Abstract
Active transport of neurotransmitters into synaptic vesicles is required for their subsequent exocytotic release. In the monoamine system, this process is carried out by the vesicular monoamine transporters (VMAT1 and VMAT2). These proteins are responsible for vesicular packaging of dopamine, norepinephrine, serotonin, and histamine. These proteins are essential for proper neuronal function; however, compared to their plasma membrane counterparts, there are few drugs available that target these vesicular proteins. This is partly due to the added complexity of crossing the plasma membrane, but also to the technical difficulty of assaying for vesicular uptake in high throughput. Until recently, reagents to enable high throughput screening for function of these vesicular neurotransmitter transporters have not been available. Fortunately, novel compounds and methods are now making such screening possible; thus, a renewed focus on these transporters as potential targets is timely and necessary.
Vesicular monoamine transporters: overview
The vesicular monoamine transporters (VMATs) are part of the Major Facilitator Superfamily (MFS) and the solute carrier family of transporters (SLC) subfamily. Like other MFS family members, VMATs contain 12 transmembrane spanning domains, with cytosolic C- and N- terminals and large glycosylated intravesicular loops. Members of the SLC18 subfamily are Drug:H+ antiporters; these transporters exchange intravesicular protons for extravesicular neurotransmitter.
The vesicular monoamine transporters are essential for proper monoaminergic neurotransmission, which requires the sequestration of transmitter into synaptic vesicles by VMAT for subsequent Ca2+-stimulated exocytotic release (1). This critical function is accomplished by the secondary active transport of neurotransmitters against their concentration gradient into synaptic vesicles (2). As proton exchangers, VMATs rely on the proton gradient generated by the V-type ATPase across the vesicular membrane and the import of chloride via the ClC-3 chloride channels. The high concentration of intravesicular protons allow for the exchange of two protons for each molecule of neurotransmitter transported (3, 4). VMATs primarily transport monoamines (dopamine, serotonin, norepinephrine and histamine), but also sequester toxicants into vesicles, shunting them away from cytosolic sites of action (5–12). This is particularly interesting given sequence homology between VMATs and the bacterial toxin extruding antiporters (TEXANs) (13).
In mammals, there are two VMAT isoforms. VMAT1 (SLC18A1) is expressed exclusively in the periphery, with expression in the sympathetic nervous system, adrenal chromaffin cells, and endocrine/paracrine cells of the gut. VMAT2 (SLC18A2) has both peripheral (enteric nervous system, adrenal chromaffin cells, and endocrine cells of the stomach, and platelets) and central nervous system (all monoaminergic neurons of the brain) expression (14). The transporters share common substrates with the exception of histamine, which is believed to be preferentially packaged by VMAT2.
Vesicular monoamine transporters in disease
Many neurological and psychiatric disorders can be linked to dysfunction of monoaminergic systems, including Parkinson’s disease (PD), Huntington’s disease, ADHD, dystonia, schizophrenia, addiction, and depression (15–20). Although the origin of monoaminergic dysfunction varies, manipulation of vesicular function could be a useful target for modulating monoamine homeostasis. Data from our lab and others suggests that direct modification of monoamine vesicular function may be beneficial in a variety of disorders, either in isolation or in conjunction with existing therapies. For purposes of this review, we will focus on dopamine packaging by VMAT2 and PD, which has been the focus of work in our lab. Data from many labs have demonstrated that proper packaging of dopamine into vesicles is critical since cytosolic dopamine is neurotoxic. Cytosolic dopamine is metabolized by enzymatic deamination or broken down by autoxidation, producing reactive, harmful oxidative products (21–28). Efficient transport of dopamine by VMAT2 prevents accumulation of these toxic byproducts.
Toxicological disruption of vesicular transport
As explored in our recent review, “Vesicular Integrity in Parkinson’s Disease,” many insults, both environmental and genetic, that lead to PD converge on vesicle function (21). Several classes of environmental toxicants, including pesticides, polychlorinated biphenyls, and brominated flame retardants, have been associated with PD pathology (21, 29–31). Epidemiological evidence linking these toxicants to disease risk is extensive (32–43). Additionally, mechanistic studies have demonstrated that these compounds exert selective toxicity to dopaminergic neurons via inhibition of synaptosomal and vesicular uptake of dopamine and resultant oxidative stress (44–60).
In vitro and animal models of modified vesicular transport
Many studies in both in vitro and animal models have also demonstrated that unregulated cytosolic dopamine is neurotoxic (61–64). In vitro experiments suggest that the relative vulnerability of dopamine neurons in PD may be mediated by cytosolic dopamine (65). Furthermore, mice that express DAT on non-dopaminergic striatal neurons, which lack VMAT2, take up dopamine into those neurons, but do not store it in vesicles, producing motor deficits and profound striatal neurodegeneration, accompanied by markers of increased dopamine oxidation (66). Additionally, transgenic mice with altered expression of VMAT2 have illustrated the critical nature of vesicular storage of dopamine for the integrity of the nigrostriatal system. VMAT2 knockout mice die soon after birth, while heterozygotes develop normally, but display increased sensitivity to amphetamine, and 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) (12, 67, 68). Mice that are hypomorphic for VMAT2 (~5% wild type expression) have been developed as a mouse model of PD (10, 69–71). These mice develop normally, but undergo progressive nigrostriatal degeneration, α-synuclein accumulation, show markers of oxidative stress, and develop motor and nonmotor symptoms of PD when they express alpha-synuclein (10, 69, 70).
In addition, genetic mutations linked to PD often affect synaptic vesicle function, leading to deficits in trafficking, transmitter storage and release. Alpha-synuclein has long been known to bind to phospholipids on the vesicle membrane (72–75). While its function is unknown, genetic ablation of the synuclein genes increases dopamine release (76, 77). In addition, dopamine influences the utilization of alternate alpha-synuclein transcripts, resulting in changes in localization of alpha-synuclein (78). Fibrillization of alpha-synuclein is promoted by oxidized dopamine; in turn, fibrillar alpha-synuclein can permeabilize the vesicular membrane, leading to further increases in cytosolic dopamine and thus more oxidative stress, creating a positive feedback loop (79). Deficiency of PINK1 function reduces synaptic efficiency by immobilizing synaptic vesicles of the reserve pool [Morais 2009]. DJ-1 interacts with synaptic vesicle proteins such as synaptophysin and Rab3A and influences the expression of VMAT2 (80, 81). Finally, DJ-1, PINK1 and parkin knock-out mouse models all show substantial presynaptic deficits in dopamine release (82–85). Together, these data suggest that vesicular dysfunction is a convergence point for both genetic and environmental risk factors of PD.
Current therapeutics targeting VMAT2
Despite the recognized importance of the vesicle in dopaminergic disease, few FDA approved drugs directly and specifically target the vesicle. Two VMAT2 inhibitors, reserpine and tetrabenazine (TBZ), have demonstrated efficacy in the treatment of disease. Although other drugs, such as amphetamine and methylphenidate, are known to affect VMAT2 function, these drugs have a complicated pharmacology due to their interaction with plasmalemmal transporters and inhibition of neurotransmitter metabolism (9, 86). As they do not exclusively act at VMAT2, we have focused on reserpine and TBZ, which specifically target the vesicle.
Reserpine
Reserpine, an alkaloid isolated from the Indian snakeroot Rauwolfia serpentine, was introduced to Western medicine in 1952, and was widely prescribed for its antihypertensive and antipsychotic properties (87). Despite these beneficial effects of reserpine, side effects were described as resembling a parkinsonian syndrome, with symptoms including depression, gastric dysmotility, and extrapyramidal symptoms (88). Although the molecular target of reserpine was not identified for decades, researchers observed that reserpine depleted dopamine in biological tissue and caused parkinsonism in rats (89, 90). The later discovery that reserpine is an irreversible and non-specific VMAT1/2 inhibitor provided a mechanistic explanation for the effects of this compound (5, 6, 91). The anti-hypertensive effect results from VMAT2 inhibition in the sympathetic nervous system and chromaffin cells, reducing sympathetic tone and, in turn, reduced blood pressure (92, 93). Despite its effectiveness for treating hypertension, the inhibition of VMAT2 within the CNS causes the aforementioned deleterious symptoms, including severe depression (88, 94). These effects are particularly problematic given the irreversible nature of the drug, since washout requires new protein synthesis and can take several weeks (95). Due to these side effects, reserpine is no longer commonly prescribed.
Tetrabenazine
TBZ is a reversible and specific VMAT2 inhibitor that was recently FDA approved for the treatment of Huntington’s disease (HD) (96–99). HD is characterized by hyperkinetic movement caused by abnormal and increased dopamine release (100). Treatment of HD with TBZ ameliorates aberrant movement through VMAT2 inhibition and depletion of dopamine (101). TBZ also shows efficacy for the treatment of other hyperdopaminergic disease states, such as Tourette’s syndrome (101). However, as with reserpine, VMAT2 inhibition leads to depletion of other monoamines, particularly serotonin, which induces a plethora of nondopaminergic effects, including depression, parkinsonism, fatigue, and GI disturbances (99, 102, 103). While many of these adverse effects are less severe and more manageable than the side effects of reserpine, due to the reversible nature of TBZ action, the therapeutic application of TBZ remains limited (99, 102, 103).
Vesicular transporters as targets for drug development
The majority of drugs used to treat PD and other monoamine disorders target receptors or plasma membrane transporters. While current treatments for PD, such as L-DOPA and dopamine agonists, may compensate temporarily for reduced endogenous dopamine signaling, they do not promote normal neurotransmission, maintain neuronal integrity, or prevent the progression of degeneration. In addition, long-term modulation of these targets often alters receptor sensitivity resulting in loss of effectiveness and increased side effects. For example, treatment with L-DOPA or other dopamine receptor agonists induce supersensitivity of D1 receptors and may contribute to side effects experienced by PD patients after chronic treatment (104).
In contrast, direct targeting of the vesicle, alone or in combination with existing therapies, may allow for modulation of neurotransmission while maintaining the proper kinetics of exocytotic release and termination of the signal. Such a strategy may prevent side effects and/or the loss of efficacy that result from compensatory changes in receptor sensitivity caused by the increased duration of transmitter in the synaptic cleft or increased receptor occupancy. In PD specifically, enhancing vesicular function may enhance dopamine transmission and confer resistance to further dopaminergic toxicity and cell death. Research suggests that pharamacological agents that indirectly increase VMAT2 function are neuroprotective. Pramiprexole (a D2 agonist) and apomorphine (a D1/D2 agonist) enhance vesicular uptake of dopamine and may be neuroprotective (105, 106). Methylphenidate increases vesicular DA uptake in rats and prevents persistent dopaminergic deficits induced by high- dose methamphetamine administration (107, 108). Furthermore, the pituitary adenylyl cyclase activating polypeptide, 38 amino acids (PACAP38) is protective against oxidative stress and dopaminergic cell damage induced by methamphetamine, likely by increasing VMAT2 expression in the striatum (109). These treatments do not directly modulate VMAT2; instead, they most likely act through upregulation of VMAT2 levels. However, the neuroprotection provided by this mechanism still suggests that pharmacological enhancement of vesicular function may both slow PD progression and improve dopaminergic function in PD patients.
Consistent with this, data from Drosophila models also suggests that enhancement of vesicular transport is a promising therapeutic target. Flies that overexpress the Drosophila isoform of VMAT in dopaminergic and serotonergic neurons show no gross defects; they are viable, grow normally, and have a typical life span (110). These flies have minor phenotypic changes including increases in stereotypic grooming behaviors and locomotion that can be reversed by reserpine, prolonged courtship behavior and decreased fertility. Furthermore, a screen for compounds that increase vesicular transport in flies with low levels of VMAT identified compounds that reversed the locomotor defect of these flies via a VMAT-dependent mechanism (111). Together, this suggests that enhancing VMAT2 function for the treatment of movement or psychiatric disorders is a strategy worth pursuing because there is no evidence, as yet, that this approach will cause major side effects.
Despite the potential utility of targeting VMAT2 to modulate vesicular function, only a few labs are developing novel ligands for this transporter. Derivatives of lobeline, ketanserin and TBZ, compounds known to bind and inhibit VMAT2, have been developed and tested for their ability to bind and/or inhibit VMAT2 (112–117). Until very recently, because of the methods and reagents available (see below), high throughput screening of vesicular transport function has not been possible. Thus, testing new compounds has been limited to small scale, derivative-based compound development for identification of more potent or specific inhibitors, rather than true high throughput screening, which enables investigation across a variety of chemical structures. Truly novel drugs cannot be identified without such screening. Novel VMAT2 inhibitors that are specific to each monoamine (i.e. a serotonergic specific VMAT2 inhibitor) may have improved efficacy for the treatment of hypermonoaminergic disorders. Additionally, direct or indirect enhancers of VMAT2 may prove highly beneficial in the treatment of hypomonoaminergic disease, such as PD.
Current methods to measure uptake by vesicular neurotransmitter transporters
Until very recently, methods for measuring the activity of vesicular neurotransmitter transporters were not amenable to the high throughput analysis that enables drug development. Vesicular transport is typically measured by radioactive neurotransmitter uptake into vesicles isolated from rat or mouse brain tissue. Vesicles can be prepared from animals treated with various drugs or toxicants to determine the systemic effect on uptake (29, 118–120). Alternatively, to determine pharmacokinetics, isolated vesicles from untreated animals can be treated directly with the drug or toxicant of interest. While this is a powerful technique, these experiments require a large amount of tissue that necessitates the use of many animals and radioactivity. This, combined with the labor intensive and time consuming protocol for vesicle isolation and the requirement for freshly prepared vesicles, make it extremely impractical to adapt this gold standard assay for high throughput screening.
Radioligand binding assays have been used to identify compounds that competitively inhibit tetrabenazine binding (112, 114, 115). The use of radioactivity limits the adaptation to high throughput screening. Additionally, binding assays only identify compounds that directly bind VMAT2 and only if the binding inhibits tetrabenazine binding by either binding the same site or stabilizing the protein in a conformation incompatible with TBZ binding. However, it is possible that compounds that bind to sites on VMAT2 without affecting TBZ binding may modulate its function. Furthermore, modulation of other vesicular proteins may indirectly affect VMAT2 function. For example, modulation of the V-type ATPase, which establishes the proton gradient that drives VMAT2 activity, would alter vesicular function. An assay that allows for direct screening of transport function would allow for identification of compounds that alter VMAT2 function both directly and indirectly and will be a useful tool for development of novel drugs that target vesicular transporters.
Two techniques for measuring vesicular uptake in cell lines expressing the transporters of interest bypass these limitations. In the first method, cells are treated with detergent to permeabilize the plasma membrane while leaving the vesicle membrane and transport machinery intact (7). In the second method, a post-nuclear fraction is isolated from cell lines expressing the vesicular transporter of interest (121, 122). Unfortunately, these assays are also not amenable to high throughput screening. In the permeabilization method, multiple, delicate washing steps are required that would be difficult to perform successfully with a high throughput liquid handler. In the fractionation protocol, adhering the isolated vesicle fraction to a plate is technically challenging. Furthermore, the use of radioactivity itself hinders adaptation to a high throughput format. An assay that enables high throughput screening would ideally use a fluorescent reagent and not require complicated fractionations or multiple wash steps.
Novel methods to enable high throughput screening
A fluorescent assay for VMAT2 transport
Our lab and others have been working to develop high throughput screening techniques for transport function in VMAT2 containing vesicles. We recently reported the development of a fluorescent assay for measuring VMAT2 function (123). The assay utilizes the Neurotransmitter Uptake Assay from Molecular Devices, which consists of a proprietary fluorescent dye that is transported by monoamine transporters and an impermeable masking dye that blocks extracellular fluorescence. While the identity of the dye is proprietary, based on its properties and behavior in the assay, it is likely to be 4-(4-dimethylamino) phenyl-1-methylpyridinium (APP+) (124). While others have determined that APP+ does not function as a fluorescent false neurotransmitter (FFN) and cannot be used to assess release kinetics like the FFNs, it is sufficient for the purposes of measuring uptake in this artificial system (125). Similarly, we have also determined that the dye in the assay is not released from vesicles in response to potassium or amphetamine, indicating that it also does not function as an FFN (data not shown). The Molecular Devices assay is marketed for assessment of transport by the monoamine plasma membrane transporters (DAT, NET and SET) using a plate reader. To adapt this assay for measuring VMAT2 function, we created a line of human embryonic kidney (HEK) cells that stably co-express human DAT and a human VMAT2-mCherry fusion protein (123). We demonstrated that the dye localizes to VMAT2-positive compartments and that this localization is inhibited by TBZ, confirming VMAT2-mediated transport.
To quantify dye accumulation in the VMAT2-positive compartment of laser scanning confocal images (Nikon A1R), we developed an image analysis method in ImageJ (126). This method identifies regions of interest defined by fluorescence of the VMAT2-mCherry fusion protein and measures fluorescence of the dye within those regions of interest. Standard deviation of pixel intensity of the dye within the mCherry-defined regions of interest provides a measure of the degree of punctate fluorescence. When VMAT2 activity is high, fluorescence within these regions is highly punctate and a high standard deviation of pixel values is calculated. As VMAT2 activity is inhibited by increasing concentrations of TBZ, fluorescence in these regions becomes less punctate and a low standard deviation of pixel values is calculated. From these values, a punctate-diffuse index can be calculated by dividing the standard deviation in each image by the mean of the standard deviations in all control (vehicle treated) images (127). As we reported, this assay measures a 2.5 fold change between positive and negative controls with a Z-factor of 0.62, which indicates the assay is amenable to a high content format (Z-factor of greater than 0.3 for high content imaging) (Table 1).
Table 1. Results of ImageJ and iDev analyses.
Top line indicates measurement and fold change between vehicle as positive control and TBZ treatment as negative control. Bottom line shows Z’ for the specified images and protocol.
| Equipment | ImageJ | iDev | ||
|---|---|---|---|---|
| Nikon A1R | Punctate-diffuse index | 2.5 | ROI_A_Target_II_ObjAvglnten | 2.3 |
| Z’ | 0.62 | Z’ | 0.44 | |
| ArrayScan | Punctate-diffuse index | 1.06 | ROI_A_CorrelationCoef | 2.3 |
| Z’ | −6.09 | Z’ | 0.01 | |
High content screen assay development
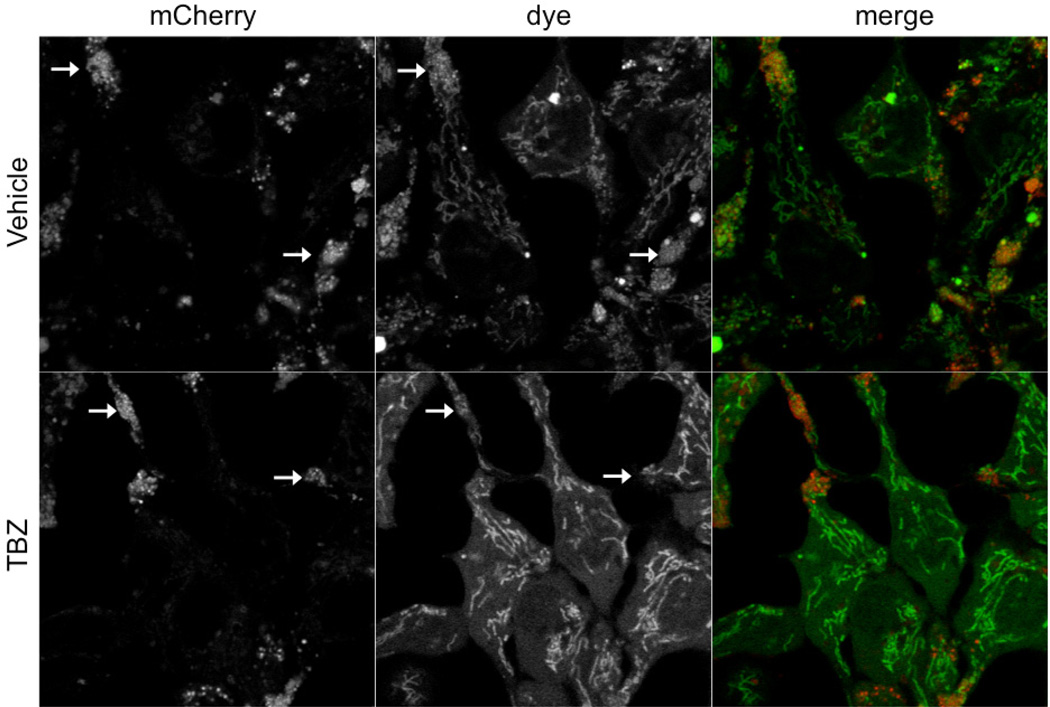
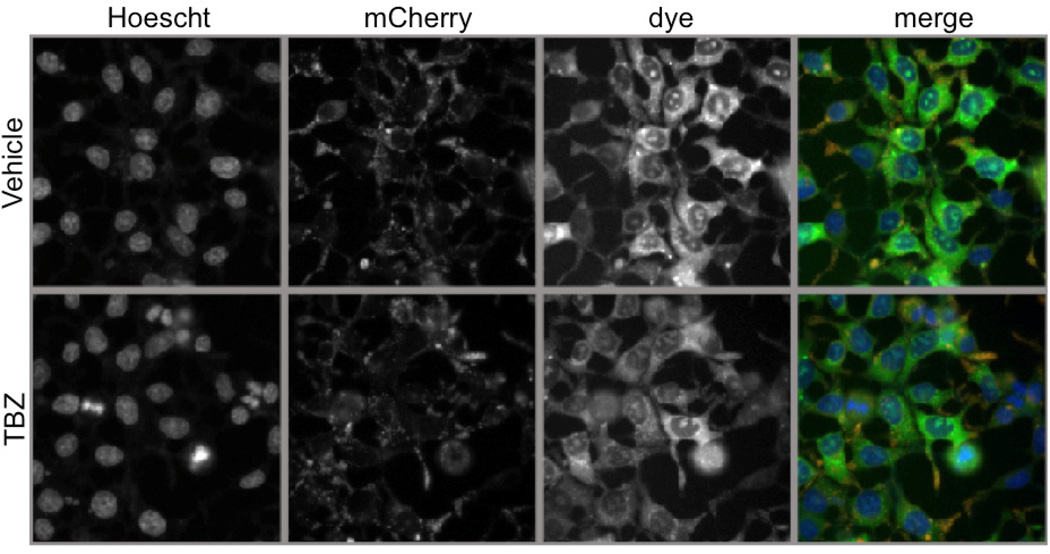
In order to develop this assay for high content analysis, we worked with a ThermoFisher ArrayScan VTI. As shown in Figures 1 and 2, the images captured with the ArrayScan VTI are acquired at a lower optical resolution than the laser scanning confocal images (Figures 1,2). As reported in our paper, images acquired with the Nikon A1R confocal show a clear punctate pattern of staining in the red channel and a similar pattern of staining in the green channel with additional staining in the mitochondria (Figure 1) (123). Furthermore, the loss of the punctate pattern of staining can be easily seen in these images (Figure 1). However, images acquired by the ArrayScan VTI, which does not have confocal capability, show a very diffuse pattern of staining in the green channel (Figure 2). Specific localization to the mitochondria and punctate vesicle-like structures are not visible at this resolution.
Figure 1. Images acquired on Nikon A1R laser scanning confocal.
Images acquired with Nikon A1R laser scanning confocal. The high resolution of this microscope allows for the identification of the punctate localization of the dye in mCherry-positive structures (indicated by arrows) that are localized mostly within extensions from the cell body. Furthermore the mitochondrial staining pattern is also visible. When VMAT2 function is inhibited by TBZ, the difference in staining is easily observed. Dye can only be seen in the mitochondrial compartment; punctate staining within the mCherry-positive puncta is lost.
Figure 2. Images acquired in high resolution acquisition mode by the ArrayScan VTI without a confocal module.
Images acquired with ArrayScan VTI. The mitochondrial pattern of staining of the dye in the cell body region and the punctate staining pattern in the extensions are not visible in these images. Images of the green dye show no difference between treated and untreated cells.
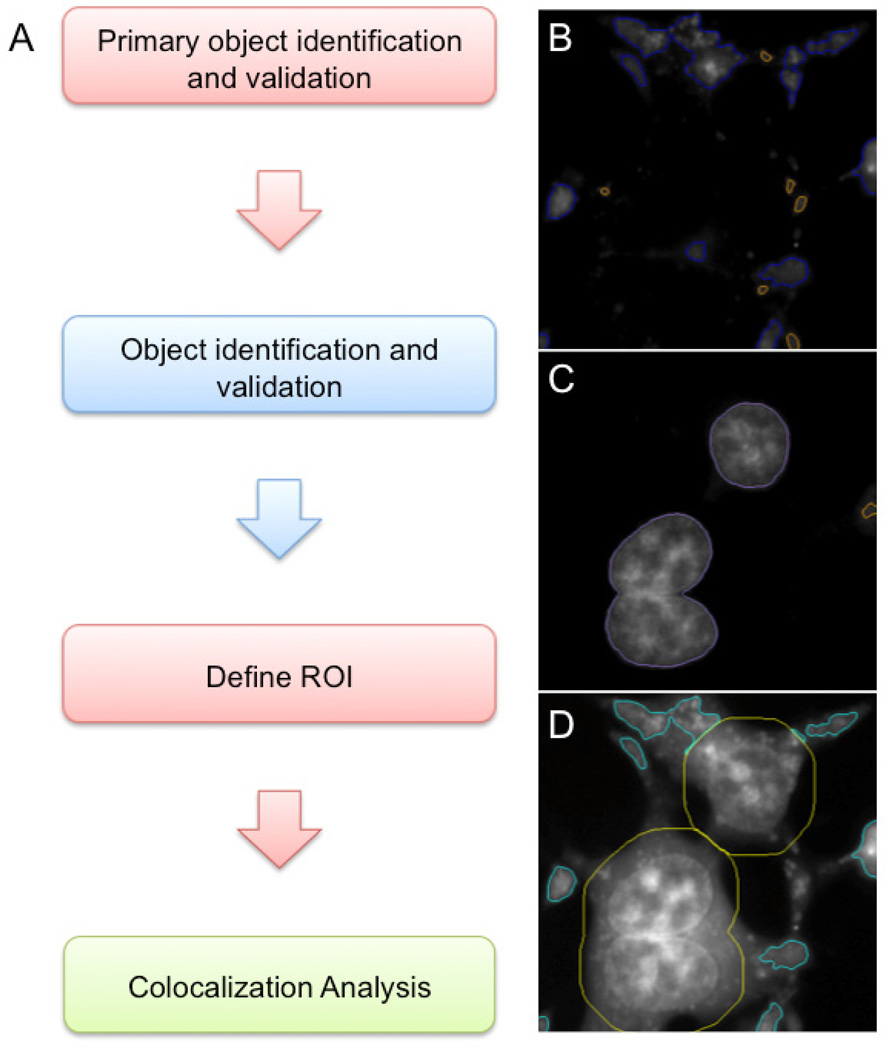
We developed a protocol in iDev, the ArrayScan image analysis software, to analyze acquired images acquired by the ArrayScan. First, similar to the ImageJ protocol, the iDev protocol identifies objects defined by mCherry fluorescence as primary objects (Figure 3). Very small objects are excluded since these do not correspond to the mCherry-positive extensions where co-localization of the dye and mCherry primarily occurs (Figure 3B). Second, nuclei are identified by staining with Hoescht 33342, a live cell nuclear stain (Figure 3C). Third, the region of interest is defined based on the mCherry positive primary objects by excluding the nuclei and an area of 30 pixels around the nuclei (Figure 3D). Finally, the protocol measures various parameters based on the green fluorescence within the defined region of interest. As shown in Table 1, while this method does detect a 2.3 fold change between positive and negative control wells, the Z factor is 0.01, indicating that there is too much variability in the assay to be useful as a high content screen.
Figure 3. iDev image analysis protocol.
(A) Flowchart of iDev protocol steps. (B) Screenshot of primary object identification and validation in channel 1 (mCherry). Primary objects are identified in the red channel. Blue outlines indicate validated objects; orange outlines indicate rejected objects. (C) Screen shot of object identification and validation for channel 2 (Hoescht 33342). Purple outlines indicate validated objects; orange outlines indicate rejected objects. (D) Screen shot of ROI definition. Yellow outlines indicate the area around the nuclei (ROI_B). Cyan outlines indicate the final ROI (ROI_A) defined as the objects validated in Ch1 with ROI_B excluded. Colocalization analysis is then carried out by measuring channel 3 (dye) within ROI_A.
We suspected that this failure was due to the resolution capabilities of the microscope on the ArrayScan compared to those of the Nikon A1R laser scanning confocal. To test this, we imported images acquired on the Nikon A1R into iDev and analyzed them using the iDev protocol, without the exclusion of the area surrounding the nucleus. Analysis of these images by the iDev protocol was successful (Table 1). This method detected a 2.3-fold change, translating to a Z-factor of 0.44. Next, we exported images acquired by the ArrayScan VTI and analyzed them in ImageJ using the protocol reported in our paper (123). This analysis did not detect any differences between vehicle and TBZ treated cells. Finally, we applied deconvolution to ArrayScan VTI images and repeated the analyses; this did not improve the results in ImageJ or iDev (data not shown). Together, the data demonstrate that the resolution of the images acquired on the ArrayScan VTI, not the analysis methods in ImageJ and iDev, is the source of the inability to detect differences in these images. Unfortunately, the lower resolution images acquired with the ArrayScan VTI are not of a sufficient resolution to be analyzed by either method. Without the submicron resolution of a laser scanning confocal, the microscope is unable to differentiate vesicular fluorescence from surrounding cytosolic fluorescence. The ArrayScan XTI is configured with a higher resolution camera and has a spinning disc confocal module. We tested our assay on this model to determine if the increased resolution of this system was sufficient to allow adaptation of our assay to a high content format. Unfortunately, images acquired on the ArrayScan XTI with confocal produced similar results to those generated on the ArrayScan VTI (data not shown). This is likely because the ArrayScan XTI confocal module is a spinning disc confocal, which does not have the submicron resolution of a laser scanning confocal.
Development of a novel FFN for high throughput screening
As discussed in our paper, FFNs existing at the time of publication were developed for use in slice preparations; they are not taken up by cells in culture or are pH-sensitive (128–130). While pH sensitive dyes are valuable tools for studying vesicular function, it is important to note that while a pH change would affect vesicular function, this additional level of complexity would complicate screens for modulators of VMAT2 function. Though the previously developed FFNs are valuable for real-time spatial analysis of VMAT2 function in brain slices, their characteristics limited their usability for high content assay development. Therefore, we hypothesized in our paper that a non-pH-sensitive FFN that was taken up in cell culture would be the ideal reagent for a high throughput screen for VMAT2 function. Recently, the Sames and Sulzer groups developed a novel FFN (FFN206) that is taken up in cell culture and is not pH-dependent (131). This dye localizes specifically to VMAT2-positive compartments and does not localize to the mitochondria, reducing non-specific noise in the assay. Because the dye localizes only to vesicle-like compartments, observed fluorescence is only due to transport into the vesicle and not mitochondrial or cytosolic staining. This specificity precludes the need for high content imaging and allows the fluorescence to be read by plate reader. The authors demonstrate that FFN206 allows for high throughput analysis with a Z-factor of 0.7–0.8 (131).
Conclusions
Radioactive neurotransmitter uptake in isolated synaptic vesicles has unquestionably enhanced the understanding of VMAT2-mediated transport. However, these assays are not amenable to high throughput screening due to the required high animal expenditure and safety restrictions of radiation usage. Development of the fluorescent high throughput assay overcomes these limitations. Cell lines are a practically unlimited resource, enabling easy replication, generation of dose response curves, and screening of multiple compounds. Such an assay also allows for observation of altered vesicular packaging in an intact cell. This is an important distinction, given that the actions of a compound at VMAT2 in an isolated vesicle are not necessarily the same as its actions at VMAT2 in an intact cell. It is possible that compounds that inhibit VMAT2 in isolated vesicles do not inhibit VMAT2 in an intact cell, if they are impermeable to the plasma membrane. Such a compound would be identified as in inhibitor in a vesicular uptake assay but not in a whole cell assay. Therefore, the whole cell assay more accurately recapitulates the pharmacological action of such a compound in vivo.
Additionally, a whole cell assay enables identification of compounds that indirectly affect vesicular packaging by VMAT2. For example, VMAT2 is known to be regulated by G-protein signaling (132–135). These pathways are unlikely to be intact in isolated vesicles and, thus, compounds that modify VMAT2 function through indirect mechanisms would not be identified. Though they do not fully recapitulate all aspects of a neuron, cell lines contain many intact pathways, allowing for identification of a broader range of compounds that alter vesicular function.
A whole cell fluorescence assay provides a more complete picture of the interplay of plasma membrane and vesicular transporters. Furthermore, fluorescent assays in cell lines can provide information that is not possible or is cumbersome to gather from traditional radioactive uptake assays, such as time courses and dose response curves. While it will not replace radioactive uptake assays for determination of pharmacokinetic profiles, overall, the low assay cost, low animal usage, the flexibility of this assay and the high content capabilities make an important addition to tools available for studying monoaminergic system. In addition, the ability to assess vesicular uptake in a high throughput format and will allow for faster screening of compounds.
As discussed above, most drugs that target monoaminergic systems act at the plasma membrane transporters (SSRIs, SNRIs) or receptors (pramiprexole). However, many of these mechanisms produce compensatory responses, such as changes in receptor density, which reduce long-term treatment efficacy and contribute to adverse effects. Modulation of synaptic vesicle function may be a valuable target for pharmaceutical manipulation since compensatory responses will likely be reduced due to preservation of release and uptake kinetics. The development of novel high throughput assays is an important step towards developing novel therapeutics that target the synaptic vesicle.
Highlights.
VMAT1 and VMAT2 transport cytosolic monoamines into synaptic vesicles
Reduced VMAT function has been linked to neurodegenerative conditions
Modulation of vesicular function may be beneficial in treating a variety of diseases
Fluorescent ligands can assess vesicular function in a high throughput format
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sudhof TC. The synaptic vesicle cycle. Annual Review of Neuroscience. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annual Review of Neuroscience. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- 3.Knoth J, Zallakian M, Njus D. Stoichiometry of H+-linked dopamine transport in chromaffin granule ghosts. Biochemistry. 1981;20:6625–6629. doi: 10.1021/bi00526a016. [DOI] [PubMed] [Google Scholar]
- 4.Parsons S. Transport mechanisms in acetylcholine and monoamine storage. The FASEB Journal. 2000;14:2423–2434. doi: 10.1096/fj.00-0203rev. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, et al. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariya S, Takahashi N, Hirano M, Ueno S. Increased vulnerability to L-DOPA toxicity in dopaminergic neurons From VMAT2 heterozygote knockout mice. Journal of Molecular Neuroscience. 2005;27:277–279. doi: 10.1385/JMN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 9.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in Neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Caudle WM, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. The Journal of Neuroscience. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. Journal of Neurochemistry. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gainetdinov RR, et al. Increased MPTP Neurotoxicity in Vesicular Monoamine Transporter 2 Heterozygote Knockout Mice. Journal of Neurochemistry. 1998;70:1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- 13.Schuldiner S. Article: Vesicular neurotransmitter transporters: from bacteria to humans. | AccessMyLibrary - Promoting library advocacy. Physiological Reviews. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 14.Weihe E, Schäfer MK, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. Journal of Molecular Neuroscience. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- 15.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nature Neuroscience. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 17.Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder — the spontaneously hypertensive rat. Behavioural Brain Research. 2002;130:191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz K, Yadid G, Weizman A, Rehavi M. Decreased limbic vesicular monoamine transporter 2 in a genetic rat model of depression. Brain Research. 2003;965:174–179. doi: 10.1016/s0006-8993(02)04167-7. [DOI] [PubMed] [Google Scholar]
- 19.Song C-H, Fan X, Exeter CJ, Hess EJ, Jinnah HA. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiology of Disease. 2012;48:66–78. doi: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SF, Koeppe RA, Tandon R, Zubieta JK, Frey KA. In vivo measurement of the vesicular monoamine transporter in schizophrenia. Neuropsychopharmacology. 2000;23:667–675. doi: 10.1016/S0893-133X(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 21.Alter SP, Lenzi GM, Bernstein AI, Miller GW. Vesicular integrity in Parkinson's disease. Current Neurology and Neuroscience Reports. 2013;13:362. doi: 10.1007/s11910-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhofer G, Kopin IJ, Goldstein DS. Leaky Catecholamine Stores: Undue Waste or a Stress Response Coping Mechanism? Annals of the New York Academy of Sciences. 2004;1018:1–7. doi: 10.1196/annals.1296.027. [DOI] [PubMed] [Google Scholar]
- 23.Burke WJ, et al. Neurotoxicity of MAO Metabolites of Catecholamine Neurotransmitters: Role in Neurodegenerative Diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 24.Rees JN, Florang VR, Eckert LL, Doorn JA. Protein Reactivity of 3,4-Dihydroxyphenylacetaldehyde, a Toxic Dopamine Metabolite, Is Dependent on Both the Aldehyde and the Catechol. Chemical Research in Toxicology. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wey MC, et al. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson's disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein DS, et al. Catechols in post-mortem brain of patients with Parkinson disease. European Journal of Neurology. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahid M, et al. Formation of dopamine quinone-DNA adducts and their potential role in the etiology of Parkinson's disease. IUBMB Life. 2011;63:1087–1093. doi: 10.1002/iub.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotoxicity Research. 1999;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 29.Hatcher JM, Delea KC, Richardson JR, Pennell KD, Miller GW. Disruption of dopamine transport by DDT and its metabolites. Neurotoxicology. 2008;29:682–690. doi: 10.1016/j.neuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson's disease. Neurotoxicology. 2012;33:178–188. doi: 10.1016/j.neuro.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry FA, Edwards RH, Fonnum F. Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annual review of pharmacology and toxicology. 2008;48:277–301. doi: 10.1146/annurev.pharmtox.46.120604.141146. [DOI] [PubMed] [Google Scholar]
- 32.Tanner CM, Langston JW. Do environmental toxins cause Parkinson's disease? A critical review. Neurology. 1990;40(suppl):17–30. discussion 30-11. [PubMed] [Google Scholar]
- 33.Tanner CM, Aston DA. Epidemiology of Parkinson's disease and akinetic syndromes. Current Opinion in Neurology. 2000;13:427–430. doi: 10.1097/00019052-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Tanner CM, et al. Rotenone, paraquat, and Parkinson's disease. Environmental Health Perspectives. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steenland K, et al. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- 36.Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 37.Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to rural environmental factors: a population based case-control study. The Canadian Journal of Neurological Sciences. 1991;18:279–286. doi: 10.1017/s0317167100031826. [DOI] [PubMed] [Google Scholar]
- 38.Ritz B. Parkinson's disease mortality and pesticide exposure in California 1984–1994. International Journal of Epidemiology. 2000;29:323–329. doi: 10.1093/ije/29.2.323. [DOI] [PubMed] [Google Scholar]
- 39.Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- 40.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson's disease: a metaanalysis. Environmental Research. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 41.Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson's disease in rural California. Environmental Health Perspectives. 2009;117:1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elbaz A, et al. Professional exposure to pesticides and Parkinson disease. Annals of Neurology. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- 43.Ascherio A, et al. Pesticide exposure and risk for Parkinson's disease. Annals of neurology. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 44.Seegal R, Bush B, Shain W. Lightly Chlorinated ortho-substituted PCB Congeners Decrease Dopamine in Nonhuman Primate Brain and in Tissue Culture. Toxicology and Applied Pharmacology. 1990;106:136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- 45.Seegal R, Brosch K, Bush B. Polychlorinated Biphenyls Produce Regional Alterations of Dopamine Metabolism in Rat Brain. Toxicology Letters. 1986;30:197–202. doi: 10.1016/0378-4274(86)90103-7. [DOI] [PubMed] [Google Scholar]
- 46.Seegal RF. The neurotoxicological consequences of developmental exposure to PCBs. Toxicogical Sciences. 2000;57:1–3. doi: 10.1093/toxsci/57.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of Dieldrin for Dopaminergic Neurons in Mesencephalic Cultures. Experimental Neurology. 1998;150:263–271. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- 48.Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicology Letters. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Richardson JR, et al. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. The FASEB Journal. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- 50.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends in Pharmacological Sciences. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 51.Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochemistry International. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 52.Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- 53.Lee DW, Opanashuk La. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Kitazawa M, Anantharam V, Kanthasamy aG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cδ in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 55.Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) Journal of Toxicology and Environmental Health. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 56.Fonnum F, Mariussen E, Reistad T. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. Journal of Neurochemistry. 2009;111:1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- 57.Caudle WM, Richardson JR, Wang M, Miller GW. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology. 2005;26:721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caudle WM, et al. Polychlorinated Biphenyl–Induced Reduction of Dopamine Transporter Expression as a Precursor to Parkinson’s Disease-Associated Dopamine Toxicity. Toxicogical Sciences. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- 59.Bradner JM, et al. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: Role of dopamine handling in neurotoxicity. Experimental Neurology. 2013;241:138–147. doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicological Sciences. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- 61.Graham DG, Tiffany SM, Bell WR, Gutknecht WF. Autoxidation Versus Covalent Binding of Quinones as Mechanism of Toxicity of Dopamine, 6-Hydroxydopamine, and Related Compounds toward C1300-Neuroblastoma Cells Invitro. Molecular Pharmacology. 1978;14:644–653. [PubMed] [Google Scholar]
- 62.Benshachar D, Zuk R, Glinka Y. Dopamine Neurotoxicity - Inhibition of Mitochondrial Respiration. Journal of Neurochemistry. 1995;64:718–723. doi: 10.1046/j.1471-4159.1995.64020718.x. [DOI] [PubMed] [Google Scholar]
- 63.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1956–1961. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease. Neurotoxicity Research. 2003;5:165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 65.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. The Journal of Neuroscience. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi N, et al. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang YM, et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 69.Colebrooke RE, et al. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson's disease. European Journal of Neuroscience. 2006;24:2622–2630. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- 70.Taylor TN, et al. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. The Journal of Neuroscience. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ulusoy A, Björklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiology of Disease. 2012;47:367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Jensen PH. Binding of alpha -Synuclein to Brain Vesicles Is Abolished by Familial Parkinson's Disease Mutation. Journal of Biological Chemistry. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 73.Davidson WS, Jonas A, Clayton DF, George JM, Jones A. Stabilization of alpha-Synuclein Secondary Structure upon Binding to Synthetic Membranes. Journal of Biological Chemistry. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 74.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. Journal of Biological Chemistry. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 75.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. Journal of Biological Chemistry. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 76.Chandra S, et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senior SL, et al. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. European Journal of Neuroscience. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rhinn H, et al. Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson's disease pathology. Nature Communications. 2012;3:1084. doi: 10.1038/ncomms2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volles MJ, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: Implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 80.Lev N, et al. DJ-1 protects against dopamine toxicity: implications for Parkinson's disease and aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2013;68:215–225. doi: 10.1093/gerona/gls147. [DOI] [PubMed] [Google Scholar]
- 81.Usami Y, et al. DJ-1 associates with synaptic membranes. Neurobiology of Disease. 2011;43:651–662. doi: 10.1016/j.nbd.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 83.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. Journal of Neurochemistry. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- 85.Kitada T, Tong Y, Gautier CA, Shen J. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. Journal of Neurochemistry. 2009;111:696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 87.Furlenmeier A, Lucas R, Macphillamy HB, Muller JM, Schlittler E. On the constitution of reserpine. Experientia. 1953;9:331–333. doi: 10.1007/BF02155832. [DOI] [PubMed] [Google Scholar]
- 88.Richman A, Tyhurst JS. An Extrapyramidal Syndrome with Reserpine. Canadian Medical Association Journal. 1955;72:457–458. [PMC free article] [PubMed] [Google Scholar]
- 89.Brodie BB, Shore PA, Silver SL. Potentiating action of chlorpromazine and reserpine. Nature. 1955;175:1133–1134. doi: 10.1038/1751133a0. [DOI] [PubMed] [Google Scholar]
- 90.Pletscher A, Shore P, Brodie B. Serotonin release as a possible mechanism of reserpine action. Science. 1955;122:364–365. doi: 10.1126/science.122.3165.374. [DOI] [PubMed] [Google Scholar]
- 91.Erickson JD, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chernow B, Zaloga GP, Lake CR, Coleman MD, Ziegler MG. Effect of antihypertensive therapy on sympathetic nervous system activity in patients with essential hypertension. Federation Proceedings. 1984;43:72–77. [PubMed] [Google Scholar]
- 93.Mahata M, Mahata SK, Parmer RJ, O'Connor DT. Vesicular monoamine transport inhibitors. Novel action at calcium channels to prevent catecholamine secretion. Hypertension. 1996;28:414–420. doi: 10.1161/01.hyp.28.3.414. [DOI] [PubMed] [Google Scholar]
- 94.Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. New England Journal of Medicine. 1954;251:1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- 95.Broadley KJ, Nicholson CD. The use of an irreversible beta-adrenoreceptor antagonist to examine reserpine- and hypothermia-induced supersensitivity of guinea-pig atria. Journal of Autonomic Pharmacology. 1980;1:27–35. doi: 10.1111/j.1474-8673.1980.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 96.Ondo WG, Tintner R, Thomas M, Jankovic J. Tetrabenazine treatment for Huntington’s disease-associated chorea. Clinical Neuropharmacology. 2002;25:300–302. doi: 10.1097/00002826-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Paleacu D, et al. Tetrabenazine treatment in movement disorders. Clinical Neuropharmacology. 2004;27:230–233. doi: 10.1097/01.wnf.0000136892.24629.96. [DOI] [PubMed] [Google Scholar]
- 98.Group HS. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366–372. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- 99.Scott LJ. Tetrabenazine: for chorea associated with Huntington's disease. CNS Drugs. 2011;25:1073–1085. doi: 10.2165/11208330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Raymond LA, et al. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fasano A, Bentivoglio AR. Tetrabenazine. Expert Opinion on Pharmacotherapy. 2009;10:2883–2896. doi: 10.1517/14656560903386292. [DOI] [PubMed] [Google Scholar]
- 102.Guay DR. Tetrabenazine, a monoamine-depleting drug used in the treatment of hyperkinetic movement disorders. The American Journal of Geriatric Pharmacotherapy. 2010;8:331–373. doi: 10.1016/j.amjopharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 103.Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clinical Therapeutics. 2012;34:1487–1504. doi: 10.1016/j.clinthera.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Olanow CW, Obeso Ja, Stocchi F. Drug insight: Continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nature Clinical Practice. Neurology. 2006;2:382–392. doi: 10.1038/ncpneuro0222. [DOI] [PubMed] [Google Scholar]
- 105.Truong JG, Rau KS, Hanson GR, Fleckenstein AE. Pramipexole increases vesicular dopamine uptake: implications for treatment of Parkinson's neurodegeneration. European Journal of Pharmacology. 2003;474:223–226. doi: 10.1016/s0014-2999(03)02080-6. [DOI] [PubMed] [Google Scholar]
- 106.Truong JG, Hanson GR, Fleckenstein AE. Apomorphine increases vesicular monoamine transporter-2 function: implications for neurodegeneration. European Journal of Pharmacology. 2004;492:143–147. doi: 10.1016/j.ejphar.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 107.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. The Journal of Neuroscience. 2002;22:8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanson GR, Sandoval V, Riddle E, Fleckenstein AE. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Annals of the New York Academy of Sciences. 2004;1025:146–150. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- 109.Guillot TS, et al. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang HY, et al. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular psychiatry. 2006;11(1):99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 111.Lawal HO, et al. Drosophila modifier screens to identify novel neuropsychiatric drugs including aminergic agents for the possible treatment of Parkinson's disease and depression. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller DK, et al. Lobeline Analogs with Enhanced Affinity and Selectivity for Plasmalemma and Vesicular Monoamine Transporters. Journal of Pharmacology and Experimental Therapeutics. 2004;310:1035–1045. doi: 10.1124/jpet.104.068098. [DOI] [PubMed] [Google Scholar]
- 113.Zheng G, Dwoskin LP, Crooks Pa. Vesicular monoamine transporter 2: role as a novel target for drug development. The AAPS Journal. 2006;8:E682–E692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yao Z, et al. Preparation and evaluation of tetrabenazine enantiomers and all eight stereoisomers of dihydrotetrabenazine as VMAT2 inhibitors. European Journal of Medicinal Chemistry. 2011;46:1841–1848. doi: 10.1016/j.ejmech.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng P, Lieberman BP, Rye S, Plöessl K, Kung HF. Synthesis and biological evaluation of 3-alkyl-dihydrotetrabenazine derivatives as vesicular monoamine transporter-2 (VMAT2) ligands. Bioorganic & Medicinal Chemistry Letters. 2011;21:3435–3438. doi: 10.1016/j.bmcl.2011.03.113. [DOI] [PubMed] [Google Scholar]
- 116.Horton DB, Nickell JR, Zheng G, Crooks Pa, Dwoskin LP. GZ-793A, a lobelane analog, interacts with the vesicular monoamine transporter-2 to inhibit the effect of methamphetamine. Journal of Neurochemistry. 2013 doi: 10.1111/jnc.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Penthala NRNR, et al. Pyrrolidine analogs of GZ-793A : Synthesis and evaluation as inhibitors of the vesicular monoamine transporter-2 (VMAT2) Bioorganic & Medicinal Chemistry Letters. 2013;23:3342–3345. doi: 10.1016/j.bmcl.2013.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu P-W, et al. Methamphetamine alters vesicular monoamine transporter-2 function and potassium-stimulated dopamine release. Journal of neurochemistry. 2010;115:325–332. doi: 10.1111/j.1471-4159.2010.06922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guillot TS, et al. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. Journal of Neurochemistry. 2008;106:2205–2217. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Volz TJ, Farnsworth SJ, Hanson GR, Fleckenstein AE. Measurement of plasmalemmal dopamine transport, vesicular dopamine transport, and K(+)-stimulated dopamine release in frozen rat brain tissue. Journal of neuroscience methods. 2009;180:317–320. doi: 10.1016/j.jneumeth.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bellocchio EE, Reimer RJ, Fremeau RT, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 122.Parra LLA, et al. The Orphan Transporter Rxt1/NTT4 (SLC6A17) Functions as a Synaptic Vesicle Amino Acid Transporter Selective for Proline, Glycine, Leucine and Alanine. Molecular Pharmacology. 2008;74:1521–1532. doi: 10.1124/mol.108.050005. [DOI] [PubMed] [Google Scholar]
- 123.Bernstein AI, Stout KA, Miller GW. A fluorescent-based assay for live cell, spatially resolved assessment of vesicular monoamine transporter 2-mediated neurotransmitter transport. Journal of Neuroscience Methods. 2012;209:357–366. doi: 10.1016/j.jneumeth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solis E, et al. 4-(4-(dimethylamino)phenyl)-1-methylpyridinium (APP+) is a fluorescent substrate for the human serotonin transporter. Journal of Biological Chemistry. 2012;287:8852–8863. doi: 10.1074/jbc.M111.267757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karpowicz RJ, Dunn M, Sulzer D, Sames D. APP+, a Fluorescent Analogue of the Neurotoxin MPP+, Is a Marker of Catecholamine Neurons in Brain Tissue, but Not a Fluorescent False Neurotransmitter. ACS Chemical Neuroscience. 2013 doi: 10.1021/cn400038u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rasband W. (ImageJ. US National Institutes of Health, Bethesda, Maryland, USA [Google Scholar]
- 127.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biology. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 128.Gubernator NG, et al. Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science. 2009;324:1441–1444. doi: 10.1126/science.1172278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee M, Gubernator NG, Sulzer D, Sames D. Development of pH responsive fluorescent false neurotransmistters. Journal of the American Chemical Society. 2010;132:8828–8830. doi: 10.1021/ja101740k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez PC, et al. Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1213569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu G, et al. New Fluorescent Substrate Enables Quantitative and High-Throughput Examination of Vesicular Monoamine Transporter 2 (VMAT2) ACS Chemical Biology. 2013;2 doi: 10.1021/cb400259n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brunk I, et al. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. Journal of Biological Chemistry. 2006;281:33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- 133.Brunk I, et al. Deletion of Go2alpha abolishes cocaine-induced behavioral sensitization by disturbing the striatal dopamine system. The FASEB Journal. 2008;22:3736–3746. doi: 10.1096/fj.08-111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Höltje M, et al. The vesicular monoamine content regulates VMAT2 activity through Galphaq in mouse platelets. Evidence for autoregulation of vesicular transmitter uptake. Journal of Biological Chemistry. 2003;278:15850–15858. doi: 10.1074/jbc.M212816200. [DOI] [PubMed] [Google Scholar]
- 135.Höltje M, et al. The Neuronal Monoamine Transporter VMAT2 Is Regulated by the Trimeric GTPase Go2. The Journal of Neuroscience. 2000;20:2131–2141. doi: 10.1523/JNEUROSCI.20-06-02131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]