Abstract
Introduction
This study estimated the cost-effectiveness of arsenic trioxide (ATO) added to all-trans retinoic acid (ATRA) when used in first-line acute promyelocytic leukemia (APL) treatment.
Methods
A Markov cohort model was developed with three states: stable disease (during first- or second-line treatment), disease event, and death. Newly diagnosed patients with low/intermediate risk APL were included and each month could remain in their current health state or move to another. Treatment consisted of ATO + ATRA, ATRA + idarubicin (IDA), or ATRA + cytarabine (AraC) + additional chemotherapy. After an initial disease event, patients discontinued first-line and switched to a second-line ATO regimen. Efficacy/safety data were obtained from published trials; quality of life/utility estimates were obtained from the literature; costs were obtained from US data sources. Costs and outcomes over time were used to calculate incremental cost-effectiveness ratios (ICERs). Deterministic and probabilistic sensitivity analyses were conducted.
Results
Compared to ATRA + AraC + additional chemotherapy, ATRA + IDA treatment had ICERs of $2,933 per life year (LY) saved and $3,122 per quality-adjusted life year (QALY) gained. Compared to the ATRA + IDA regimen, first-line ATO + ATRA treatment had ICERs of $4,512 per LY saved and $5,614 per QALY gained. Results were sensitive to changes in pharmacy costs of the ATO + ATRA regimen during consolidation.
Conclusion
The ATO + ATRA regimen is highly cost-effective compared to ATRA + AraC + additional chemotherapy or ATRA + IDA in the treatment of newly diagnosed low to intermediate risk APL patients.
Keywords: acute promyelocytic leukemia, arsenic trioxid, all-trans retinoic aci, cost-effectiveness
INTRODUCTION
It is estimated that acute promyelocytic leukemia (APL), a distinct subtype of acute myeloid leukemia (AML), accounts for between 5 and 12% of all AML cases1. In the United States (US), approximately 1,000 to 1,500 new cases of APL are diagnosed each year1. The average age at diagnosis of 40 years for APL is considerably younger than the average age of 65 years for AML.
APL was once the most fatal of the acute leukemias, but treatment was revolutionized in the 1980s by the introduction of all-trans retinoic acid (ATRA) which, given in conjunction with chemotherapy transformed APL into the most highly curable acute leukemia2. Previous multicenter trials examining the efficacy of ATRA used in combination with chemotherapy have reported remission rates as high as 95% and long-term disease-free survival (DFS) as well as cure rates exceeding 80%3–5.
Approximately 10–12% of newly diagnosed patients will relapse, and 30–50% of relapsed patients will relapse a second time6,7. In 2000, arsenic trioxide (ATO; Trisenox®) was approved in the US for the treatment of patients with APL who are refractory to, or have relapsed from, previous treatment with ATRA and anthracycline-based chemotherapy4. Studies reported that approximately 85% of relapsed patients initially treated with ATRA achieved sustained molecular remission with second-line ATO therapy8–10 as well as complete remission (CR) rates of approximately 80–90% and overall survival (OS) rates of 50–70% at 1 to 3 years10–12. In addition, 50% of APL patients with an initial relapse could be cured with ATO salvage therapy followed by consolidation therapy13.
Furthermore, studies have also reported that patients with newly diagnosed APL benefit from ATO therapy either alone or in combination with ATRA6,14,15. For eampleclinical trials have reported that patients receiving ATO plus ATRA induction therapy experienced fewer relapses, achieved CR faster, had lower hematologic toxicity, and significantly better 2-year OS rates compared to patients receiving standard ATRA-chemotherapy16–18. The use of ATO in conjunction with chemotherapy during consolidation therapy is also highly effective in these patients19,20. In fact, ATRA and ATO-based frontline therapy without chemotherapy can be considered the new standard of care in low- or intermediate-risk patients3.
A cost-effectiveness model was developed to evaluate the relative value of ATO + ATRA compared to two other widely used regimens for the treatment of low to intermediate risk APL: ATRA + idarubicin (IDA) and ATRA + cytarabine (AraC) + additional chemotherapy, from the perspective of a US third-party payer. This type of analysis is critical to help payers understand what the incremental economic cost of a new health technology will be, including expenditures on pharmacy, medical, and other related healthcare costs, per life year (LY) saved and quality-adjusted life year (QALY) gained.
MATERIALS AND METHODS
A Markov cohort model was developed to evaluate the cost-effectiveness of first-line treatment regimens for patients with APL in the US. Based on National Comprehensive Cancer Network (NCCN) guidelines21, newly diagnosed patients with low to intermediate risk (white blood cell [WBC] count ≤10,000/mcL) were treated with one of the following three first-line regimens: 1) ATO + ATRA (induction and consolidation with ATO + ATRA; no maintenance), 2) ATRA + IDA (induction and consolidation with ATRA + IDA, consolidation adds mitoxantrone, and maintenance with 6-mercaptopurine [6MP] + methotrexate [MTX] + ATRA), or(3) ATRA + AraC + additional chemotherapy (induction with ATRA + AraC + daunorubicin [DA], consolidation with ATRA + DA, and maintenance with 6MP + MTX + ATRA). The NCCN guidelines also recommend that relapsed or refractory APL patients receive an ATO regimen; all patients treated with a second-line regimen in the model received induction and consolidation with ATO + MTX + AraC + prednisolone, followed by autologous stem-cell transplant (SCT).
The model uses a base case set of parameters based on data from a variety of sources; most parameters were estimated from published literature and other secondary sources including research databases and publicly available data. Each of the patient health states in the model has associated economic costs and health outcomes (including utilities); estimation of the model involves following patients over time and tabulating cumulative costs and outcomes associated with each treatment. The incremental costs and outcomes compared to the reference scenario are then used to calculate an incremental cost-effectiveness ratio (ICER) for each treatment regimen.
Model Structure
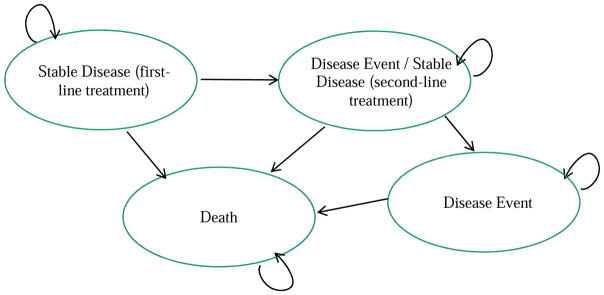
Standard modeling methods were used to construct and estimate the Markov cohort model. The clinical decision at model initiation was choice of first-line treatment strategy. Figure 1 provides an overview of the health states that patients may occupy at any given time and potential transitions during each monthly cycle. These health states are mutually exclusive and defined by disease status during treatment and disease progression.
Figure 1.
Markov Model Schematic: Health States
All patients began in the first-line stable disease health state; during subsequent cycles, patients could remain in the first-line stable disease state or progress to the second-line stable/first-line disease event state depending on clinical probabilities. Once in this state, the patient could not move back to the first-line stable disease state. A patient who has experienced a disease event following first-line treatment discontinues their initial treatment and immediately begins second-line treatment with the ATO regimen, hence a single health state for disease event and stable disease on second-line treatment. From there, patients could remain in the second-line stable disease state or move to the disease event state following second-line treatment. Patients who failed both first- and second-line treatments were assumed to enter a clinical trial. Patients could die from any state due to the effects of APL, treatment-related mortality, or natural causes.
In addition to the state transitions detailed above, patients may experience treatment-related adverse events (AEs) during each cycle on treatment. It was assumed that patients do not discontinue their treatment regimen due to an AE; the probability of experiencing each AE was multiplied by the cost of treating that AE. Finally, cost-effectiveness outcomes were estimated over a lifetime horizon. Based on the clinical trial populations17, patients started in the model at 45 years of age and the analysis continued to run until patients reached 100 years of age or died, whichever came first. Both outputs and cost inputs were discounted at a rate of 3.0% per year after the first year in the model.
Target Population
The target population for the present model included adult APL patients (at least 18 years of age) who were similar to the clinical trial populations of interest: patients who had been newly diagnosed with APL and were of low to intermediate risk based on WBC count.
Clinical Parameters and Survival Estimates
The main clinical parameters included in the model were EFS (event-free survival) and OS. Kaplan-Meier (KM) curves were obtained from published clinical trials for each of the comparators in the first-line (ATO + ATRA and ATRA + IDA treatments17; ATRA + AraC + additional chemotherapy treatment20) as well as second-line22 settings. The KM curves in the second-line trials showed a distinct difference between the probabilities for the initial two years of patient treatment compared to treatment after two years. Therefore two sets of transition probabilities were estimated for the second-line treatment Markov states; it was assumed that the second set of transition probabilities came into effect at the 24th month.
The monthly estimates from the KM curves for EFS and OS were used to estimate the following monthly transition probabilities: 1) from first-line stable disease to second-line stable/first-line disease event, 2) from first-line stable disease to death, 3) from second-line stable/first-line disease event to second-line disease event, 4) from second-line stable/first-line disease event to death, 5) from second-line disease event to death (see Table 1). Calibration was conducted using Microsoft Excel Solver where the calibration process manipulated all of the transition probabilities so that the deviation between the observed clinical trial data and the predicted data (model-produced outcomes) was minimized.
Table 1.
Clinical Transition Probabilities
| Parameter | ATO + ATRA (first-line)1 | ATRA + IDA (first-line)1 | ATRA + AraC + Additional Chemotherapy (first-line)2 | ATO Regimen (second-line at 0–2 years)3 | ATO Regimen (second- line at >2 years)3 |
|---|---|---|---|---|---|
|
| |||||
| Probability of a disease event from stable disease | 0.00084 | 0.00202 | 0.00530 | 0.01099 | 0.00000 |
| Probability of APL-related death from stable disease | 0.00058 | 0.00444 | 0.00510 | 0.01099 | 0.00000 |
| Probability of treatment-related death due to a disease event | N/A | N/A | N/A | 0.01447 | 0.00000 |
Microsoft Excel Solver calculated the transition probabilities such that the sum of the percent absolute difference between EFS and OS in step 1 and step 2 was as small as possible. This process was repeated for each treatment arm in each stage (i.e., first- or second-line) of the analysis.
The probabilities of AEs in each treatment phase of first-line therapy were also taken from the published clinical trials of the comparators, with a specific focus on AEs that required significant treatment: neutropenia, thrombocytopenia, hepatic toxicity, and fever of unknown origin (FUO; see Table 2). Powell20 did not report any AEs associated with ATRA + AraC + additional chemotherapy; therefore the AE rates were set to zero for this regimen. Similarly, in the second-line setting, AEs were not included in the clinical trial publications. There was an exception for late secondary leukemia in ATRA + IDA patients who had event-free disease in the first- and second-line settings: because there is a small but serious risk of these patients developing secondary leukemia, years later and without experiencing an event, this probability was incorporated into the model at six years after induction remission23. During each monthly cycle, patients were at risk of dying from causes other than APL, based on age-specific mortality rates for the US population.
Table 2.
Adverse Event Probabilities
| Parameter | Probability of Grade 3 or 4 Adverse Event | ||
|---|---|---|---|
|
| |||
| Treatment Phase | Adverse Event | ATO + ATRA | ATRA + IDA |
|
| |||
| Induction1 | Neutropenia | 46.0% | 79.0% |
| Thrombocytopenia | 59.0% | 88.0% | |
| Hepatic toxicity | 2.5% | 0.4% | |
| Fever of unknown origin | 1.1% | 9.8% | |
|
| |||
| Consolidation (first cycle)1 | Neutropenia | 6.0% | 35.0% |
| Thrombocytopenia | 6.0% | 18.0% | |
| Hepatic toxicity | 2.5% | 0.4% | |
| Fever of unknown origin | 1.1% | 9.8% | |
|
| |||
| Consolidation (second cycle)1 | Neutropenia | 6.0% | 76.0% |
| Thrombocytopenia | 6.0% | 65.0% | |
| Hepatic toxicity | 2.5% | 0.4% | |
| Fever of unknown origin | 1.1% | 9.8% | |
|
| |||
| Consolidation (third cycle)1 | Neutropenia | 4.0% | 25.0% |
| Thrombocytopenia | 3.0% | 15.0% | |
| Hepatic toxicity | 2.5% | 0.4% | |
| Fever of unknown origin | 1.1% | 9.8% | |
|
| |||
| Post-induction remission (at 6 years)2 | Late secondary leukemia | N/A | 2.2% |
Utility Estimates
In order to calculate QALYs as a measure of disease burden, the model requires quality of life (QOL)/utility estimates for each health state. Utility values can range from 1.00 (perfect health) to 0.00 (death) in the model. Published disease-specific utilities for patients with APL were not availabletherefore it was assumed that chronic lymphocytic leukemia (CLL) utility estimates from Woods28 and Ferguson29 were applicable to patients with APL, an approach that has been used in previous leukemia research30. To address a limitation of using CLL utilities (i.e., that CLL patients are typically older and have worse outcomes than APL patients), age-adjusted utilities were calculated for the model. Furthermore, since the CLL utility estimates used background UK utility values31, the APL utility estimates were adjusted for the US population. The utility estimates for each health state are as follows: first-line stable disease = 0.7828, first-line disease event/second-line stable disease = 0.6529, second-line disease event = 0.4729.
Costs
The model used a US third-party payer perspective and excluded indirect costs, including those due to lost productivity. Patients were assumed to receive the treatment regimens as outlined in the relevant clinical trials17,20,22. Pharmacy costs for each treatment regimen were estimated using wholesale acquisition costs (WAC; see Table 3). Medical costs were estimated from standard US costing sources by treatment phase. In the induction phase, each treatment arm had the same estimated medical cost because the Inpatient Prospective Payment System (IPPS) from Medicare bundles costs from similar diagnostic categories into a diagnosis-related group (DRG) and assigns an average cost. Induction is performed in the inpatient setting for all three treatment regimens. All fees for lab tests and medications are included in this cost, whereas clinician fees based on Current Procedural Terminology (CPT) codes were added. It was assumed that patients are hospitalized after a disease event following first-line treatment and any additional costs associated with tests and procedures would be included in the DRG cost for second-line treatment. Patients enrolled in a clinical trial after failing second-line treatment did not accrue any treatment costs. Costs associated with AEs that were included in the model are as follows: neutropenia = $8,734, thrombocytopenia = $1,376, hepatic toxicity = $6,382, FUO = $8,734, late secondary leukemia (post-induction remission) = $25,220. Since patients are already hospitalized for induction, the costs of treating their AEs were not calculated separately from the overall cost of their inpatient stay in the base-case; the costs for late secondary leukemia were based on the inpatient cost for acute leukemia and the additional cost of a stem-cell transplant.
Table 3.
Pharmacy and Medical Costs (Model Inputs)
| Parameter | ATO + ATRA (first-line)1 | ATRA + IDA (first-line)1 | ATRA + AraC + Additional Chemotherapy (first-line)2 | ATO Regimen (second-line)3 |
|---|---|---|---|---|
|
| ||||
| Total pharmacy4 | $61,797 | $13,146 | $38,008 | $33,241 |
| Induction phase: medical5 | $20,503 | $20,503 | $20,503 | $20,503 |
| Consolidation phase: medical5 | $778 | $778 | $778 | $778 |
| Maintenance phase: medical5 | N/A | $40 | $40 | N/A |
| Off-treatment medical monitoring6 | $177 | $177 | $177 | $177 |
| Disease event: medical7 | N/A | N/A | N/A | $483 |
| Autologous stem-cell transplant8 | N/A | N/A | N/A | $4,717 |
Source = Lo-Coco, 201317;
Source = Powell, 201020;
Source = Yanada, 201322;
Pharmacy costs were estimated using wholesale acquisition costs (WAC);
Medical costs were estimated from standard US costing sources using the average diagnosis-related group (DRG) cost for all inpatient services plus Current Procedural Terminology (CPT) codes by treatment phase;
Patients\assumed to have an oncologist office visit every 3 months;
One-time costs associated with a disease event post second-line treatment calculated in consultation with a clinical expert;
Patients who received a autologous stem-cell transplant post second-line treatment incurred costs for procedures assumed to be on an outpatient basis.
Note: It was assumed that patients are hospitalized after a disease event post first-line treatment and any costs would be included in the DRG cost for second-line treatment.
Model Outcomes
Model outputs included total costs, as well as LYs and QALYs gained for treatment starting with each regimen for first-line APL. Model outputs were used to estimate ICERs in terms of the incremental cost per LY saved and incremental cost per QALY gained. If a more costly strategy provided no additional benefit (i.e., is both more costly and less effective) compared to an alternative strategy, then it is said to be “dominated” by the alternative strategy and no ICER is calculated. If a more costly strategy provided additional benefit, then the two strategies are compared by dividing the incremental cost by the incremental benefit.
Sensitivity Analyses
A series of one-way (deterministic) sensitivity analyses were performed to assess how changes in key input parameter values or model assumptions affected the results. As 95% confidence intervals were not available for the included parameters, base-case inputs were varied by +/−25%. One-way sensitivity analyses were performed only for the cost-effectiveness analyses with QALYs as the outcome measure; results were presented in the form of a tornado diagram.
Uncertainty in cost-effectiveness results was also assessed by performing probabilistic sensitivity analyses (PSA) using second-order Monte Carlo simulations. Uncertainty in treatment efficacy (i.e., transition probabilities) was characterized by probability distributions around each of their base-case values; parameters of these distributions were derived from published literature. The incremental cost per QALY was calculated for each set of parameter values, as in the base-case. Results were generated as cost-effectiveness acceptability curves, which show the fraction of the 1,000 simulations in which each treatment was incrementally cost-effective over a range of ICER thresholds.
RESULTS
Base-Case Results
The base-case results for first-line APL treatment are presented in Table 4, ordered from least expensive to most expensive. Total costs per patient ranged from $96,940 for the ATRA + AraC + additional chemotherapy regimen to $136,170 for the ATO + ATRA regimen. Health outcomes per patient ranged from 8.57 LYs saved and 6.71 QALYs gained for the ATRA + AraC + additional chemotherapy regimen to 17.79 LYs saved and 14.33 QALYs gained for the ATO + ATRA regimen. Compared to the least expensive regimen, ATRA + AraC + additional chemotherapy, ATRA + IDA treatment had an incremental cost of $4,457 and incremental LYs and QALYs of 1.52 and 1.43, respectively; the ICERs were $2,933 per LY saved and $3,122 per QALY gained. Compared to the ATRA + IDA regimen, ATO + ATRA treatment had an incremental cost of $34,773 and incremental LYs and QALYs of 7.71 and 6.19, respectively; the ICERs were $4,512 per LY saved and $5,614 per QALY gained.
Table 4.
Base-Case Cost-Effectiveness Results
| Regimen | Total | Incremental | ICER | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cost | LYs Saved | QALYs Gained | Cost | LYs Saved | QALYs Gained | Cost per LY Saved | Cost per QALY Gained | |
| ATRA + AraC + Additional Chemotherapy | $96,940 | 8.57 | 6.71 | N/A | N/A | N/A | N/A | N/A |
| ATRA + IDA | $101,396 | 10.09 | 8.13 | $4,457 | 1.52 | 1.43 | $2,993 | $3,122 |
| ATO + ATRA | $136,170 | 17.79 | 14.33 | $34,773 | 7.71 | 6.19 | $4,512 | $5,614 |
Note: LY = life year; QALY = quality-adjusted life year; ICER = incremental cost-effectiveness ratio.
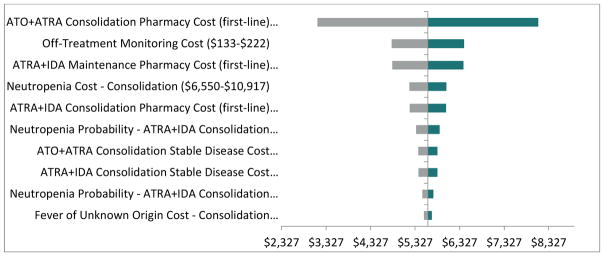
Sensitivity Analyses
The one-way sensitivity analyses were performed on ICER for ATO + ATRA compared to ATRA + IDA. The results of these sensitivity analyses are shown in Figure 2, which displays the 10 most sensitive parameters per comparison in terms of the ICERs per QALY gained (i.e., the parameters with the largest difference between results for the low-case and high-case) as tornado diagrams. Figure 2 shows that when the pharmacy cost of ATO + ATRA consolidation (first-line) was varied +/−25% around the base-case value of $8,828, the ICER varied from $3,136 to $8,093.
Figure 2.
One-Way Sensitivity Analyses Based on Incremental Cost per QALY Gained (ATO + ATRA versus ATRA + IDA)
Using the Markov cohort model, a second-order PSA with 1,000 samples was conductedAt a willingness-to-pay threshold of $9,000 per QALY gained, the probability of ATO + ATRA being cost-effective compared to all other treatment regimens was 83%, whereas ATO + ATRA was cost-effective compared to all other treatment regimens in 100% of the simulations at a willingness-to-pay threshold of $14,000 per QALY gained.
DISCUSSION
First-line ATO + ATRA therapy has been shown to provide excellent clinical outcomes in the treatment of low to intermediate risk APL, with improvements in patients’ QOL as well as survival.17,25 In the analysis described here, ATO + ATRA is highly cost-effective, with base-case ICERs of $4,512 per LY saved and $5,614 per QALY gained, compared to ATRA + IDA in the first-line setting. With an ICER of under $6,000 per QALY, the cost-effectiveness of ATO + ATRA is substantially below willingness-to-pay thresholds ($50,000–$150,000 per QALY) that have been historically or are currently being used in the US34. Of note, several oncolytic therapies have recently been associated with ICERs that are well above $100,000 per QALY.35,36
Presented another way, compared with ATRA + AraC + additional chemotherapy, the ATO + ATRA regimen was associated with more than double the QALYs, while total costs were only 40% more. When evaluated against the ATRA + IDA regimen, treatment with ATO + ATRA displayed an approximately 75% increase in QALYs with a corresponding 35% increase in total costs. While there is uncertainty in the results of any cost-effectiveness analysis, extensive one-way deterministic and probabilistic sensitivity analyses showed that whereas the model results were most sensitive to changes in the pharmacy cost of the ATO + ATRA regimen during the consolidation phase of treatment, ATO + ATRA is very likely to be cost-effective at willingness-to-pay thresholds applicable to the U.S. setting.
Limitations
This analysis has several limitations related to the input data used to populate the model. Due to a lack of published data, some estimates of resource use during regular clinical treatment of APL were based on clinical expert opinion and could reasonably be expected to vary, with an associated impact on costs. Additionally, the clinical parameters were taken from a small number of multi-country randomized clinical trials; the extent to which these values will translate to real-world US practice is unknown. The AE rates were set to zero in the model for first-line ATRA + AraC + additional chemotherapy, as well as the second-line ATO regimen, due to the fact that the clinical trial publications and supplemental materials did not report the AEs associated with these regimens. While it seems unlikely that patients in these studies did not experience AEs, there were no alternate sources available to estimate these rates, thus the true costs for the ATRA + AraC + additional chemotherapy comparator can be expected to have been somewhat higher if AEs were included. Furthermore, it was assumed that AEs (and their treatment) would last substantially less than an entire one month treatment cycle and the corresponding utility decrements would be too transient to include in a meaningful way; therefore the utility values included in the model were only those associated with the disease state.
Conclusions
This paper summarizes the methods and results of a cost-effectiveness model for ATO + ATRA in the first-line treatment of low to intermediate risk APL patients from the perspective of a third-party US payer. These models are valuable to payers making formulary decisions regarding the access and affordability of medicines. The ATO + ATRA regimen was found to be highly cost-effective compared to ATRA + AraC + additional chemotherapy or ATRA + IDA in the treatment of this APL patient cohort.
Clinical Practice Points.
Arsenic trioxide (ATO) has been shown to provide excellent clinical outcomes in the treatment of low to intermediate risk APL patients who are refractory to, or have relapsed from, previous treatment.
The current study found that ATO is highly cost effective compared to all-trans retinoic (ATRA) + cytarabine + additional chemotherapy in the treatment of newly diagnosed low to intermediate risk APL patients
ATO should be considered the treatment of choice in newly diagnosed low to intermediate risk APL patients
Acknowledgments
Sponsorship for this study and article processing charges were funded by Teva Pharmaceuticals, Frazer, PA, USA. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors wish to thank Dr. Milton Weinstein and Dr. Ashutosh Pathak for their contribution to the manuscript.
Editorial assistance in the preparation of this manuscript was provided by Dr. Jason Allaire, PhD of Generativity Solutions Group, Cary, NC, USA. Support for this assistance was funded by Teva Pharmaceuticals, Frazer, PA, USA.
Footnotes
CONFLICT OF INTEREST
Lo Coco received consultancy honoraria from Teva and has been in the speaker bureau of Teva and Lundbeck. Tallman received consultancy honoraria from Teva. Barnes, Mueller, and Tang are full time employees of Teva and have no other competing interests to declare. Kruse is a full time employee for Optum. Wildner and Martin are employees of MAPI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Martin Tallman, Email: tallmanm@mskcc.org.
Francesco Lo-Coco, Email: francesco.lo.coco@uniroma2.it.
Gisoo Barnes, Email: gisoo.barnes@tevapharm.com.
Morgan Kruse, Email: morgan.kruse@optum.com.
Rebecca Wildner, Email: rebecca.wildner@optum.com.
Monique Martin, Email: monique.martin@optum.com.
U Udo Mueller, Email: Udo.Mueller@teva.de.
Boxiong Tang, Email: boxiong.tang@tevapharm.com.
References
- 1.Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:391–399. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 2.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the european LeukemiaNet. Blood. 2009;113(9):1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 3.Watts JM, Tallman MS. Acute promyelocytic leukemia: What is the new standard of care? Blood Rev. 2014;28(5):205–212. doi: 10.1016/j.blre.2014.07.001. S0268-960X(14)00052-6. [DOI] [PubMed] [Google Scholar]
- 4.Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood. 2010;115(25):5137–5146. doi: 10.1182/blood-2010-01-266007. [DOI] [PubMed] [Google Scholar]
- 5.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: Long-term outcome and prognostic factor analysis from the north american intergroup protocol. Blood. 2002;100(13):4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 6.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Durable remissions with minimal toxicity. Blood. 2006;107(7):2627–2632. doi: 10.1182/blood-2005-08-3532. 2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 7.Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20(1):57–65. doi: 10.1016/j.beha.2006.11.002. S1521-6926(06)00083-1 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 9.Soignet SL, Frankel SR, Douer D, et al. United states multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 10.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: Remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324. [PubMed] [Google Scholar]
- 11.Shigeno K, Naito K, Sahara N, et al. Arsenic trioxide therapy in relapsed or refractory japanese patients with acute promyelocytic leukemia: Updated outcomes of the phase II study and postremission therapies. Int J Hematol. 2005;82(3):224–229. doi: 10.1532/IJH97.05044. [DOI] [PubMed] [Google Scholar]
- 12.Raffoux E, Rousselot P, Poupon J, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21(12):2326–2334. doi: 10.1200/JCO.2003.01.149. [DOI] [PubMed] [Google Scholar]
- 13.Lengfelder E, Lo-Coco F, Ades L, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: Registry results from the european LeukemiaNet. Leukemia. 2015 doi: 10.1038/leu.2015.12. [DOI] [PubMed] [Google Scholar]
- 14.Ghavamzadeh A, Alimoghaddam K, Rostami S, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29(20):2753–2757. doi: 10.1200/JCO.2010.32.2107. [DOI] [PubMed] [Google Scholar]
- 15.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469–3473. doi: 10.1182/blood-2005-10-4006. 2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 16.Shen ZX, Shi ZZ, Fang J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(15):5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 18.Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120(8):1570–80. doi: 10.1182/blood-2012-02-410746. blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 19.Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: A multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–1243. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 20.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North american leukemia intergroup study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network (NCCN) 2014 Feb 19; http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf.
- 22.Yanada M, Tsuzuki M, Fujita H, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013;121(16):3095–3102. doi: 10.1182/blood-2012-11-466862. [DOI] [PubMed] [Google Scholar]
- 23.Montesinos P, Gonzalez JD, Gonzalez J, et al. Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic acid and anthracycline-based chemotherapy. J Clin Oncol. 2010;28(24):3872–3879. doi: 10.1200/JCO.2010.29.2268. [DOI] [PubMed] [Google Scholar]
- 24.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. 26/4/391 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Efficace F, Mandelli F, Avvisati G, et al. Randomized phase III trial of retinoic acid and arsenic trioxide versus retinoic acid and chemotherapy in patients with acute promyelocytic leukemia: Health-related quality-of-life outcomes. J Clin Oncol. 2014;32(30):3406–3412. doi: 10.1200/JCO.2014.55.3453. [DOI] [PubMed] [Google Scholar]
- 26.Johnsen AT, Tholstrup D, Petersen MA, Pedersen L, Groenvold M. Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol. 2009;83(2):139–148. doi: 10.1111/j.1600-0609.2009.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Else M, Smith AG, Cocks K, et al. Patients’ experience of chronic lymphocytic leukaemia: Baseline health-related quality of life results from the LRF CLL4 trial. Br J Haematol. 2008;143(5):690–697. doi: 10.1111/j.1365-2141.2008.07407.x. [DOI] [PubMed] [Google Scholar]
- 28.Woods B, Hawkins N, Dunlop W, O’Toole A, Bramham-Jones S. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in england and wales: A cost-utility analysis. Value Health. 2012;15(5):759–770. doi: 10.1016/j.jval.2012.03.1389. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson J, Tolley K, Gilmour L, Priaulx J. PCN79 health state preference study mapping the change over the course of the disease process in chronic lymphocytic leukemia (CLL) Value Health. 2008;11(6):A485. [Google Scholar]
- 30.Lu L, Peters J, Roome C, Stein K. Cost-effectiveness of alemtuzumab for T-cell prolymphocytic leukemia. Int J Technol Assess Health Care. 2012;28(3):241–248. doi: 10.1017/S0266462312000244. [DOI] [PubMed] [Google Scholar]
- 31.Szende A, Janssen B, Cabases J. Self-reported population health: An international perspective based on EQ-5D. Netherlands: Springer; 2014. p. 196. [PubMed] [Google Scholar]
- 32.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 33.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14(6):836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Reed SD, Li Y, Anstrom KJ, Schulman KA. Cost effectiveness of ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2009;27(13):2185–2191. doi: 10.1200/JCO.2008.19.6352. [DOI] [PubMed] [Google Scholar]