Abstract
Background
Treatment of acute myeloid leukemia (AML) remains difficult due to the development of treatment resistance which might be overcome through antagonists of inhibitors of apoptosis proteins (IAPs).
Patients and methods
This multi-center, open-label, dose escalation study aimed to evaluate tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of Debio1143 (formerly AT-406), a new IAP antagonist, when given along with a standard "7 plus 3 regimen" of daunorubicin and cytarabine to poor-risk patients with AML during the induction cycle. Consecutive patient cohorts received once daily 100, 200, 300, or 400 mg of oral Debio1143 on treatment days 1–5. Blood samples were collected regularly until hematological recovery or response; bone marrow samples on day 0, 14, and 29; and PK and PD samples on days 1, 3, 5, 8, 10 and 1, 2, 8, respectively.
Results
Of 29 enrolled patients, 23 completed the study. Most common adverse events of any grade deemed related to treatment were nausea (31% of patients), diarrhea (14%), and febrile neutropenia (14%). Exposure exceeded dose-proportionality, without accumulation over 5 days. Inhibition of cIAP-1 was detectable in CD34/CD117+ cells and blasts. A total of 11 (38%) patients achieved complete remission, the majority in the 100 mg dose cohort. Of these, 6 (56%) relapsed still within the study period. Responders more frequently showed plasma increases of TNFα and IL-8 post-first dose of Debio1143.
Conclusion
Debio1143 up to 400 mg/day showed good tolerability in combination with daunorubicin and cytarabine; further studies in subsets of patients with AML are warranted.
Keywords: Pharmacokinetic, treatment resistance, cIAP1, TNF, interleukin, Debio1143
Introduction
Apoptosis is the process of programmed cell death.1 Resistance of tumor cells to apoptosis poses a major problem for treatment as mutations that trigger tumor formation by suppressing cell death, also reduce treatment sensitivity and inevitably contribute to treatment failure and relapse.2–4 Key factors in the inhibition of apoptosis of cancer cells may thus be a promising target for drug development.1, 5 Among such key factors are the inhibitors of apoptosis proteins (IAPs).
IAPs primarily inhibit caspases, cysteine proteases involved in the intrinsic and extrinsic pathways of apoptosis.1 They are also involved in the regulation of nuclear factor κB,6, 7 mitogenic kinase signaling, proliferation, and mitosis as well as in the activation of cell motility kinases and metastasis.8 Over-expression of IAPs was found in many types of cancer to be associated with chemoresistance, disease progression, and poor prognosis.3, 4 By contrast, inactivation of IAPs, particularly when combined with other treatments, resulted in the death of tumor cells without apparent detrimental effects on normal cells.7, 9, 10
Overexpression of IAPs and its impact on survival and chemoresistance were also shown in acute myeloid leukemia (AML),11, 12 which is the most common acute leukemia in adults. Treatment remains challenging particularly in poor-risk patients such as the elderly,13, 14 as responsiveness to standard therapy declines with age.15, 16 Unfavorable cytogenetics, multi-drug resistance, low performance status, and comorbidity also contribute to poor prognosis.13, 14
Standard treatment for remission induction of AML are “7 plus 3” regimens that include daunorubicin 45–90 mg/m2 given as iv bolus for 3 days along with 100 mg/m2 cytarabine iv daily for 7 days.17, 18 Variations of this regimen have mostly proven disappointing.19–23 However, in patients with rapidly relapsed or refractory AML, idarubicin and high-dose cytarabine were combined with escalating doses of X-chromosome associated IAP (XIAP) antisense oligonucleotides24 resulting in decreases of XIAP mRNA and an increased overall response rate: In the highest dose group, 15 out of 32 (47%) patients achieved complete remission (CR) as compared with only 1 out of 24 (4%) receiving lower doses. Thus, use of an adjunct treatment antagonizing IAPs may help to resolve therapy resistance of AML.
Debio1143 is a small molecule mimetic of the second mitochondria-derived activator of caspase C. It blocks a highly conserved 70 amino acid domain on the N-terminal end of IAPs known as the Baculovirus IAP Repeat (BIR) domain. In vivo and in vitro studies have demonstrated that Debio1143 induces cell death in several tumor models by inhibiting XIAP and cellular IAPs (cIAPs) 1 and 2. Here we present the results of a Phase I study of Debio1143 in patients with poor-risk AML in combination with standard induction therapy of daunorubicin and cytarabine.
Patients and Methods
Study population
Patients aged ≤75 years were eligible if they had AML other than acute promyelocytic leukemia.25 Relapsing patients had to have completed treatment with any anthracycline based regimens or hematopoietic stem cell transplantation by >6 months. Patients with first-time diagnosis had to have ≥1 poor-risk factor (unfavorable cytogenetics; hematologic disease for ≥3 months based on bone marrow aspiration/biopsy or treatment-related secondary AML; gene mutations associated with poor survival; age ≥70 years; WBC >100 k/μL) and no favorable cytogenetic variant according to the modified UK MRC/NCRI criteria.26 All patients had to have finished any cancer drug therapy for ≥21 days and any radiation, systemic corticosteroids, or minor surgery for ≥14 days from entry (28 days for thoracic radiation or major surgery). Moreover, patients had to have recovered from previous treatments and to demonstrate an Eastern Cooperative Oncology Group (ECOG) Performance Status ≤2 as well as adequate cardiac, renal, and liver function. Main exclusion criteria were primary refractory AML, central nervous system leukemia, any graft versus host disease or preventive treatment, any infection or chronic inflammation.
Study design and treatments
Patients were enrolled in sequential cohorts of ≤6 patients of whom ≥3 had to complete the 30-day induction treatment. If none of them demonstrated Debio1143-related dose-limiting toxicity (DLT) the dose was escalated to the next level. DLT mainly comprised any CTC grade 5 toxicity, grade 4 thrombocytopenia or neutropenia by day 42 in the absence of AML or any grade 3 or 4 non-hematologic toxicity (except anorexia, fatigue) if neither abating until day 42 nor attributable to persistent AML. In case of DLT, this cohort was expanded until there were ≥6 evaluable patients. If no additional DLT occurred, the next dose cohort was opened. If ≥2 patients experienced DLT, the maximum tolerated dose (MTD) was exceeded. Patients remained on study until hematological recovery, requirement for re-induction, or unacceptable toxicity occurred.
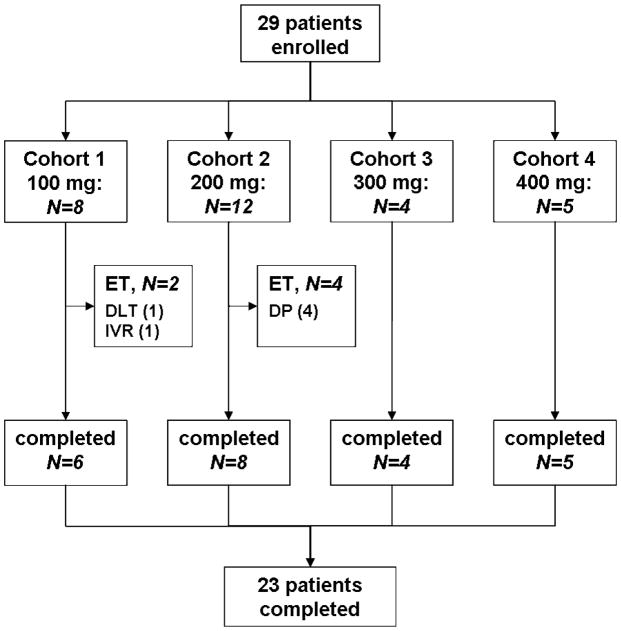
The Debio1143 starting dose was 100 mg/day which was increased in consecutive cohorts by increments of 100 mg/day up to 400 mg/day. Patients received daunorubicin 90 mg/m2 iv on days 1–3 and cytarabine 100 mg/m2 iv by continuous infusion, on days 1–7 of the induction cycle. Oral Debio1143 was scheduled on days 1–5. Patients who showed clear evidence of persistent leukemia (≥20% blast, no hypocellularity) on day 15 were removed from the study and replaced (Fig. 1). To assess treatment response bone marrow aspirates and biopsies were obtained at baseline and days 14 and 29.
Figure 1.
Patient flow-chart according to CONSORT
ET: early termination; DLT: dose-limiting toxicity; DP: disease progression; IVR: investigator request
The study was compliant with all applicable legal obligations, the requirements of the Declaration of Helsinki and Good Clinical Practice. It was approved by the Institutional Review Boards of all participating sites.
Pharmacokinetic (PK) and pharmacodynamic (PD) analysis
Blood samples were drawn during the first 8 days of the induction therapy to determine PKs of Debio1143, daunorubicin, daunorubicinol, and cytarabine using LC-MS/MS validated methods. Expression of cIAP-1, XIAP, CD34 and CD117 were measured in PBMC using validated multiplex immunohistochemistry assay. Plasma biomarkers (TNFα, MCP-1 and IL-8) were measured through commercial ELISA.
Statistical analysis
Data was compared over time to assess changes from baseline during treatment and follow-up. If sample size allowed, Wilcoxon matched pairs test was used for inferential comparisons. Dose proportionality was explored using a regression model for log transformed AUC and Cmax data.
Results
From 29 March 2011 until 21 January 2013, 29 patients, 17 men (59%) and 12 women (41%) with a mean age of 55.5±12.9 years, were enrolled in 4 cohorts and treated with oral Debio1143 doses ranging from 100 to 400 mg daily (Figure 1); 26 patients were white (90%), 3 were African Americans (10%); 21 patients (72%) had untreated AML while in 8 patients (28%) it had relapsed after 1 to 6 lines of therapy (n= 3, 1, 2, 1, 0, 1, respectively). Seven patients (24%) had prior stem cell transplantation; in 3 patients, extramedullary disease was present. Based on WHO criteria, 5 patients (17%) had AML with genetic abnormalities, 11 patients (38%) AML with MDS-related features, 5 patients (17%) therapy-related AML, and 8 patients (28%) had AML not otherwise specified. Cytogenetic results were favorable in 1 (3%) patient, intermediate in 11 (38%), unfavorable in 16 (55%) and missing in 1 (3%). All patients completed the induction cycle. Three (10%) patients did not receive the planned dose of Debio1143. There were no dose delays of Debio1143 or daunorubicin, but one of cytarabine. Eventually, the MTD was not reached and 400 mg was the highest tested dose.
Dose limiting toxicities (DLT)
In addition to the typical toxicities of the “7 plus 3” induction therapy of daunorubicin and cytarabine, we observed DLTs in 3 (10%) patients that were attributed to treatment with 100, 200, and 400 mg Debio1143, respectively. One patient developed asymptomatic grade 3–4 transient elevation of liver enzymes after a single dose of 100 mg. A second patient experienced grade 3–4 mucositis, grade 3 atrial fibrillation and grade 4 hypokalemia and hypocalcemia two weeks after completion of the initiation cycle with 200 mg Debio1143. Atrial tachycardia and electrolyte imbalance were successfully treated and considered sequelae of mucositis, which persisted though. A third patient developed heart failure along with a neutropenic septic episode at the end of the initiation cycle with 400 mg/d Debio1143. The patient was reported to have expired 3 months later due to disease progression.
General Safety
Overall, there were 441 AEs with all patients having at least one. The majority of these was of CTC grade 1 or 2; 28 (97%) patients experienced ≥1 AE of grade 3, 8 (28%) of grade 4, and 2 (7%) of grade 5. Two patients discontinued study drug treatment primarily because of an AE. Eleven patients (38%) had overall 20 SAEs; 2 patients (7%) experienced a fatal SAE (fungal pneumonia; persistent/recurrent AML), but both episodes were considered unrelated to Debio1143. Neither incidence, nor severity of AEs increased with dose; 96 AEs (21.8%) including 10 SAEs (50%) were considered related to Debio1143 (Table 1); 19 (66%) patients experienced ≥1 related AE and 4 (14%) a related SAE. Most affected organ systems were gastrointestinal and general disorders with nausea, diarrhea, and febrile neutropenia being the most common ADRs (Table 1). No clinically meaningful trends were observed in safety laboratory or ECG measurements, vital signs, body weight measurements, or ECOG performance status.
Table 1.
ADRs of Debio1143 with more than one occurrence, by System Organ Class and Preferred Term (PT)
| Dose cohort | 100 mg (N=8) | 200 mg (N=12) | 300 mg (N=4) | 400 mg (N=5) | AE, total 441 (100%) | Pts, total 29 (100%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| System Organ Class / • PT | Pts | AE | Pts | AE | Pts | AE | Pts | AE | AE | % | Pts | % |
| Blood / lymphatic system | 2 | 2 | 1 | 1 | 3 | 1 | 3 | 2 | 9 | 2.0 | 6 | 20.7 |
|
| ||||||||||||
| •Febrile neutropenia | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 6 | 1.4 | 4 | 13.8 |
| •Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0.5 | 1 | 3.4 |
|
| ||||||||||||
| Cardiac disorders | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 0.5 | 2 | 6.9 |
|
| ||||||||||||
| Eye disorders | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.5 | 1 | 3.4 |
|
| ||||||||||||
| Gastrointestinal disorders | 12 | 7 | 11 | 4 | 3 | 1 | 0 | 0 | 26 | 5.9 | 12 | 41.4 |
|
| ||||||||||||
| •Diarrhoea | 3 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 0.9 | 4 | 13.8 |
| •Nausea | 5 | 5 | 4 | 4 | 0 | 0 | 0 | 0 | 9 | 2.0 | 9 | 31.0 |
| •Stomatitis | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0.5 | 2 | 6.9 |
| •Vomiting | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 0.7 | 3 | 10.3 |
|
| ||||||||||||
| General / admin. site | 4 | 3 | 5 | 4 | 1 | 1 | 0 | 0 | 10 | 2.3 | 8 | 27.6 |
|
| ||||||||||||
| •Fatigue | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 3 | 0.7 | 3 | 10.3 |
| •Mucosal inflammation | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 0.7 | 3 | 10.3 |
| •Pyrexia | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 3 | 0.7 | 2 | 6.9 |
|
| ||||||||||||
| Infections / infestations | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 6 | 1.4 | 5 | 17.2 |
|
| ||||||||||||
| Investigations | 5 | 3 | 2 | 2 | 3 | 1 | 3 | 1 | 13 | 2.9 | 7 | 24.1 |
|
| ||||||||||||
| •ALT increased | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0.7 | 3 | 10.3 |
| •AST increased | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 0.7 | 2 | 6.9 |
| •Blood bilirubin increased | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0.5 | 2 | 6.9 |
| •ECG QT prolonged | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 3 | 0.7 | 1 | 3.4 |
|
| ||||||||||||
| Metabolism / nutrition | 0 | 0 | 5 | 3 | 1 | 1 | 7 | 1 | 13 | 2.9 | 5 | 17.2 |
|
| ||||||||||||
| •Decreased appetite | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0.5 | 2 | 6.9 |
| •Hypocalcaemia | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 0.7 | 1 | 3.4 |
| •Hypokalaemia | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 4 | 0.9 | 2 | 6.9 |
|
| ||||||||||||
| Nervous system disorders | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.5 | 2 | 6.9 |
|
| ||||||||||||
| Respiratory, thoracic mediastinal disorders | 2 | 1 | 3 | 3 | 3 | 1 | 0 | 0 | 8 | 1.8 | 5 | 17.2 |
|
| ||||||||||||
| Skin, subcutaneous tissue | 2 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 5 | 1.1 | 4 | 13.8 |
|
| ||||||||||||
| Total ADRs | 8 | 32 | 7 | 33 | 2 | 15 | 2 | 16 | 96 | 21.8 | 19 | 65.5 |
Pts: patients
Pharmacokinetics
Cmax and AUC increased greater than dose proportional in the range from 100 to 400 mg (Table 2). Average Tmax was about 2 h. On day 1, T1/2 was about 5.5 h, independent of the dose. No drug accumulation was observed over the 5-day dosing period.
Table 2.
Pharmacokinetics of Debio1143, means ± standard deviation
| Dose | Day | N | Cmax [mg/l] | Tmax° [h] | T1/2 [h] | AUC0-inf [mg*h/l] |
|---|---|---|---|---|---|---|
| 100 mg | 1 | 8 | 1.2 ± 0.7 | 2.6 | 5.0 ± 1.1 | 6.0 ± 3.5 |
| 5 | 8 | 8.3 ± 0.8 | 2.9 | 13.0 ± 2.9 | 8.5 ± 5.7 | |
|
| ||||||
| 200 mg | 1 | 12 | 3.0 ± 1.8 | 1.5 | 5.6 ± 0.9 | 18 ± 9 |
| 5 | 12 | 2.4 ± 1.3 | 1.5 | 18.0 ± 5.1 | 24 ± 14 | |
|
| ||||||
| 300 mg | 1 | 4 | 4.4 ± 1.7 | 1.2 | 4.9 ± 1.0 | 25 ± 12 |
| 5 | 4 | 2.5 ± 4.0 | 2.0 | 15.9 ± 3.4 | 24 ± 11 | |
|
| ||||||
| 400 mg | 1 | 4 | 9.5 ± 4.5 | 1.0 | 5.9 ± 1.4 | 55 ± 20 |
| 5 | 4 | 5.6 ± 1.8 | 2.5 | 25.5 ± 3.7 | 72 ± 20 | |
Median
Pharmacodynamics
cIAP1 and XIAP staining in PBMCs
Eighteen patients had pre- and post-first dose assessments of IAP in PBMCs. In general, XIAP levels were low (H-score≤20) and cIAP1 varied without pattern or significant differences over time (Figure 2). There was no obvious correlation between changes in cIAP1 and types of AML or treatment success. In 13 patients, cIAP1 levels measured in CD34/CD117+ cells were significantly lower on days 1 (6 h) and 8 as compared to baseline (Figure 2).
Figure 2.
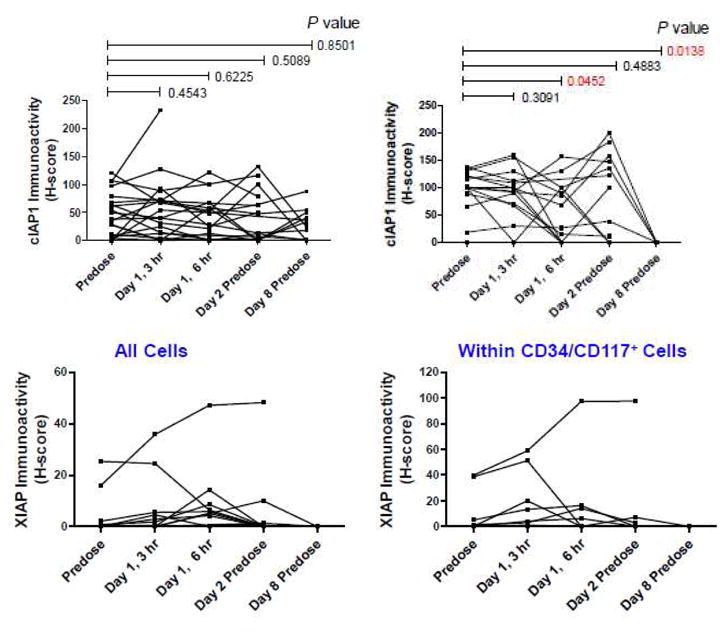
Change over time in the expression of cIAP-1 and XIAP in PBMCs
Plasma biomarker levels
TNFα w as measurable in 19 patients but remained below the limit of detection in 10 patients. Baseline values ranged up to 36 pg/ml. In 13 patients (68.4%) levels 3 h post-first dose were greater than at baseline, across doses. Those included all 4 patients with treatment-related AML (100%). An increase in plasma TNF7agr; was also detected at 3 h on Day 1 in 9 out of 11 patients with CR, but only in 3 out of 6 patients with resistant disease. Sample size was insufficient to assess any dose-dependencies.
IL-8 and MCP-1 were measurable in 22 patients. IL-8 levels significantly increased over 6 h postdose (Figure 3), highest in the lowest dose group. There was no obvious difference in IL-8 changes among AML types, however, 8 patients (72.7%) with IL-8 above median at 3 h post-first dose achieved CR as compared to only 3 (27.3%) with values below median. With the exception of 3 patients MCP-1 decreased post-first dose until day 2 with decreases being significant at 3 h and 24 h post-first dose (Figure 3). On day 8, values had re-increased to a level significantly above baseline. MCP-1 changes did neither depend on dose or AML type, nor was there any striking correlation with treatment response.
Figure 3.
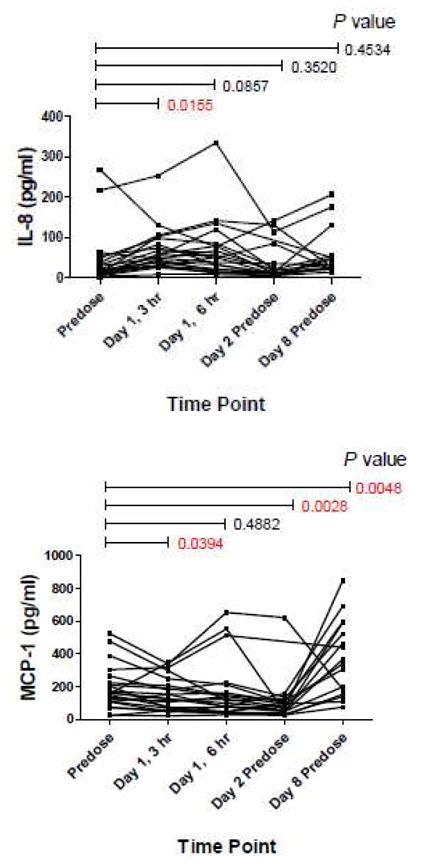
Change over time in plasma biomarker concentrations
Efficacy
A total of 14 (48%) patients achieved disease remission, 11 (38%) a CR, 2 (7%) a CR with incomplete hematological recovery (CRi) and 1 (3%) a partial response. The majority of remissions occurred in cohort 1, 100 mg: 7 (88%) versus 200 mg: 4 (33%); 300 mg: 1 (25%); 400 mg: 2 (40%). Of the 13 patients with CR/CRi, 8 (62%) relapsed within the study period. In 15 (52%) patients, treatment failed due to resistant disease. For 26 (90%) patients overall survival was ≥30 days. Correlative subgroup analyses between disease remission and cytogenetic variants were inconclusive.
Discussion
Debio1143 is one of six SMAC mimetic IAP antagonists that have entered clinical development. LCL-161 and birinapant (TL-32711) have even entered phase II.27, 28 Debio1143 was shown to be well tolerated when given as monotherapy to patients with advanced cancer and to elicit PD effects seen in animals at linear PK at doses >80 mg.29 This was the first trial using Debio1143 concomitantly to a standard “7 plus 3” chemotherapy regimen consisting of daunorubicin and cytarabine for the treatment of AML. Our findings indicate that such a triple combination is clinically feasible and revealed good general safety and tolerability, and low mortality rate. An incidence of DLTs in about 10% of patients is rather low for a combination of antineoplastic drugs.
For all three DLTs possibly related to Debio1143, there were other at least contributing factors: The patient with transient grade 3–4 liver enzyme increases had previously experienced a similar event under chemotherapy with clofarabine. Some hepatotoxicity appears to be inherent to the mode of action of IAP inhibitors though.30, 31 Mucositis is a common complication of cytarabine, 32 but based on its severity, the investigator felt it to be associated with increased apoptosis through Debio1143 which indeed might have contributed to the successful remission in this patient. Sepsis itself is a contributing factor to heart failure regardless of age 33–35 and heart failure is a known and labeled side effect of daunorubicin.
The general safety profile of Debio1143 with gastrointestinal AEs being most common is not of concern, particularly as the incidence and severity of AEs did not increase with dose. Debio1143 is likely to exhibit non-linear PKs, although the drug did not accumulate over 5 days. Robust PK interpretation of daunorubicin and cytarabine data remained difficult because the study was not designed to accurately explore a potential interaction between Debio1143 and cytarabine or daunorubicin. In general, PK of daunorubicin appeared not to be affected by Debio1143 dose. Cytarabine clearance in some patients was higher than reported in the literature,36 however sampling conditions were not properly controlled and an interaction thus would remain to be further investigated.
There was no obvious association between PK and PD, however sample sizes were small and both PK and PD data varied considerably. Accordingly, PD data was inconclusive when exploring associations with disease characteristics or treatment outcomes, although the significant decrease of cIAP1 levels in CD34/CD117+ cells and the significant changes in plasma IL-8 and MCP-1 may indicate some clinical on-target activity. The changes of IL-8 and MCP-1 were not in line though with those in patients receiving Debio1143 monotherapy for mostly solid tumors in the first-in-man trial.29
In terms of efficacy, addition of Debio1143 as an adjunct to cytarabine and daunorubicin did not result in strikingly higher remission rates than observed in studies on the combination of cytarabine and daunorubicin only.37 Our overall CR rate was 38% after the induction cycle without any difference between younger and older patients. CR rates reported for “7 plus 3” combinations ranged from 53% to 58% in AML patients with considerably more favorable cytogenetic factors.38 Interestingly, in our study the CR rate after the induction cycle with the 100 mg dose of Debio1143 was 62%. Although the validity of such comparisons with historical data might generally be doubtful, it at least warrants further, controlled studies.
In conclusion, tolerability and safety of Debio1143 at oral doses up to 400 mg once daily for 5 days as an adjunct to standard cytarabine and daunorubicin regimen was acceptable. Controlled studies evaluating the efficacy of the 100 mg dose in subgroups of AML patients are warranted.
Clinical Practice Points
Several SMAC mimitics inactivating IAPs have entered early clinical development. First-in-man studies in compassionate use patients with advanced, refractory cancer of any type revealed rather weak anti-tumor activity when given as single agent. By contrast, first results of combination approaches were promising in that SMACs may overcome treatment resistance to common chemotherapy regimens. Specifically, acute myeloid leukemia (AML) often develop treatment resistance. The combination of oral Debio1143 doses up to 400 mg q5wk along with standard induction therapy of daunorubicin and cytarabine proved feasible in poor-risk AML patients and was well tolerated. Clinical on-target activity was indicated by a significant decrease of cIAP1 levels in CD34/CD117+ cells and blasts as well as by significant changes in plasma cytokine levels. Complete remission rate after the induction cycle was 38% overall and 62% with the 100 mg dose. This was slightly higher than for “7 plus 3” combinations in AML patients despite less favorable cytogenetic factors.
Acknowledgments
The authors would like to thank patients and their families, and the study coordinators and research nurses who executed the study. We thank Uwe Totzke for medical writing support sponsored by Debiopharm International SA, Lausanne.
Footnotes
ClinicalTrials.gov identifier: NCT01265199
Conflicts of interest
This work was supported by Ascenta Therapeutics, Malvern, PA, USA. GV, ER and CZ are employed by Debiopharm International SA; JMB is employed by Ascenta Therapeutics; JMS is a founder of Ascenta Therapeutics and has stocks. All remaining authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John F. DiPersio, Email: jdipersi@dom.wustl.edu.
Harry P. Erba, Email: harry.erba@ccc.uab.edu.
Richard A. Larson, Email: rlarson@medicine.bsd.uchicago.edu.
Selina M. Luger, Email: selina.luger@uphs.upenn.edu.
Martin S. Tallman, Email: tallmanm@mskcc.org.
Jeffrey M. Brill, Email: jbrill@ascenta.com.
Gregoire Vuagniaux, Email: gregoire.vuagniaux@debiopharm.com.
Elisabeth Rouits, Email: elisabeth.rouits@debiopharm.com.
J. Mel Sorensen, Email: msorensen@ascenta.com.
Claudio Zanna, Email: claudio.zanna@debiopharm.com.
References
- 1.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.LaCasse EC, Mahoney DJ, Cheung HH, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 4.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 5.Fulda S. Inhibitor of apoptosis proteins as targets for anticancer therapy. Expert Rev Anticancer Ther. 2007;7:1255–1264. doi: 10.1586/14737140.7.9.1255. [DOI] [PubMed] [Google Scholar]
- 6.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 7.Varfolomeev E, Goncharov T, Fedorova AV, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra S, Languino LR, Raskett CM, et al. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw TJ, Lacasse EC, Durkin JP, et al. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122:1430–1434. doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 11.Schimmer AD, Welsh K, Pinilla C, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 12.Carter BZ, Milella M, Tsao T, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081–2089. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- 13.British Committee for Standards in H. Milligan DW, Grimwade D, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135:450–474. doi: 10.1111/j.1365-2141.2006.06314.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control. 2008;19:379–390. doi: 10.1007/s10552-007-9097-2. [DOI] [PubMed] [Google Scholar]
- 16.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yates JW, Wallace HJ, Jr, Ellison RR, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–488. [PubMed] [Google Scholar]
- 18.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchner T, Hiddemann W, Wormann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93:4116–4124. [PubMed] [Google Scholar]
- 20.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 21.Feldman EJ, Alberts DS, Arlin Z, et al. Phase I clinical and pharmacokinetic evaluation of high-dose mitoxantrone in combination with cytarabine in patients with acute leukemia. J Clin Oncol. 1993;11:2002–2009. doi: 10.1200/JCO.1993.11.10.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hann IM, Stevens RF, Goldstone AH, et al. Randomized comparison of DAT versus ADE as induction chemotherapy in children and younger adults with acute myeloid leukemia. Results of the Medical Research Council's 10th AML trial (MRC AML10). Adult and Childhood Leukaemia Working Parties of the Medical Research Council. Blood. 1997;89:2311–2318. [PubMed] [Google Scholar]
- 23.Russo D, Malagola M, de Vivo A, et al. Multicentre phase III trial on fludarabine, cytarabine (Ara-C), and idarubicin versus idarubicin, Ara-C and etoposide for induction treatment of younger, newly diagnosed acute myeloid leukaemia patients. Br J Haematol. 2005;131:172–179. doi: 10.1111/j.1365-2141.2005.05745.x. [DOI] [PubMed] [Google Scholar]
- 24.Schimmer AD, Estey EH, Borthakur G, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 26.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 27.Dubrez L, Berthelet J, Glorian V. IAP proteins as targets for drug development in oncology. Onco Targets Ther. 2013;9:1285–1304. doi: 10.2147/OTT.S33375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulda S. Molecular Pathways: Targeting Inhibitor of Apoptosis Proteins in Cancer-From Molecular Mechanism to Therapeutic Application. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0227. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz HI, Smith DC, Pitot HC, et al. Safety, pharmacokinetics, and pharmacodynamic properties of oral DEBIO1143 (AT-406) in patients with advanced cancer - results of a first-in-man study. Cancer Chemother Pharmacol. 2014 doi: 10.1007/s00280-015-2709-8. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson RI, Tarrant J, Cain G, et al. Toxicity profile of small-molecule IAP antagonist GDC-0152 is linked to TNF-alpha pharmacology. Toxicol Sci. 2013;131:247–258. doi: 10.1093/toxsci/kfs265. [DOI] [PubMed] [Google Scholar]
- 31.Akazawa Y, Guicciardi ME, Cazanave SC, et al. Degradation of cIAPs contributes to hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2013;305:G611–619. doi: 10.1152/ajpgi.00111.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niscola P, Romani C, Cupelli L, et al. Mucositis in patients with hematologic malignancies: an overview. Haematologica. 2007;92:222–231. doi: 10.3324/haematol.10232. [DOI] [PubMed] [Google Scholar]
- 33.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 34.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 35.Young JD. The heart and circulation in severe sepsis. Br J Anaesth. 2004;93:114–120. doi: 10.1093/bja/aeh171. [DOI] [PubMed] [Google Scholar]
- 36.Wan SH, Huffman DH, Azarnoff DL, et al. Pharmacokinetics of 1-beta-D-arabinofuranosylcytosine in humans. Cancer Res. 1974;34:392–397. [PubMed] [Google Scholar]
- 37.Holowiecki J, Grosicki S, Robak T, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18:989–997. doi: 10.1038/sj.leu.2403336. [DOI] [PubMed] [Google Scholar]
- 38.Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012;30:2425–2428. doi: 10.1200/JCO.2011.38.9601. [DOI] [PubMed] [Google Scholar]