Abstract
Background
Multiple randomized trials have demonstrated a benefit for all-trans retinoic acid (ATRA) in patients with acute promyelocytic leukemia (APL). Pseudotumor cerebri (PTC) is an infrequently reported adverse effect of ATRA.
Methods
We examined the incidence, clinical course, and outcomes of patients with APL treated on Intergroup Protocol 0129 (I0129) who developed PTC. This trial evaluated the role of ATRA alone during induction and/or as maintenance therapy.
Results
Of the patients on trial, 240 received ATRA during induction, maintenance, or both; 8 had a clinical suspicion for PTC. Upon review of individual cases, this was felt to be “probable” in 4 patients, “possible” in 1 and “unlikely” in 3 due to lack of diagnostic criteria or presence of a more likely alternate diagnosis.
Conclusions
“Probable” PTC occurred in 1.7% of patients who received ATRA during induction and/or maintenance therapy. In agreement with previous reports, the incidence of PTC in APL patients receiving ATRA was higher in the pediatric population. Here, we discuss the method for diagnosing PTC in the setting of ATRA therapy and management strategies.
Keywords: Acute promyelocytic leukemia, pseudotumor cerebri, all-trans retinoic acid, ATRA
MICRO-ABSTRACT
Pseudotumor cerebri (PTC) is a rare side effect of all-trans retinoic acid (ATRA). We examined patients with acute promyelocytic leukemia (APL) treated on I0129 who developed PTC. This trial evaluated the role of ATRA alone during induction and/or as maintenance therapy. We found that 1.7% of patients receiving ATRA developed “probable” PTC. We review the literature on PTC in APL and discuss diagnostic criteria.
INTRODUCTION
Multiple randomized trials have demonstrated that all-trans retinoic acid (ATRA) significantly improves the outcome of patients with acute promyelocytic leukemia (APL)1,2. While ATRA is usually well tolerated, multiple side effects may occur including neurologic toxicities such as headache and more rarely, pseudotumor cerebri syndrome (PTC). The mechanism of ATRA neurotoxicity may be similar to vitamin A toxicity, which is a known cause of PTC, as high doses of ATRA can lead to retinoids enhancing the production of CSF. In a study comparing CSF retinol levels in patients with idiopathic PTC to those without, a higher level of vitamin A was noted in the CSF of affected patients, none of whom had known vitamin A toxicity3. Additionally, ATRA may alter the lipid constituents of the arachnoid villi which disturbs the normal transport system and therefore CSF cannot be absorbed4.
PTC may be a primary disorder, most commonly seen in obese female patients of childbearing age, or it may be secondary to other causes such as cerebral venous abnormalities, or associated with various medical conditions (such as Addison’s Disease), or medications (such as ATRA therapy). In 2013, the diagnostic criteria for the syndrome were revised, with requirements for diagnosis including papilledema, normal neurologic exam except for cranial nerve abnormalities, normal neuroimaging without evidence of hydrocephalus, mass, or structural lesion, and no abnormal meningeal enhancement on MRI or contrast-enhanced CT, normal CSF composition, and elevated lumbar puncture (LP) opening pressure (≥250 mm Hg or ≥280 mm Hg for non-obese children). Further, the diagnosis may be made in the absence of papilledema if all of the above criteria are satisfied and the patient has unilateral or bilateral abducens nerve palsy. If there is neither papilledema nor a sixth cranial nerve palsy, the diagnosis can be suggested, but not confirmed with additional neuroimaging characteristics5. While headache is the most common presenting symptom of PTC6, presence of headache is not part of the 2013 diagnostic criteria.
PTC has frequently been described in the literature as an adverse effect of ATRA therapy, usually in single case reports4,7–29, making an estimation of its true incidence difficult. Here, we discuss the series of patients on clinical trial I0129 in which there was a clinical suspicion for PTC, focusing on diagnostic criteria in this select population, and how to treat such patients.
METHODS
Treatment with ATRA
Intergroup 0129 was a prospective trial conducted between April 1992 and February 1995 in which patients with newly diagnosed APL by morphology were randomized to receive either ATRA 45 mg/m2/day divided into two daily doses or to daunorubicin 45 mg/m2 (1.5 mg/kg for children <3 years of age) by intravenous (IV) bolus on days 1–3 and cytosine arabinoside 100 mg/m2 (3.3 mg/kg for children <3 years of age) by continuous IV infusion on days 1–7 for induction. Patients on the ATRA induction arm were required to have WBC ≤10,000/µL at presentation, or after hydroxyurea, prior to initiating ATRA. Patients who failed to respond to ATRA for induction after a maximum of 90 days, or failed before 90 days of ATRA because of toxicity or progressive disease, were permitted to cross over to the chemotherapy arm. Patients randomized to induction chemotherapy who did not achieve CR after 2 cycles did not cross over to the ATRA arm but were removed from the study and treated at the physician’s discretion. For consolidation, patients who had a CR with chemotherapy or ATRA received two cycles of consolidation therapy2.
For maintenance, patients in CR after both cycles of consolidation chemotherapy were randomly assigned to either 45 mg of ATRA per square meter per day given orally in divided doses every 12 hours for one year or to observation2.
Patients
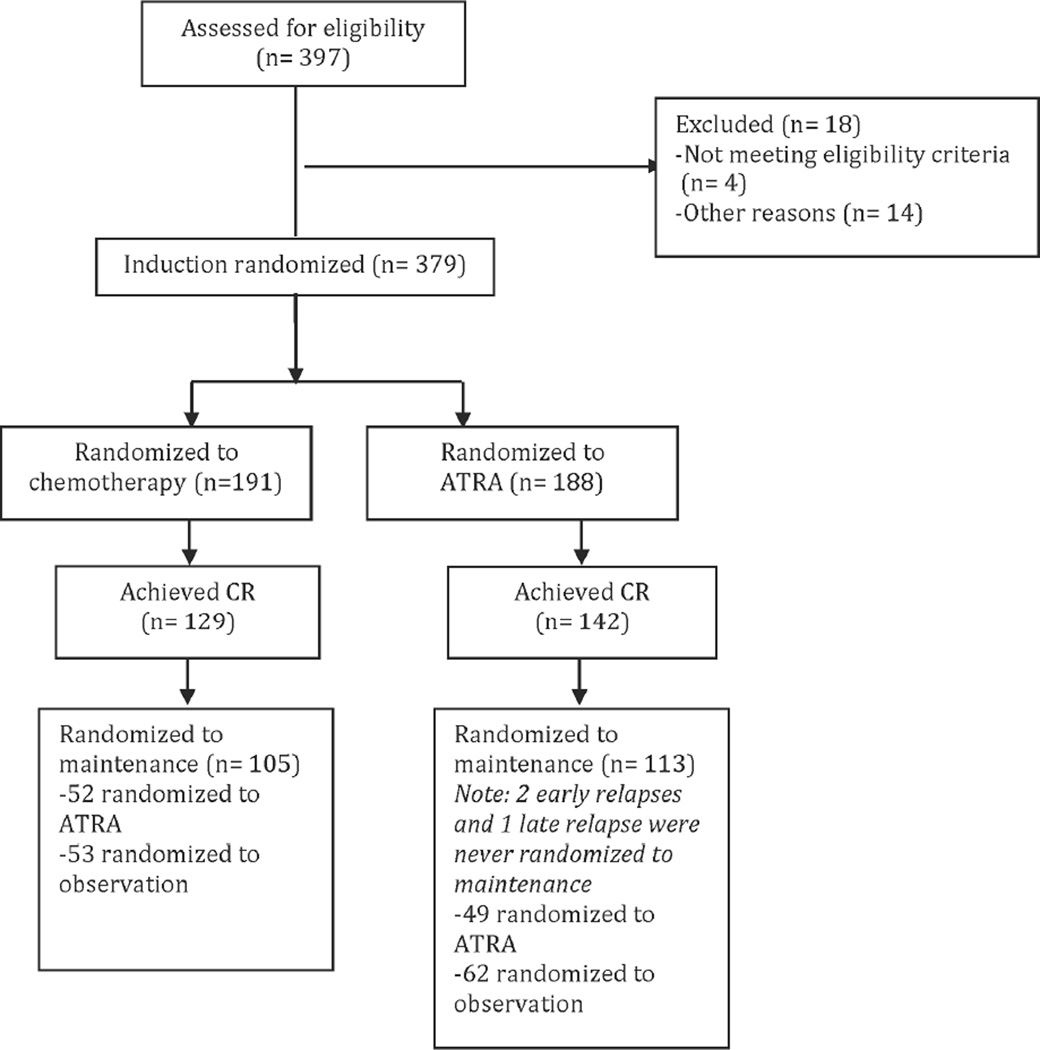
A total of 397 patients were assessed for eligibility, 18 excluded leaving 379 patients for induction randomization. For induction therapy, 191 were randomized to receive chemotherapy and 188 were randomized to receive ATRA. Of the 191 patients randomized to chemotherapy for induction, 129 achieved a complete remission with 105 randomized for maintenance (52 randomized to ATRA maintenance and 53 randomized to observation). Of the 188 patients randomized to ATRA induction, 142 obtained a complete remission with 113 randomized for maintenance (49 randomized to ATRA maintenance and 64 randomized to observation). In total, 240 patients were evaluable for analysis on the ATRA arms (induction, maintenance, or both)(Figure 1)30. Details of patient characteristics and outcomes have previously been reported2.
Figure 1.
Flow diagram depicting distribution of patients for induction and maintenance randomization.
Diagnosis
The diagnosis of PTC was made in the presence of signs and symptoms of intracranial hypertension without clinical or radiological evidence of infective or space occupying lesions. “Definite” PTC met all of the 2013 diagnostic criteria: (1) presence of papilledema, (2) normal neurologic exam except for cranial nerve abnormalities, (3) normal neuroimaging without evidence of hydrocephalus, mass, or structural lesion, and no abnormal meningeal enhancement on MRI or contrastenhanced CT, (4) normal cerebral spinal fluid (CSF) composition with elevated lumbar puncture (LP) opening pressure. Because of the clinical circumstances associated with APL and the frequent inability to perform a lumbar puncture safely in some of these patients, we constructed additional criteria to address the patients in this cohort so we could delineate those in whom PTC was probable even though they did not meet the full 2013 diagnostic criteria. Neurological exams and CSF composition were assumed to be normal unless explicitly stated otherwise, and in cases where the opening pressure was not available from the LP, it is assumed that pressure was elevated to such a degree to meet diagnostic criteria. Thus, patients with “probable” PTC met all but one of the diagnostic criteria. “Possible” cases were missing two of the criteria, and “unlikely” cases were those in which an alternate diagnosis was more likely than PTC or when only 0–1 criteria were present (Table 1).
Table 1.
Proposed criteria for diagnosing PTC in APL patients.
| Diagnosis category | Number of criteria present |
Criteria |
|---|---|---|
| Definite | 4 |
|
| Probable | 3 | |
| Possible | 2 | |
| Unlikely | 0–1 |
Treatment of pseudotumor cerebri
Treatment of PTC, when suspected, was variable. In some instances, patients were provided with symptomatic therapy such as dexamethasone and narcotics, and in other cases the ATRA was held and restarted either at the same dose or a lower dose. Data were not available regarding response to specific interventions within this series.
RESULTS
Clinical characteristics of patients with pseudotumor cerebri
Probable cases
Case 1
During induction, an 8 year-old girl developed a grade 2 headache on day 4 of ATRA therapy. The headache persisted until day 20 when a head CT scan was performed, which was normal. An ophthalmologist, saw the patient, and bilateral papilledema was noted on funduscopic exam. For treatment of presumed PTC, this patient was given dexamethasone. This patient meets all criteria for PTC except an LP was not performed, making the diagnosis “probable.”
Case 2
During induction, a 19 year-old boy developed headache on day 9 of ATRA therapy. He underwent an LP for workup, which showed an elevated CSF pressure of 420 mm water. Papilledema was noted in the temporal region of his optic discs. ATRA was held, but resumed on day 12. Neuroimaging was not documented, though this patient met all other criteria for PTC, making this case “probable.”
Case 3
During induction, an 11 year-old boy developed diplopia with papilledema on day 13 of ATRA therapy, with a 6th nerve palsy on day 14 of therapy. Head CT scan was negative on day 14. The patient was placed on dexamethasone on day 19 for treatment of presumed PTC. An MRI on day 29 confirmed no structural abnormalities, and the patient underwent an LP, which showed “increased intracranial pressure,” exact measurement was not available. The patient underwent repeated LPs but developed right and left lateral rectus palsies. Consideration was made whether these symptoms could be due to leukemic infiltrates and hence the patient received radiotherapy to bilateral globes and anterior skull base on day 47. By day 53, his vision improved. ATRA was discontinued on day 55. The patient achieved a CR. It is not clear whether this patient truly had leukemic involvement of orbit and optic nerve. This patient meets all criteria for “definite” PTC; however, due to confounding factors including suspicion for an alternate diagnosis and administration of radiotherapy, this case is also best considered as “probable.”
Case 4
A 15 year-old girl received cytarabine and daunorubicin for induction therapy, and subsequently received ATRA maintenance. She developed a headache, blurred vision, and diplopia on day 19 of ATRA therapy. An LP on day 47 showed “increased intracranial pressure.” A head CT scan was normal on day 50. ATRA was held intermittently and restarted at a decreased dose, and therapy was completed without difficulty. This case meets all criteria for PTC diagnosis except that papilledema was not described, making the diagnosis “probable.”
Possible case
Case 5
A 24 year-old woman developed a grade 1 headache on day 2 of induction ATRA therapy, treated with acetaminophen/propoxyphene. However, the headache increased to grade 2 by day 7, in addition to a “dark spot” in her visual field. The visual symptoms resolved although neurology and ophthalmology documented “blurring of the disc margin.” This patient also developed polymicrobial bacteremia during this time frame. While the patient meets some of the criteria for PTC, the diagnosis is only considered “possible” as neither neuroimaging nor LP were performed, and the presence of an alternate diagnosis (bacteremia) may have contributed to symptoms.
Unlikely cases
Case 6
An 11 year-old girl developed nausea and vomiting on day 4 of induction therapy with ATRA. A CT head scan was performed on day 7, which was normal. Headache began on day 9. On day 12, there is a note in the chart saying the patient had PTC. However, neither papilledema nor increased CSF pressure were documented, so there is insufficient evidence to make this diagnosis.
Case 7
On day 2 of ATRA therapy, this 4 year-old girl developed a headache with a normal neurologic exam. The headache persisted and on day 13 of therapy, she developed acute right 6th nerve palsy. Head CT scan showed a right subdural hematoma. On day 15, an MRI showed a transverse sinus thrombosis. The patient was treated with dexamethasone for “presumed PTC or conceivably from her thrombosis.” The patient improved after dexamethasone. On day 20, an LP confirmed elevated pressure of 520 mm water, which persisted on day 21, at 470 mm water. A shunt was placed with some improvement in headache and neurologic exam. On day 28, the patient developed bilateral 6th nerve palsies, and head CT scan showed hydrocephalus, and subsequent head CT scan showed an increase in subdural hematomas. The patient then developed bilateral pneumonias, was transferred to pediatric ICU, and was intubated for respiratory failure. She died on day 30 of therapy. Though PTC was suspected in this case, it is unlikely, as this patient had multiple other diagnoses including bilateral subdural hematomas, a transverse sinus thrombosis and hydrocephalus as cause for her symptoms.
Case 8
A 14 year-old boy developed severe muscular pain and headache on day 14 of ATRA therapy, prompting brief discontinuation of therapy. His symptoms were attributed to PTC. Therapy was resumed two days later at 75% dose then decreased to 50% dose. In this case, there was insufficient evidence to make a diagnosis of PTC, and this may instead be simply ATRA-related headache.
Summary of all cases
Three of the 188 patients developed “probable” PTC during induction therapy with ATRA, with an additional one patient with “possible” PTC. In addition, one patient of the 101 receiving maintenance ATRA developed “probable” PTC during maintenance. In total, of the 240 patients who received ATRA either during induction or maintenance there were 4 patients with “probable” PTC (1.7%). In three additional cases during induction, there was a clinical suspicion for PTC; however, upon review, PTC was “unlikely” as alternate diagnoses were more likely (Table 2).
Table 2.
Characteristics of patients with suspected pseudotumor cerebri (ND: Not documented).
| Patient | Age | Gender | Day # of ATRA symptoms began |
Headache | Papilledema | Neuroimaging | LP | WBC | Hb | Plt | Diagnosis category |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 4 | Yes | Yes | Normal | ND | 4.5 | 7 | 12 | Probable |
| 2 | 19 | M | 9 | Yes | Yes | ND | 420 mm H2O |
550 | 2.1 | 19 | Probable |
| 3 | 11 | M | 13 | ND | Yes | Normal | “Increased” pressure |
1.6 | 7.2 | 77 | Probable |
| 4 | 15 | F | 19 | Yes | ND | Normal | “Increased” pressure |
1.1 | 8.2 | 11 | Probable |
| 5 | 24 | F | 2 | Yes | Yes | ND | ND | 3.6 | 11.8 | 77 | Possible |
| 6 | 11 | F | 4 | Yes | ND | Normal | ND | 1.2 | 9.8 | 20 | Unlikely |
| 7 | 4 | F | 2 | Yes | ND | Abnormal | 520 mm H20 |
6 | 8.8 | 24 | Unlikely |
| 8 | 14 | M | 14 | Yes | ND | ND | ND | 1.1 | 7.2 | 14 | Unlikely |
DISCUSSION
Our findings indicate that PTC was suspected in 3.3% of patients who received ATRA on I0129. However, upon detailed review of individual cases, the diagnosis was determined to be unlikely in a subset of these patients due to lack of sufficient diagnostic criteria or presence of a more likely alternate diagnosis. Overall, only 1.7% of patients receiving ATRA either during induction or maintenance developed “probable” PTC by the criteria we have proposed. The cases of “probable” PTC that we have described were sufficiently symptomatic for this diagnosis to be evaluated or considered. Ultimately, we do not know the true incidence of PTC on I0129, given that not every patient with headache receiving ATRA underwent neuroimaging, LP, and ophthalmic exam, and therefore, we may be underestimating the true incidence of this complication.
PTC has been described in single case reports as an adverse effect of ATRA therapy, but its true incidence is unknown. Some publications have focused on development of PTC in the setting of drug interactions between ATRA and agents such as voriconazole or fluconazole7,8. The largest series of patients (n= 32) in which PTC was characterized in newly diagnosed APL included three consecutive Programa Espanol de Tratamientos en Hematologia (PETHEMA) group trials: LPA96, LPA99, and LPA200531, and was published in abstract form only. The authors diagnosed PTC “in the presence of signs and symptoms of intracranial hypertension without clinical or radiological evidence of infective or space occupying lesions,” without specifying further regarding diagnostic criteria within the abstract. They reported a PTC incidence of 3% in APL patients receiving ATRA, with the highest incidence amongst the youngest patients31. Ten of the 124 pediatric patients (8%) on the multicentric Gruppo Italiano per le Malattie Ematologiche dell'Adulto (GIMEMA)-Italian Pediatric Hematology and Oncology Group (AIEOP) "AIDA" (ATRA and idarubicin) trial developed PTC32. Similarly, de Botton et al. reported 5 of 31 pediatric patients (16%) on the APL93 trial developed PTC33. However, the exact criteria used for the PTC diagnosis and the details of individual PTC cases are not available within these publications32,33. Ultimately, owing to the higher observed incidence of adverse effects including PTC in children, most pediatric oncologists begin ATRA at a lower dose of 25 mg/m2 per day for patients below age 1833.
In a recent case report of PTC during ATRA therapy, Holmes et al. performed a systematic review of peer-reviewed publications using Medical Subject Headings (MeSH), PubMed/Medline, and Google Scholar databases, as well as examining the bibliographies of each article for additional references. Including the case presented, the authors identified 23 patients with PTC during ATRA treatment for APL, 11 of whom were adults8–27,34,35. Since that publication, an additional three case reports and one case series have been published4,28,29,36. Compiling all these patients and our four probable PTC patients (n= 35), the mean age at diagnosis of PTC was 23.5 years with a median age of 18 years, with a female to male ratio of 1.03:14,10,28,29,36. The mean age of diagnosis amongst these case reports may be an overestimate due to publication bias, as PTC is considered a more common complication in the pediatric population, as seen in our series. The median time to the diagnosis of PTC after initiation of ATRA, when occurring during induction (n= 28), was 14.8 days (range 1–35 days). PTC also occurred during consolidation in two cases and maintenance in seven cases (occurring during both induction and maintenance for 2 patients and only during maintenance for 5 patients)4,10,28,29,36. Regarding treatment, ATRA was held in 30 of the 35 cases (86%) (in some cases after initial attempts to dose reduce), and in three additional cases after other therapeutic interventions failed4,10,28,29,36. In 8 of the 30 cases (27%) where ATRA was held, PTC resolved without further treatment. In the other cases, signs and symptoms resolved following therapeutic LPs and/or the use of medications, such as mannitol, glycerin, acetazolamide, topiramate, corticosteroids, and analgesics4,10,28,29. The most recent report of PTC in APL discussed the utility of topirimate in patients failing acetazolamide36. Interestingly, this most recent report described a high incidence of PTC (50%) in the setting of dual differentiation therapy with ATRA and arsenic trioxide (ATO) (5 of 10 patients). The small numbers limit generalizability of these findings36. Importantly, PTC was not reported in any of the landmark clinical trials combining ATRA with ATO37–39.
In the setting of APL, there are numerous limitations to establishing the diagnosis of PTC; specifically, there are safety concerns in obtaining an LP in the setting of thrombocytopenia and coagulopathy that occur frequently in this disease. Further, there are concerns for introduction of circulating leukemia cells into the CSF. Notably, in the compiled 35 PTC patients receiving ATRA therapy, only 26 (74%) underwent a LP and in only 17 patients (49%) did the LP opening pressure meet modern diagnostic criteria for PTC4,10,28,29,36.
Due to these limitations, we propose modification of diagnostic criteria for the APL population to account for the risks of performing an LP. Our suggested modified criteria are based on clinical experience; due to rarity of PTC in APL, it will likely not be feasible to validate these revised criteria. In all patients, diagnostic criteria should include the presence of papilledema, with a normal neurologic exam except for cranial nerve abnormalities. We advise a formal neurology consultation. All patients should have neuroimaging that reveals no evidence of hydrocephalus, mass, or structural lesion, and no abnormal meningeal enhancement on MRI or contrast-enhanced CT. It is imperative that neuroimaging be performed prior to LP. Regarding CSF examination, this should be performed only when safe (i.e. in patients without severe thrombocytopenia, without high percent circulating leukemic cells, and without active coagulopathy). The CSF criteria for diagnosis are the same for patients without APL in that composition of CSF (sugar, protein, culture, and cytology) should be normal and elevated opening pressure should be documented. In patients who cannot safely get an LP performed, but satisfy all other criteria, a diagnosis of “probable” PTC should be made, and the patients should be treated appropriately.
Regarding management, multiple strategies have been utilized. In the majority of published cases, ATRA therapy is held, and often reinitiated once symptoms have resolved, although at a lower dose. Symptomatic relief may be accomplished by the administration of analgesics. Other therapies that have been utilized include corticosteroids, acetazolamide, mannitol, glycerin, topiramate, or therapeutic LP. Data are insufficient to suggest superiority of any specific approach given that all reports are anecdotal.
Limitations of this series include its retrospective nature and limited information regarding details of patient presentations such as details of CSF composition and pressure. However, given our concordance with other series, we feel that the reported cases of PTC captured within this patient population are a close reflection the true incidence of this complication.
CONCLUSIONS
In summary, PTC is a rare complication of ATRA therapy, which definitely appears to be more frequent in children and tends to develop shortly after ATRA initiation, most commonly during induction. Diagnostic challenges include the frequent inability to obtain a lumbar puncture in the setting of coagulopathy or severe thrombocytopenia, in which case we propose a provisional diagnosis of “probable” PTC. Clinicians should be vigilant regarding this possible complication of ATRA therapy.
CLINICAL PRACTICE POINTS.
PTC is a rare complication of ATRA therapy which is more common in children
Neurologic consultation should be considered when this diagnosis is suspected
PTC generally resolves with combination of holding ATRA and supportive measures, and ATRA may often be resumed
Acknowledgments
The authors acknowledge the patients for participation on this clinical trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
There are no competing interests to declare.
REFERENCES
- 1.Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82:3241–3249. [PubMed] [Google Scholar]
- 2.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. The New England journal of medicine. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 3.Warner JE, Bernstein PS, Yemelyanov A, Alder SC, Farnsworth ST, Digre KB. Vitamin A in the cerebrospinal fluid of patients with and without idiopathic intracranial hypertension. Annals of neurology. 2002;52:647–650. doi: 10.1002/ana.10377. [DOI] [PubMed] [Google Scholar]
- 4.Anoop TM, Jain N, Nair SG, Narayanan G. All-trans-retinoic acid-induced pseudotumor cerebri in acute promyelocytic leukemia. Journal of neurosciences in rural practice. 2014;5:273–275. doi: 10.4103/0976-3147.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 6.Mallery RM, Friedman DI, Liu GT. Headache and the pseudotumor cerebri syndrome. Current pain and headache reports. 2014;18:446. doi: 10.1007/s11916-014-0446-z. [DOI] [PubMed] [Google Scholar]
- 7.Dixon KS, Hassoun A. Pseudotumor cerebri due to the potentiation of alltrans retinoic acid by voriconazole. Journal of the American Pharmacists Association : JAPhA. 2010;50:742–744. doi: 10.1331/JAPhA.2010.09133. [DOI] [PubMed] [Google Scholar]
- 8.Vanier KL, Mattiussi AJ, Johnston DL. Interaction of all-trans-retinoic acid with fluconazole in acute promyelocytic leukemia. Journal of pediatric hematology/oncology. 2003;25:403–404. doi: 10.1097/00043426-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Visani G, Bontempo G, Manfroi S, Pazzaglia A, D'Alessandro R, Tura S. All-trans- retinoic acid and pseudotumor cerebri in a young adult with acute promyelocytic leukemia: a possible disease association. Haematologica. 1996;81:152–154. [PubMed] [Google Scholar]
- 10.Holmes D, Vishnu P, Dorer RK, Aboulafia DM. All-Trans Retinoic Acid-Induced Pseudotumor Cerebri during Induction Therapy for Acute Promyelocytic Leukemia: A Case Report and Literature Review. Case reports in oncological medicine. 2012;2012:313057. doi: 10.1155/2012/313057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh YC, Tang HF, Fang IM. Pseudotumor cerebri caused by all-trans-retinoic acid treatment for acute promyelocytic leukemia. Japanese journal of ophthalmology. 2006;50:295–296. doi: 10.1007/s10384-005-0300-2. [DOI] [PubMed] [Google Scholar]
- 12.Naderi S, Nukala S, Marruenda F, Kudarvalli P, Koduri PR. Pseudotumour cerebri in acute promyelocytic leukemia: improvement despite continued ATRA therapy. Annals of hematology. 1999;78:333–334. doi: 10.1007/s002770050524. [DOI] [PubMed] [Google Scholar]
- 13.Selleri C, Pane F, Notaro R, et al. All-trans-retinoic acid (ATRA) responsive skin relapses of acute promyelocytic leukaemia followed by ATRA-induced pseudotumour cerebri. British journal of haematology. 1996;92:937–940. doi: 10.1046/j.1365-2141.1996.411948.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen HY, Tsai RK, Huang SM. ATRA-induced pseudotumour cerebri--one case report. The Kaohsiung journal of medical sciences. 1998;14:58–60. [PubMed] [Google Scholar]
- 15.Schroeter T, Lanvers C, Herding H, Suttorp M. Pseudotumor cerebri induced by all-trans-retinoic acid in a child treated for acute promyelocytic leukemia. Medical and pediatric oncology. 2000;34:284–286. doi: 10.1002/(sici)1096-911x(200004)34:4<284::aid-mpo16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Guirgis MF, Lueder GT. Intracranial hypertension secondary to all-trans retinoic acid treatment for leukemia: diagnosis and management. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2003;7:432–434. doi: 10.1016/j.jaapos.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Naithani R, Kumar R, Mishra P. Pseudotumor cerebri in a child in early phase of induction therapy for APL with ATRA. Indian journal of pediatrics. 2009;76:439–440. doi: 10.1007/s12098-009-0134-x. [DOI] [PubMed] [Google Scholar]
- 18.Tiamkao S, Sirijirachai C. Pseudotumor cerebri caused by all-trans-retinoic acid: a case report. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2000;83:1420–1423. [PubMed] [Google Scholar]
- 19.Ganguly S. All-trans retinoic acid related headache in patients with acute promyelocytic leukemia: prophylaxis and treatment with acetazolamide. Leukemia research. 2005;29:721. doi: 10.1016/j.leukres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Colucciello M. Pseudotumor cerebri induced by all-trans retinoic acid treatment of acute promyelocytic leukemia. Archives of ophthalmology. 2003;121:1064–1065. doi: 10.1001/archopht.121.7.1064. [DOI] [PubMed] [Google Scholar]
- 21.Varadi G, Lossos A, Or R, Kapelushnik J, Nagler A. Successful allogeneic bone marrow transplantation in a patient with ATRA-induced pseudotumor cerebri. American journal of hematology. 1995;50:147–148. doi: 10.1002/ajh.2830500215. [DOI] [PubMed] [Google Scholar]
- 22.Gallipoli P, Drummond MW. Pseudotumour cerebri as a manageable side effect of prolonged all-trans retinoic acid therapy in an adult patient with acute promyelocytic leukaemia. European journal of haematology. 2009;82:242–243. doi: 10.1111/j.1600-0609.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 23.Machner B, Neppert B, Paulsen M, Hofmann C, Sander T, Helmchen C. Pseudotumor cerebri as a reversible side effect of all-trans retinoic acid treatment in acute promyelocytic leukaemia. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2008;15:e68–e69. doi: 10.1111/j.1468-1331.2008.02152.x. [DOI] [PubMed] [Google Scholar]
- 24.Sano F, Tsuji K, Kunika N, et al. Pseudotumor cerebri in a patient with acute promyelocytic leukemia during treatment with all-trans retinoic acid. Internal medicine (Tokyo, Japan) 1998;37:546–549. doi: 10.2169/internalmedicine.37.546. [DOI] [PubMed] [Google Scholar]
- 25.Mishra SK, Melinkeri SR, Dabadghao S. Benign thymic hyperplasia after chemotherapy for acute myeloid leukemia. European journal of haematology. 2001;67:252–254. doi: 10.1034/j.1600-0609.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- 26.Decaudin D, Adams D, Naccache P, Castagna L, Munck JN. Maintained all-trans retinoic acid therapy in a patient with pseudotumour cerebri despite aggravated symptoms. Leukemia & lymphoma. 1997;27:373–374. doi: 10.3109/10428199709059694. [DOI] [PubMed] [Google Scholar]
- 27.Jeddi R, Kacem K, Ben Lakhal R, et al. Pseudotumor cerebri with all-trans retinoic acid. A case report. La Tunisie medicale. 2006;84:827–829. [PubMed] [Google Scholar]
- 28.Labrador J, Puig N, Ortin A, Gutierrez NC, Gonzalez-Diaz M. Multiple cranial neuropathy and intracranial hypertension associated with all-trans retinoic acid treatment in a young adult patient with acute promyelocytic leukemia. International journal of hematology. 2012;96:383–385. doi: 10.1007/s12185-012-1134-6. [DOI] [PubMed] [Google Scholar]
- 29.Rasul FT, Toma AK, Khan AA, Plant GT, Watkins LD. Pseudotumor cerebri presenting with visual failure in promyelocytic leukemia: a case report. Journal of medical case reports. 2012;6:408. doi: 10.1186/1752-1947-6-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douer D, Zickl LN, Schiffer CA, et al. All-trans retinoic acid and late relapses in acute promyelocytic leukemia: very long-term follow-up of the North American Intergroup Study I0129. Leukemia research. 2013;37:795–801. doi: 10.1016/j.leukres.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montesinos P, Vellenga E, Holowiecka A, et al. Incidence, Outcome and Risk Factors of Pseudotumor Cerebri after All-Trans Retinoic Acid and Anthracycline- Based Chemotherapy in Patients with Acute Promyelocytic Leukemia. American Society of Hematology meeting. 2008:2992. [Google Scholar]
- 32.Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–453. doi: 10.1182/blood-2004-05-1971. [DOI] [PubMed] [Google Scholar]
- 33.de Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1404–1412. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Smith MA, Adamson PC, Balis FM, et al. Phase I and pharmacokinetic evaluation of all-trans-retinoic acid in pediatric patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10:1666–1673. doi: 10.1200/JCO.1992.10.11.1666. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto O, Yoshinari M, Rikiishi T, et al. Hypercalcemia due to all-trans retinoic acid therapy for acute promyelocytic leukemia: a case report of effective treatment with bisphosphonate. Pediatrics international : official journal of the Japan Pediatric Society. 2001;43:688–690. doi: 10.1046/j.1442-200x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith MB, Griffiths EA, Thompson JE, Wang ES, Wetzler M, Freyer CW. High pseudotumor cerebri incidence in tretinoin and arsenic treated acute promyelocytic leukemia and the role of topiramate after acetazolamide failure. Leukemia research reports. 2014;3:62–66. doi: 10.1016/j.lrr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. The New England journal of medicine. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 38.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]