Abstract
Background
The nematodes Dirofilaria immitis and D. repens are enzootic in Greece. In the light of evidence of dirofilariosis spreading to new areas around the world, the aim of the present study was to update and enrich the current knowledge on the prevalence of Dirofilaria infections in dogs in Greece, to assess the risk factors of heartworm infection, and to initiate the drawing of the epizootiological map of canine dirofilariosis, investigating Dirofilaria infections in five locations along the north-south axis of the country, i.e. municipalities of Thessaloniki, Larissa, Achaia, Attica and Heraklion, associated with the five largest urban centres of Greece.
Methods
Blood samples collected from 750 dogs in total, were examined by the modified Knott’s method and by serology. A questionnaire including information about each examined dog was filled in and statistical analysis of the results was performed using the Chi-square test and a Binary Multiple Univariate Generalized Linear Model.
Results
In total, 31 (4.1 %) out of 750 examined animals were found positive for D. immitis by any of the tests applied (Knott’s method and serological examination). Moreover, microfilariae of D. repens and Acanthocheilonema reconditum were detected by the Knott’s method in 17 (2.3 %) and 10 (1.3 %) of the animals, respectively. At the municipality level, the prevalence of infection was 14, 7, 5.3, 0.7 and 0 % for D. immitis, 1, 2, 8.7, 0.3 and 0 % for D. repens, and 0, 3, 2.7, 0.7 and 1 % for A. reconditum in Thessaloniki, Larissa, Achaia, Attica and Heraklion, respectively. In addition, in three dogs (one each in Thessaloniki, Achaia and Attica) mixed D. immitis - D. repens infections were detected by the Knott’s method. The area of the country, dog’s usage and age were determined as risk factors for heartworm infection.
Conclusions
Northern areas of Greece have higher Dirofilaria prevalence and the prevalence in a western province (Achaia) is recorded for the first time. The mosquito population dynamics recorded in the past is likely to play an important role in the distribution of Dirofilaria infections in Greece, and needs further investigation. Similarly, the role of wild reservoirs of filarial parasites in different areas needs to be clarified. Promotion by veterinarians of preventive treatment and compliance by pet owners is essential in all parts of Greece, regardless of the recorded prevalence of infection.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1797-6) contains supplementary material, which is available to authorized users.
Keywords: Dirofilaria immitis, Dirofilaria repens, Acanthocheilonema reconditum, Dog, Greece, Prevalence, Risk factors
Background
Dirofilaria infections in dogs are caused by the filarial nematode parasites Dirofilaria immitis and Dirofilaria repens, both transmitted by the bite of infected mosquitoes. Dirofilaria immitis, also known as “heartworm”, is the causative agent of dirofilariosis (heartworm disease), one of the most serious parasitic diseases affecting dogs and some other carnivores [1], while D. repens parasitizes the subcutaneous and intramuscular connective tissue [2]. Moreover, these parasites, and in particular D. repens, have zoonotic implications: D. immitis is the causative agent of pneumonic dirofilariosis and D. repens can cause subcutaneous or ocular dirofilariosis in humans [3].
Both D. immitis and D. repens are enzootic in Greece. There are only a few relevant, and, in most cases, outdated epizootiological surveys, most of them conducted in areas of northern Greece, revealing a prevalence ranging from 5 to 34 % and from 18 to 33 % for D. immitis and D. repens, respectively [4–8]. Only two surveys have been carried out in other areas of the country, i.e. in Attica and Crete (southern Greece), where both species were much less prevalent, i.e. 0.7 and 0 % for D. immitis and 0.4 and 0.7 % for D. repens in Attica and Crete, respectively [9, 10].
There is also some anecdotal, often contradictory information derived from veterinarians throughout the country, suggesting that heartworm infection is non-existent in some areas or, on the contrary, that cases were confirmed in areas considered as non-enzootic so far. At the same time, there is recent research activity in Europe, introducing the idea of “spreading dirofilariosis” towards northern areas, or to areas that were not considered enzootic until recently [11–15].
In this context, the aim of the present study was to update and enrich the current knowledge on the prevalence of Dirofilaria infections in dogs in Greece, to confirm or reject the anecdotal information about the prevalence of heartworm disease in different areas of the country, to assess the risk factors of heartworm infection, and finally, to establish the basis of the current epizootiological map of canine dirofilariosis in Greece.
Methods
Study areas
In order to form a basic and representative picture of the prevalence of filarial infection in different areas of Greece, five locations, distributed along the north-south axis of the country, associated with the five biggest urban centres were selected. These were the municipalities of Thessaloniki, Larissa, Attica, Achaia and Heraklion, where the cities of Thessaloniki (40°38′N, 22°56′E), Larissa (39°38′N, 22°25′E), Patras (38°25′N, 21°73′E), Athens (37°97′N, 23°73′E) and Heraklion (35°19′N, 25°8′E) are situated.
Sampling population
The number of animals to be examined was determined by the number of veterinarians and estimated number of dogs in the area and according to the existing information about the prevalence of Dirofilaria spp. in the area (stratified sampling) [16]. The dogs included in the survey were all owned animals, older than 18 months and were not receiving any kind of preventive treatment for dirofilariosis or macrocyclic lactones for other reason. The dogs were sampled during a routine visit to the veterinary practice or were presented by their owners in order to participate in the survey, after notification by the veterinarian. In total, 750 dogs were examined, i.e. 100 from Thessaloniki, 100 from Larissa, 150 from Achaia, 300 from Athens and 100 from Heraklion.
A questionnaire was designed, including information about the age, gender, breed, hair length, movement to other areas and lifestyle (outdoor living, usage) of the dog, and the environment (type of vegetation and existence of ponds or wetlands).
Samples and examinations
All animals were examined with the consent of their owner. From each dog, 2 ml of blood was collected from a peripheral vein (jugular, cephalic or saphenous) in EDTA tube. The samples were kept refrigerated (4 °C), and sent within a maximum of 5 days to the Laboratory of Parasitology and Parasitic Diseases (Aristotle University of Thessaloniki).
All the samples were examined by two methods: the modified Knott’s method [17] for the detection and identification of microfilariae (first stage larvae, L1) and the serological commercial kit Pet Check® (IDEXX), for the detection of adult D. immitis antigen. The microfilariae retrieved by the Knott’s test were identified under light microscopy at 100× and 400× magnifications on the basis of their morphometric (i.e. length and width) and morphological (i.e. anterior and posterior extremities) features [17–19].
Statistical analysis
Statistical analysis was performed for D. immitis infections as they are the most important from a clinical point of view. The risk factors affecting D. immitis occurrence were determined using the Chi-square test of independence between the D. immitis test results (positive or negative) and a variety of animal characteristics [20]. The Chi-square testing was securely performed as the sample size was big enough (n = 750) and thus the average expected frequency was at least 10 for the significance level of α = 0.01 [21]. Moreover, all the categories significant at the P < 0.2 level (risk factors) for the Chi-square test, were selected to be entered in a Binary (D. immitis infection) Multiple (many risk factors) Univariate (one output variable) Generalized Linear Model (GLM). This procedure can estimate the risk factors while allows the comparison of the percentages of the infected animals within the values of each characteristic (odds ratio). The statistical analysis was implemented using the R package version 3.2.2 [22].
Results
By the modified Knott’s method, D. immitis microfilariae were found in 19 (2.5 %) out of the 750 dogs. At municipality level, D. immitis microfilariae were found in 7 (7 %), 5 (5 %), 7 (4.6 %), 0 and 0 of the dogs from Thessaloniki, Larissa, Achaia, Attica and Heraklion, respectively. Dirofilaria repens microfilariae were recovered in 17 (2.3 %) of the dogs, i.e. in 1 (1 %), 2 (2 %), 13 (8.7 %), 1 (0.3 %) and 0 of the dogs from Thessaloniki, Larissa, Achaia, Attica and Heraklion, respectively. Moreover, Acanthocheilonama reconditum microfilariae were found in 10 (1.3 %) of the dogs in total and 0, 3 (3 %), 4 (2.7 %), 2 (0.7 %) and 1 (1 %) of the dogs from Thessaloniki, Larissa, Achaia, Attica and Heraklion, respectively. In addition, mixed D. immitis and D. repens infections were revealed by the Knott’s method overall in 3 cases, i.e. in 1 (1 %), 1 (0.7 %), and 1 (0.3 %) of the dogs from Thessaloniki, Achaia and Attica, respectively. The results of the Knott’s method are presented in details in Table 1.
Table 1.
Microfilariae detected by the modified Knott’s method in different areas of Greece
| Municipality (No. of dogs examined) | No. of dogs with D.i. (% ± CI) | No. of dogs with D.r. (% ± CI) | No. of dogs with A.r. (% ± CI) | No. of dogs with D.i. + D.r. (% ± CI) |
|---|---|---|---|---|
| Thessaloniki (n = 100) | 7 (7.0 ± 5.0) | 1 (1.0 ± 2.0) | 0 | 1 (1.0 ± 2.0) |
| Larissa (n = 100) | 5 (5.0 ± 4.3) | 2 (2.0 ± 2.7) | 3 (3.0 ± 3.3) | 0 |
| Achaia (n = 150) | 7 (4.7 ± 3.5) | 13 (8.7 ± 4.5) | 4 (2.7 ± 2.6) | 1 (0.7 ± 1.3) |
| Attica (n = 300) | 0 | 1 (0.3 ± 0.7) | 2 (0.7 ± 0.9) | 1 (0.3 ± 0.7) |
| Heraklion (n = 100) | 0 | 0 | 1 (1.0 ± 2.0) | 0 |
| Total (n = 750) | 19 (2.5 ± 1.1) | 17 (2.3 ± 1) | 10 (1.3 ± 0.8) | 3 (0.4 ± 0.5) |
Abbreviations: D.i. D. immitis, D.r. D. repens, A.r. Acanthocheilonema reconditum, D.i. + D.r. D. immitis and D. repens mixed infections; CI 95 % confidence interval
Dirofilaria immitis antigen was detected by the serological examination in 28 (3.7 %) of the 750 dogs. At municipality level, 14 (14 %), 4 (4 %), 8 (5.3 %), 2 (0.7 %) and 0 dogs were found positive from Thessaloniki, Larissa, Achaia, Attica and Heraklion, respectively. However, three dogs from Larissa, although found negative in serology, were positive for D. immitis microfilariae by the Knott’s method, subsequently increasing the number of positive dogs to 7 (7 %) in Larissa and to 31 (4.1 %) overall (Table 2). In 19 out of 31 D. immitis positive samples the Knott’s method was in accordance with the serological test. However, in 9 samples, although D. immitis antigen was detected in serology, no microfilariae were found. The overall prevalence for each filarial species, in each location of the survey is shown in Fig. 1. None of the positive dogs originated from or had moved to other areas of the country.
Table 2.
Dogs positive for Dirofilaria immitis by at least one method (Knott’s, serology) in different areas of Greece
| Municipality | No. of dogs examined | No. of D. immitis-positive dogs (% ± CI) |
|---|---|---|
| Thessaloniki | 100 | 14 (14.0 ± 6.8) |
| Larissa | 100 | 7 (7.0 ± 5.0) |
| Achaia | 150 | 8 (5.3 ± 3.6) |
| Attica | 300 | 2 (0.7 ± 0.9) |
| Heraklion | 100 | 0 |
| Total | 750 | 31 (4.1 ± 1.4) |
Fig. 1.
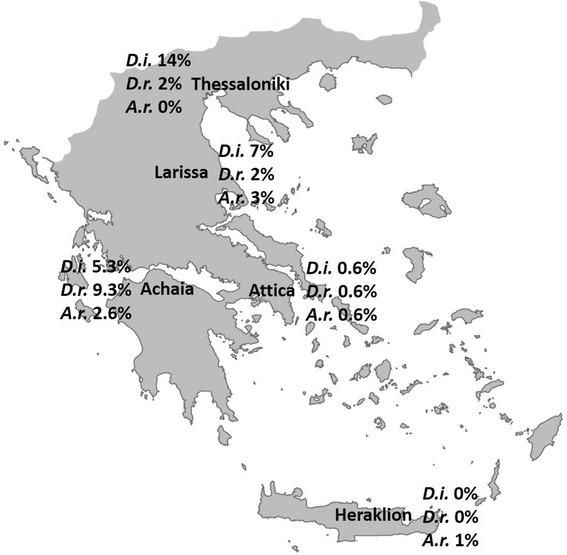
The map of Greece showing the areas of investigation (Municipalities of Thessaloniki, Larissa, Achaia, Attica and Heraklion) and the prevalence of Dirofilaria immitis (D.i., as found both in Knott’s and serology), D. repens (D.r.) and Acanthocheilonema reconditum (A.r.) infections in each area
Regarding the statistical analysis of the risk factors of D. immitis infection (Table 3), the municipality where the dog lived, its usage and its age were the most important risk factors (Chi-square test of independence for municipality: χ2 = 40.22, df = 4, P < 0.001; for usage: χ2 = 27.51, df = 7, P < 0.001; for age: χ2 = 6.09, df = 2, P = 0.048). On the other hand, factors gender, breed, hair length, body size, living together with other animals, water collections and type of vegetation in the area were not associated with higher risk of infection. The statistically significant characteristics by the Chi-square test were entered in the multiple GLM showing the same statistically significant results (Table 3).
Table 3.
Assessment of risk factors of Dirofilaria immitis infection and results of multiple Generalized Linear Model (GLM) for the risk factors driving Dirofilaria immitis
| Variable | Odds ratio | 95 % CI | GLM (multiple) P-value |
|---|---|---|---|
| City | |||
| Thessaloniki vs Attica | 26.8 | 6.4–185.5 | < 0.001*** |
| Larisa vs Attica | 11.9 | 2.6–85.5 | 0.003** |
| Achaia vs Attica | 6.0 | 1.3–42.1 | 0.033* |
| Heraklion vs Attica | ns | 0.989 | |
| Age category | |||
| 2–7 vs ≤ 2 | 6.4 | 1.6–43.0 | 0.019* |
| > 7 vs ≤ 2 | 9.5 | 2.2–67.4 | 0.007** |
| Usage | |||
| Guard vs Pet | 8.2 | 2.4–33.0 | 0.001** |
| Hunter vs Pet | 5.3 | 1.6–20.8 | 0.009** |
| Dogs | |||
| Yes vs No | ns | 0.331 | |
| Day living | |||
| Outside vs Inside | ns | 0.356 | |
| Night living | |||
| Outside vs Inside | ns | 0.733 | |
| Outside life | |||
| Partially vs None | ns | 0.464 | |
| Exclusively vs None | ns | 0.363 | |
| Water pools | |||
| Yes vs No | ns | 0.910 | |
*P < 0.05; **P < 0.01; ***P < 0.001
According to this model, a dog living in Thessaloniki, Larissa or Achaia was 26.8, 11.9 and 6 times, respectively, more likely to be infected than a dog living in Attica. Moreover, guard and hunting dogs were almost 8.2 and 5.3 times, respectively, more likely to be infected than pet dogs. Regarding the age, a dog between 2 and 7 years and one older than 7 years were 6.4 and 9.5 times, respectively, more likely to be infected than a dog of an age up to 2 years.
Discussion
Many European countries are enzootic for Dirofilaria infections [23]. During recent years, filarial nematodes seem to have spread in areas previously considered free of these parasites [14]. The expansion of distribution of filarial parasites is attributed to climate change, the increasing abundance of mosquitoes, the expanding movement of the main hosts, i.e. microfilaraemic dogs between different areas and the abundance of wild reservoirs [11]. In this context, it is important to monitor the prevalence and risk factors of filarial infections both in enzootic and in non-enzootic areas in order to ensure a minimum of surveillance of these pathogens that are of both veterinary and medical interest. In Europe, the areas with high prevalence in filarial infections in dogs are located in the south, e.g. Portugal (15.1 %), Spain (2–35 %), southern France (15 %) and Italy (2.3– > 50 % along the Po River Valley) [11, 14, 24–26]. Furthermore, reports of autochthonous cases and prevalence of infection from previously non-enzootic areas are being constantly reported [14].
The findings of the present study are in accordance with previous reports [4–10] and confirm that the prevalence of dirofilariosis is higher in northern than in southern areas of Greece. Our results suggest that the percentage of infected animals lowers progressively towards the south. Indeed, D. immitis was found in 14 % of the dogs in the northernmost location (Thessaloniki) and was recorded progressively lower in the locations with smaller latitude, i.e. 7 % in Larissa, 5.3 % in Achaia, 0.6 % in Attica and 0 % in Heraklion. Despite there being a recent confirmed autochthonous case of heartworm infection in a dog in Heraklion (Diakou, unpublished data), it seems that the disease is practically non-existent, at least to date, as both in the present study and in the survey of sheltered stray dogs 20 years ago [10], no D. immitis-positive dog was recorded. The prevalence found in Achaia is a new record, in the sense that, to date, filarial infections have never been investigated in this area of Greece (western Greece). However, our communication with the local vets revealed that at least since the 1990s, heartworm infections have been present in the area.
Similarly, D. repens infection was also found in higher prevalence in northern and western areas of the country, while in southern areas the occurrence of the infection was very low or even non-existent. Interestingly, there is a recent report of 8 cases of human ocular dirofilariosis due to D. repens, in which all patients were residents of northwest Greece [27]. Although this parasite is of known endemicity in areas of southern Europe, it has been shown that infection rates are increasing, both in well-known endemic areas and in northern and eastern areas formerly considered free of the infection [15, 28].
Microfilariae of A. reconditum were also found in different areas in the present survey. This is a filarial nematode with global distribution and fleas or lice as intermediate hosts. Acanthocheilonema reconditum adults are found beneath the subcutaneous tissues mainly in the limbs and back, and are considered less pathogenic than other filarial nematodes of the dog [29]. The distribution of A. reconditum infections as found in the present study reflects the different epizootiological characteristics of this parasite, as its prevalence did not follow the respective for Dirofilaria sp. and remained in relatively low levels in all areas (0–3 %). This species has been found in previous surveys in Greece, ranging from 0 to 12 % in different areas [4, 6, 7, 9, 10]. It is worth noting that the highest prevalence (12 %) recorded to date in Greece, was found in sheltered dogs in Heraklion, Crete [10], a fact that could be attributed to the critical role of reservoir dogs in a confined environment, as the closeness to a microfilaraemic animal is crucial for the transmission of infection, which is due to the nature of intermediate hosts [29].
The discordant result of nine samples being antigen positive for D. immitis but negative in the Knott’s method was rather expected, as occult heartworm infections are common [30]. The risk of some of these dogs being infected with Angiostrongylus vasorum and thus giving a cross reaction to the serological test used here [31] can be considered minimum, as A. vasorum is so far considered very rare in Greece [32].
Conversely, in three samples although D. immitis microfilariae were detected in the Knott’s method, serology was negative. There are quite a few similar reports of false negative serological tests in the literature [24, 33]. Various factors can lead to a false negative serological test [29, 33], one of them being the antigen-antibody complex formation, that in some cases occurs to such an extent that it does not leave enough antigen circulating to be detected by the serological tests [34]. Consequently, a combination of these two diagnostic tests (Knott and serology) is recommended for higher sensitivity in heartworm diagnosis.
Among the risk factors evaluated here, the area where the dog lived (municipality) was the most determinative factor. The usage of the animal was also very important, thus, guard and hunting dogs were at higher risk of infection, probably as a consequence of the outdoor lifestyle and higher exposure to mosquito bites. Age was also identified as an important risk factor, most likely related to the accumulation of transmission periods and, subsequently, opportunities for an infection to occur in hosts that are not under preventive treatment. Age has been found to be among the recognised risk factors for filarial infection in dogs [35, 36]. However, in some similar studies, no risk factors for filarial infection could be identified [26, 37].
The results of the present study suggest that the situation of heartworm disease in the country has remained quite similar in recent decades, with D. immitis and D. repens infections being more prevalent in the north compared to the rest of the country, while there is evidence, that in western areas like Achaia, there is an important prevalence of filarial infections. The situation is not the same in Italy, where, until the end of the 1980s, heartworm infection was also mainly a problem in the northern areas of the country, but during recent years its distribution patterns have changed, as more cases of D. immitis infection were detected in the south of the country [12].
The distribution of Dirofilaria infections in Greece, at first glance, seems unexpected and odd for several reasons: first of all, obviously, the definitive hosts (mainly dogs and other carnivores) as well as the vectors (mosquitoes) are present throughout the country. In addition, the mean temperatures all year round would facilitate faster extrinsic development and a higher number of transmission cycles in the southern parts than in the northern parts of the country. In fact, according a Dirofilaria Development Unit (DDU) -based forecast model applied to different areas of Europe, a whole extra month of transmission (i.e. November) is expected in southern Greece, compared to the rest of the areas examined [15]. Attica and the Island of Crete (where the municipality of Heraklion is located) in particular, are among the areas with the second highest yearly average predicted number of Dirofilaria generations in Europe, i.e. 8–10 generations, according to the Linear Kriging interpolation [23]. Thus, the recorded distribution of Dirofilaria prevalence in northern and southern areas of Greece is contradictory to what is expected based on the models mentioned above, and requires further attempts to explain.
It is known that although temperature is an important factor for the establishment and the prevalence of infection in an area, there are other factors that influence the transmission risk of Dirofilaria spp. [38] and, of course, all of these factors are linked to the availability and abundance of vectors (mosquitoes) and reservoirs (definitive hosts). Mosquito population dynamics may be essential in the transmission of pathogens [35, 39]. Mosquitoes are present in all parts of Greece but their populations are not equal, neither in terms of abundance nor stability throughout the country. Indeed, as was revealed by a CO2 traps network, sampled twice a month at 106 constant sampling sites throughout Greece, during the summer of 2011, total mosquito populations were significantly higher in northern than in southern Greece [40]. In particular, populations of Culex spp. in the region of Central Macedonia were found to be 20 times higher than in the Peloponnese region and even 31 times higher than in Attica region. Also Aedes spp. populations in the region of Eastern Macedonia and Thrace, are ten times higher than in the Peloponnese region and western Greece [40].
The existence of wetlands and rice fields, as expected, seems closely related to elevated mosquito populations and, subsequently, higher risk for Dirofilaria transmission. Indeed, the bigger and most important wetlands in Greece are located in the north and west of the country [41]. Thus, the map of the wetlands in the country interestingly matches the map of dirofilariosis as illustrated in the present study. Similarly, 91 % of the total rice cultivation areas on a national level are located in northern Greece [42]. Rice field abundance was acknowledged as one of the possible elements contributing to the higher Dirofilaria infection rates in certain areas of Portugal [24].
Another factor that should be considered regarding the heartworm geographical distribution in Greece, is the role of wild canids. Although little information is available on the microfilaraemia of these animals, it cannot be excluded that they may contribute to the spreading of the infection [43]. As populations of jackals and wolves are widely distributed in the central and northern part of the country (jackals are also present in Peloponnese), but absent elsewhere [44, 45] the role of these species in the prevalence configuration of heartworm infections in Greece is worth investigating.
Conclusions
The results of the present study suggest that Dirofilaria infections in Greece are more prevalent in the northern areas compared to the southern areas and that the occurrence of Dirofilaria infections in the west of the country is not negligible. Moreover, A. reconditum infections have been recorded in low prevalence in various areas of the country. Targeted investigations about the role of mosquito populations, and definitive hosts other than the dog, would provide evidence based interpretations for the Dirofilaria distribution in Greece. Finally, it is necessary to emphasize that prevention is essential because of both veterinary and medical importance of Dirofilaria infections, and should be applied in all dogs and cats in Greece, regardless of the prevalence of infection, considering that conditions permit the completion of the filarial life cycle in all areas of the country.
Acknowledgments
The authors express their acknowledgments to Mr. Stefanos Papaioannou, Sales Manager at Petline SA and to IDEXX for providing the kit Pet Check®. The authors also thank all the collaborators from ELANCO for their valuable help in the communication with the veterinarians, and to Ms Anne Beavan for editing the English language. Finally, the authors would like to express their sincerest thanks and appreciation to all the veterinarians that participated in the survey. Without their contribution, this survey would not have been realised.
Funding
The study was partially funded by ELANCO.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its Additional file 1.
Authors’ contributions
AD designed the study protocol, supervised the collection of dog material, participated in the examinations of samples and wrote the manuscript. EK organised the collection of dog material and participated in examinations of samples. AT performed the statistical analyses and wrote the corresponding parts of the manuscript. VG and NS participated in the protocol design, facilitated the communication with the veterinarians that provided the samples and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The study was supported by ELANCO, of which VG and NS are employees.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All animals were examined with the consent of their owner. The mild procedure of blood sampling was performed taken into consideration the Directive 2010/63/EU in Europe.
Abbreviations
- DDU
Dirofilaria Development Unit
- GLM
Generalized Linear Model
Additional file
Data collected from the questionnaires filled in for each examined dog. (XLSX 91 kb)
Contributor Information
Anastasia Diakou, Email: diakou@vet.auth.gr.
Emmanouil Kapantaidakis, Email: kaptis88@hotmail.com.
Androniki Tamvakis, Email: atamvaki@mar.aegean.gr.
Vassilios Giannakis, Email: vgiannakis@elanco.gr.
Nina Strus, Email: strus_nina@elanco.com.
References
- 1.Bowman DD, Atkins CE. Heartworm biology, treatment, and control. Vet Clin North Am Small Anim Pract. 2009;39:1127–58. doi: 10.1016/j.cvsm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Canestri Trotti G, Pampiglione S, Rivasi F. The species of the genus Dirofilaria, Railliet & Henry, 1911. Parassitologia. 1997;39:369–74. [PubMed] [Google Scholar]
- 3.Simon F, Morchon R, Gonzalez-Miguel J, Marcos-Atxutegi C, Siles-Lucas M. What is new about animal and human dirofilariosis? Trends Parasitol. 2009;25:404–9. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Papazahariadou MG, Koutinas AF, Rallis TS, Haralabidis ST. Prevalence of microfilaraemia in episodic weakness and clinically normal dogs belonging to hunting breeds. J Helminthol. 1994;68:243–5. doi: 10.1017/S0022149X00014413. [DOI] [PubMed] [Google Scholar]
- 5.Lefkaditis M, Koukeri S, Cozma V. An endemic area of Dirofilaria immitis seropositive dogs at the eastern foothills of Mt Olympus, Northern Greece. Helminthologia. 2010;47:3–7. doi: 10.2478/s11687-010-0001-3. [DOI] [Google Scholar]
- 6.Founta A, Theodoridis Y, Frydas S, Chliounakis S. The presence of filarial parasites of dogs in Serrae Province. J Hell Vet Med Soc. 1999;50:315–20 (In Greek).
- 7.Diakou A. Epidemiological study of dog parasitosis diagnosed by blood and serological examinations. Anima. 2000;8:9–17 (In Greek).
- 8.Vakalis N, Himonas C. Human and canine dirofilariasis in Greece. Parassitologia. 1997;39:389–91. [PubMed] [Google Scholar]
- 9.Diakou A. The prevalence of canine dirofilariosis in the region of Attiki. J Hell Vet Med Soc. 2001;52:152–6 (In Greek).
- 10.Plevraki A, Savvas I, Papadopoulos B, Diakou A, Haralabidis S. Parasites of the dogs in an animal shelter in Ahlada (Heraklion) (In Greek) Stock Farm Develop. 1996;21:24–6. [Google Scholar]
- 11.Genchi C, Rinaldi L, Cascone C, Mortarino M, Cringoli G. Is heartworm disease really spreading in Europe? Vet Parasitol. 2005;133:137–48. doi: 10.1016/j.vetpar.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Otranto D, Capelli G, Genchi C. Changing distribution patterns of canine vector borne diseases in Italy: leishmaniosis vs. dirofilariosis. Parasit Vectors. 2009;2 Suppl 1:S2. [DOI] [PMC free article] [PubMed]
- 13.Giangaspero A, Marangi M, Latrofa MS, Martinelli D, Traversa D, Otranto D, Genchi C. Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol Res. 2013;112:1357–61. doi: 10.1007/s00436-012-3206-1. [DOI] [PubMed] [Google Scholar]
- 14.Morchón R, Carretón E, González-Miguel J, Mellado-Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe–new distribution trends. Front Physiol. 2012;3:196. doi: 10.3389/fphys.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genchi C, Mortarino M, Rinaldi L, Cringoli G, Traldi G, Genchi M. Changing climate and changing vector-borne disease distribution: the example of Dirofilaria in Europe. Vet Parasitol. 2011;176:295–9. doi: 10.1016/j.vetpar.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Ardilly P, Tillé Y. Sampling methods: exercises and solution. New York: Springer; 2006. [Google Scholar]
- 17.Boch J, Supperer R. Veterinarmedizinische Parasitologie. Berlin: Verlang Paul Parey; 1992. [Google Scholar]
- 18.Lindsey LR. Identification of canine microfilariae. J Am Vet Med Assoc. 1965;146:1106–14. [PubMed] [Google Scholar]
- 19.McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 20.Zar JH. Biostatistical analysis. 4. New Jersey: Prentice-Hall; 1998. [Google Scholar]
- 21.Roscoe JT, Byars JA. Sample size restraints commonly imposed on the use of the chi-square statistic. J Am Stat Assoc. 1971;66:755–9. doi: 10.1080/01621459.1971.10482341. [DOI] [Google Scholar]
- 22.R Development Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 23.Genchi C, Rinaldi L, Mortarino M, Genchi M, Cringoli G. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009;163:286–92. doi: 10.1016/j.vetpar.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Alho AM, Landum M, Ferreira C, Meireles J, Goncalves L, de Carvalho LM, Belo S. Prevalence and seasonal variations of canine dirofilariosis in Portugal. Vet Parasitol. 2014;206:99–105. doi: 10.1016/j.vetpar.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Simon L, Afonin A, Lopez-Diez LI, Gonzalez-Miguel J, Morchon R, Carreton E, et al. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet Parasitol. 2014;200:257–64. doi: 10.1016/j.vetpar.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Traversa D, Aste G, Milillo P, Capelli G, Pampurini F, Tunesi C, et al. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet Parasitol. 2010;169:128–32. doi: 10.1016/j.vetpar.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Kalogeropoulos CD, Stefaniotou MI, Gorgoli KE, Papadopoulou CV, Pappa CN, Paschidis CA. Ocular dirofilariasis: a case series of 8 patients. Middle East Afr J Ophthalmol. 2014;21:312–6. doi: 10.4103/0974-9233.142267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasić-Otaševć SA, Trenkić Božinović MS, Gabrielli SV, Genchi C. Canine and human Dirofilaria infections in the Balkan Peninsula. Vet Parasitol. 2015;209:151–6. doi: 10.1016/j.vetpar.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Brianti E, Gaglio G, Napoli E, Giannetto S, Dantas-Torres F, Bain O, Otranto D. New insights into the ecology and biology of Acanthocheilonema reconditum (Grassi, 1889) causing canine subcutaneous filariosis. Parasitology. 2012;139:530–6. doi: 10.1017/S0031182011002198. [DOI] [PubMed] [Google Scholar]
- 30.Genchi C, Venco L, Genchi M. Guideline for the laboratory diagnosis of canine and feline Dirofilaria infections. Mappe Parassitologiche. 2007;8:137–44. [Google Scholar]
- 31.Schnyder M, Deplazes P. Cross-reactions of sera from dogs infected with Angiostrongylus vasorum in commercially available Dirofilaria immitis test kits. Parasit Vectors. 2012;5:258. [DOI] [PMC free article] [PubMed]
- 32.Diakou A. Angiostrongylus vasorum and angiostrongylosis in Greece. J Hell Vet Med Soc. 1995;46:51–6 (In Greek).
- 33.Drake J, Gruntmeir J, Merritt H, Allen L, Little SE. False negative antigen tests in dogs infected with heartworm and placed on macrocyclic lactone preventives. Parasit Vectors. 2015;8:68. [DOI] [PMC free article] [PubMed]
- 34.Little SE, Raymond MR, Thomas JE, Gruntmeir J, Hostetler JA, Meinkoth JH, Blagburn BL. Heat treatment prior to testing allows detection of antigen of Dirofilaria immitis in feline serum. Parasit Vectors. 2014;7:1–4. [DOI] [PMC free article] [PubMed]
- 35.Iglodyova A, Miterpakova M, Hurnikova Z, Antolova D, Dubinsky P, Letkova V. Canine dirofilariosis under specific environmental conditions of the Eastern Slovak Lowland. Ann Agric Environ Med. 2012;19:57–60. [PubMed] [Google Scholar]
- 36.Selby L, Corwin R, Hayes H., Jr Risk factors associated with canine heartworm infection. J Am Vet Med Assoc. 1980;176:33–5. [PubMed] [Google Scholar]
- 37.Tasic A, Tasic-Otasevic S, Gabrielli S, Miladinovic-Tasic N, Ignjatovic A, Dordevic J, et al. Canine Dirofilaria infections in two uninvestigated areas of Serbia: epidemiological and genetic aspects. Vector Borne Zoonotic Dis. 2012;12:1031–5. doi: 10.1089/vbz.2011.0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown HE, Harrington LC, Kaufman PE, McKay T, Bowman DD, Nelson CT, et al. Key factors influencing canine heartworm, Dirofilaria immitis, in the United States. Parasit Vectors. 2012;5:1–9. [DOI] [PMC free article] [PubMed]
- 39.Parham PE, Michael E. Modelling the effects of weather and climate change on malaria transmission. Environ Health Perspect. 2010;118:620. doi: 10.1289/ehp.0901256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diakou A, Gewehr S, Kapantaidakis E, Mourelatos S. Can mosquito population dynamics predict Dirofilaria hyperendemic foci? E-SOVE 2014 the 19th Conference. 2014; Thessaloniki. p. 76.
- 41.Ramsar Convention Secretariat. The Ramsar Convention Manual: a guide to the Convention on Wetlands (Ramsar, Iran, 1971) 6th ed. Gland: Ramsar Convention Secretariat; 2013. http://www.ramsar.org/sites/default/files/documents/library/manual6-2013-e.pdf.
- 42.Tsochatzis DE. Development of analytical methods for the determination of residues of pesticides used in rice cultures: application for the assessment of their environmental implications. Thessaloniki: Aristotle University of Thessaloniki; 2012. p. 201. [Google Scholar]
- 43.Otranto D, Cantacessi C, Dantas-Torres F, Brianti E, Pfeffer M, Genchi C, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet Parasitol. 2015;213:24–37. doi: 10.1016/j.vetpar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Papageorgiou N, Vlachos C, Sfougaris A, Tsachaldis E. Status and diet of wolves in Greece. Act Theriol. 1994;39:411–6. doi: 10.4098/AT.arch.94-48. [DOI] [Google Scholar]
- 45.Chapron G, Kaczensky P, Linnell JD, von Arx M, Huber D, Andrén H, López-Bao JV, Adamec M, Álvares F, Anders O. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science. 2014;346:1517–9. doi: 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its Additional file 1.


