast. Chinese Journal of Traditional Medical Science and Technology. 2012;19(4):318–319. [Google Scholar]
- 78.Yin X., Li L., Zheng L.-L., et al. Influence of aqueous extract of Aralia echinocaulis Hand.-Mazz on the expression of fracture healing-related factor receptors. Zhongguo Gu Shang. 2011;24(9):761–765. [PubMed] [Google Scholar]
- 79.Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research. 2005;51(2):117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Sun J., Yu S. Research progress of n pharmacological action of quercetin. Chinese Medicines Journal of Research and Practice. 25(3):85–88. [Google Scholar]
- 81.Luo M., Luo D., Zhao W. Research progress on pharmacological action of quercetiin. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2014;25(17):12–14. [Google Scholar]
- 82.Teixeira A., Baenas N., Dominguez-Perles R., et al. Natural bioactive compounds from winery by-products as health promoters: a review. International Journal of Molecular Sciences. 2014;15(9):15638–15678. doi: 10.3390/ijms150915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan Z., Dong Q. Study of the effect of Tangshukang for the VDR, TGF-β1, CaBp-D9K mRNA expression on the diabetic osteoporosis rats. Modern Medicine Journal of China. 2010;12(4):4–6. [Google Scholar]
- 84.Xing H. An Experimental Study on the Effect of Qianggubao on the Metabolism of Bone Collagen in Diabetic Osteoporosis Rats. Chinese Internal Medicine, Fujian College of TCM; 2005. [Google Scholar]
- 85.Yang X., Mostafa A. J., Appleford M., Sun L. W., Wang X. Bone formation is affected by matrix advanced glycation end products (AGEs) in vivo. Calcified Tissue International. 2016 doi: 10.1007/s00223-016-0153-3. [DOI] [PubMed] [Google Scholar]
- 86.Huovinen V., Saunavaara V., Kiviranta R., et al. Vertebral bone marrow glucose uptake is inversely associated with bone marrow fat in diabetic and healthy pigs: [18F]FDG-PET and MRI study. Bone. 2014;61:33–38. doi: 10.1016/j.bone.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Leung A. Y. Traditional toxicity documentation of Chinese materia medica—an overview. Toxicologic Pathology. 2006;34(4):319–326. doi: 10.1080/01926230600773958. [DOI] [PubMed] [Google Scholar]
- 88.Le P. M., Benhaddou-Andaloussi A., Elimadi A., Settaf A., Cherrah Y., Haddad P. S. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. Journal of Ethnopharmacology. 2004;94(2-3):251–259. doi: 10.1016/j.jep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 89.Kalus U., Pruss A., Bystron J., et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytotherapy Research. 2003;17(10):1209–1214. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 90.Maji A. K., Pandit S., Banerji P., Banerjee D. Pueraria tuberosa: a review on its phytochemical and therapeutic potential. Natural Product Research. 2014;28(23):2111–2127. doi: 10.1080/14786419.2014.928291. [DOI] [PubMed] [Google Scholar]
- 91.Santosh N., Mohan K., Royana S., Yamini T. B. Hepatotoxicity of tubers of Indian Kudzu (Pueraria tuberosa) in rats. Food and Chemical Toxicology. 2010;48(4):1066–1071. doi: 10.1016/j.fct.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Y., Su X., Cheng B., Jiang J., Chen H. Comparative study on pharmacological effects of various species of Pueraria . Zhongguo Zhongyao Zazhi. 1995;20(10):619–621. [PubMed] [Google Scholar]
- 93.Zhang Z., Lam T.-N., Zuo Z. Radix puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. Journal of Clinical Pharmacology. 2013;53(8):787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 94.Yu S.-Y., OuYang H.-T., Yang J.-Y., et al. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. Journal of Ethnopharmacology. 2007;110(2):352–355. doi: 10.1016/j.jep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 95.Lee W. Y. W., Chiu L. C. M., Yeung J. H. K. Cytotoxicity of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on HepG2 cells in relation to glutathione perturbation. Food and Chemical Toxicology. 2008;46(1):328–338. doi: 10.1016/j.fct.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 96.Xu M., Zhang M., Yao H. E., Chen L., Xie X. Study on the toxic effect of the liposoluble components of Salvia on rat retinal Müller cells. West China Journal of Pharmaceutical Sciences. 2013;28(5):453–455. [Google Scholar]
- 97.Hu C., Huang Y., Wang X., Sun C., Luo T., Yang X. A toxicity comparative study of eucommia ulmoides seed and bark. Journal of Jinggangshan University (Natural Science) 2015;36(1):95–100. [Google Scholar]
- 98.Zhang J., Du H., Li Q., Ding Y. Study advancement about pharmacological andtoxicological of Eucommia ulmoides Oliv. Journal of Henan University (Medical Science) 2014;33(3):217–222. [Google Scholar]


