Abstract
A large variety of vesicles is actively secreted into the extracellular space by most type of cells. The smallest nanoparticles (30-120 nm), called exosomes, are known to transport their cargo (nucleic acids, proteins and lipids) between diverse locations in the body. Specific content of exosomes and their influence on recipient cells depends primarily on the type of the secretory (donor) cell, yet several studies highlight the importance of environmental stress on which the donor cells are exposed. Ionizing radiation, which induces damage to DNA and other structures of a target cell, is one of well-recognized stress conditions influencing behavior of affected cells. A few recent studies have evidenced radiation-induced changes in composition of exosomes released from irradiated cells and their involvement in radiation-related communication between cells. Inducible pathways of exosome secretion activated in irradiated cells are regulated by TSAP6 protein (the transmembrane protein tumor suppressor-activated pathway 6), which is transcriptionally regulated by p53, hence cellular status of this major DNA damage response factor affects composition and secretion rate of exosomes released from target cells. Moreover, exosomes released from irradiated cells have been shown to mediate the radiation-induced bystander effect. Understanding radiation-related mechanisms involved in exosome formation and “makeup” of their cargo would shed light on the role of exosomes in systemic response of cells, tissues and organisms to ionizing radiation which may open new perspectives in translational medicine and anticancer-treatment.
Keywords: Exosome, ionizing radiation, intercellular communication, bystander effect
1. INTRODUCTION
Cells release three major types of vesicles into the extracellular microenvironment: exosomes, microvesicles and apoptotic bodies [1]. The largest attention is recently dedicated to exosomes, mainly due to their involvement in cell-to-cell communication [2], but also in immunological responses [3] and in rescue from apoptosis [4]. Exosomes are nanometer-sized (30-120 nm) membrane vesicle structures actively secreted by various types of normal and cancer cells [5]. Their content and their influence on recipient cells primarily depends on the cell type they are derived from. However, recent studies highlight the importance of stress conditions on mechanisms involved in exosome-mediated inter-cellular communication. Among the major environmental factors, ionizing radiation (IR) was proven to significantly influence cell to cell communication through different signal transduction systems. Radiation-induced signals may be passed between normal cells or between tumor cells as well as from tumor to normal cells or vice versa. This phenomenon is called Radiation-Induced Bystander Effect (RIBE) and leads to observation of radiation-related effects in cells that were not exposed to radiation themselves. Currently, there is growing evidence showing exosomes as key players in RIBE which will be discussed in more details in the related section [6-8]. The most evident influence of IR on exosome release is connected with radiation-induced DNA damage and activation of p53-related additional pathway of exosome formation and secretion which leads to evident increase of exosome release [9]. Similar upregulation of exosome secretion due to p53 activation by targeting DNA is induced by treating the host cells with chemotherapeutic agents such as cisplatin or doxorubicin [10]. However, other cellular stress-related agents may also act on different levels of the process of exosome formation and with different outcomes. Decrese of exosome release may be induced by influencing endo-lysosomal stability after targeting microtubules with taxol or vinca alkaloids, or by targeting ubiquitous proton pumps with their inhibitors [11]. Exosome composion was reported to be modulated by influencing protein sorting after targeting multivesicular bodies with curcumin. Since exosomes are reported to be influenced by various types of stress agents it will not be possible to cover in details the influence of all of them. Here, we review the current knowledge on the influence of ionizing radiation on exosome composition and pathways involved in their formation, secretion, and interactions with the recipient cells.
2. EXOSOME BIOGENESIS AND SECRETION
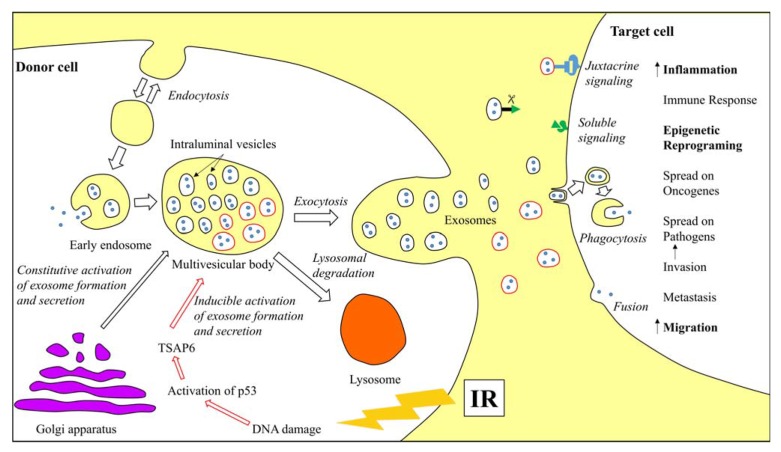
Exosomes originate from the intraluminal vesicles (ILVs), which are formed inside the early multivesicular bodies, also called endosomes, by inward budding from the limiting membrane (Fig. 1). Formation of ILVs usually involves the ESCRT (Endosomal Sorting Complex Required for Transport) machinery [12], but there is also some evidence of ESCRT-independent pathways during ILVs formation, depending on the cargo that is sorted within a given cell [13]. Most of the late endosomes fuse with lysosomes where their content is degraded. However, MVBs containing vesicles marked with tetraspanins (CD9, CD63 or CD81) or lysosomal-associated membrane proteins (LAMP1 and LAMP2) may also fuse with the plasma membrane and release their content outside the cell in a controlled manner. The released vesicles are then called exosomes.
The constitutive intracellular vesicular trafficking is controlled by the RAB family of small GTPase proteins. However there is no clear evidence whether the particular components of this family act at different steps of exosome secretion or are used differently by distinct cell types, because different molecules have been described in different cells. RAB11 is responsible for Ca2+-induced exosome secretion by the erythroleukemia cell line [14], Rab35 is involved in secretion of PLP-enriched exosomes by oligodendroglial cells [15], whereas RAB27A and RAB27B possess complementary functions in spontaneous secretion of MHC class II-bearing exosomes by HeLa-CIITA cells [16]. The last step of exosome secretion involves fusion of MVB with the plasma membrane. This process requires a specific combination of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs): vesicular SNAREs localized on MVBs and target SNAREs localized on the intracellular side of the plasma membrane. Their interaction results in a membrane-bridging SNARE complex, which is responsible for membrane fusion [17]. Regulation of exosome secretion was also shown to involve lipids, particularly phosphatidic acid, produced by phospholipase D2 or diacylglycerol kinase [18- 20].
3. EXOSOME COMPOSITION
Exosomes, beyond specific size range, may also be identified on the basis of their molecular composition. It was confirmed by numerous studies that they contain specific molecular components in contrast to plasma membrane-derived extracellular vesicles containing a random set of cell debris components. Composition of exosomes released from different sources is currently being systematized in the database ExoCarta 2012 listing exosomal proteins, lipids, and RNA [21].
Because of the endosomal origin, exosomes carry protein families associated with multivesicular bodies (MVB) formation (e.g., Alix and tumor susceptibility gene 101), membrane transport and fusion (e.g., Rab GTPases, annexins, and flotillins), and protein families mostly associated with lipid microdomains, such as integrins and tetraspanins (e.g., CD63, CD9, CD81, and CD82). Other proteins identified in almost all exosomes independent of their origin (“conserved” proteins) are heat shock proteins (e.g. HSC70), cytoskeleton proteins (β-actin, myosin, cofilin and tubulins), metabolic proteins (e.g., GADPH and ENO1), and major histocompatibility complex (MHC) class I and II molecules. Apart from conserved proteins, exosomes contain a wide range of proteins that are cell-type specific and depend on physiological or pathophysiological conditions, such as exposure to environmental stress.
The presence of micro RNAs (miRNA) in exosomes was initially described in human and murine mast cells. Some of the miRNA species were present at higher levels in exosomes than in cells, implying that some miRNAs may be uniquely packed into exosomes [22]. Indeed, the loading of miRNA into exosomes seems to be a tightly regulated process in the endo-lysosomal compartment [23]. After secretion and uptake of the exosomes by the target cell, the secretory miRNAs can act as a master silencer of gene expression, as reported for their cellular counterparts [24]. Since exosomal miRNAs shuttle between tumor cells and also between normal and tumor cells [25] and vice versa [26]; exosomal miRNA can influence not only tumor cell functions but also modify their niche and host reaction.
In terms of lipid species, exosomes usually have more cholesterol, phosphatidylinositol (PI), ceramide, sphingomyelin (SM), and monosialoganglioside (GM3) than the donor cells [27]. On the other hand, the amounts of phosphatydilcholine (PC) and lysobisphosphatidic acid (LBPA) are lower [28].
4. BIOLOGICAL FUNCTIONS OF EXOSOMES
First attempts to understand the biological functions of exosome were undertaken when studying the expulsion of proteins during the process of reticulocytes maturing into erythrocytes. The authors concluded that exosomes provided the major route for removal of obsolete plasma membrane proteins during the cell maturation process [29]. This observation was confirmed later, yet initially only for exosomes released from similar type of cells. Exosomal release of unnecessary proteins and RNA instead of their lysosomal processing is beneficial to cells that do not have efficient degradation capability or are located toward a drainage system, such as the tubules of the kidney or the gut [30].
Additional analyses of exosomes released from different types of cells revealed more functions. Importantly, exosomes are secreted not only by normal cells, but also from cells in pathological conditions such as tumor. Exosomes are currently most recognized for their involvement in the intercellular communication both in physiological and pathological conditions. Traditionally, cell communication was classified as contact-dependent (juxtacrine), paracrine, endocrine, exocrine or synaptic. In all these types of communication, cells secrete signaling molecules that are incorporated via a specific receptor-mediated uptake or can passively diffuse into recipient cells due to their small size. Discovery of exosome involvement in cell-to-cell communication provided new insights about cell signaling. Exosomes may transfer more complex information (signals for apoptosis, survival, division, growth, differentiation, etc.) targeting multiple and specific distant cells [31]. At the moment three main mechanisms are proposed for exosome-mediated intercellular communication. The first one involves juxtacrine signaling and relies on interaction of exosome membrane proteins with corresponding target cell receptors activating intracellular signaling. This mechanism was observed for exosomes derived from mature dendritic cells (DC), where the intercellular adhesion molecule 1 (ICAM1) located on the exosome surface bound to the lymphocyte function-associated antigen 1 receptor located on the surface of activated T-cells [32] or antigen-presenting cells (APCs) [33]. The second mechanism of interaction between exosome and target cell involves soluble ligands – proteins cleaved from the exosome membrane, which was observed in case of tumor necrosis factor receptor 1 (TNFR1) in vascular endothelial cells [34] or CD46 receptor in ovarian adenocarcinoma cell lines [35]. The third and most commonly observed mechanism of exosome-mediated signaling is its internalization by a recipient cell. Exosome fusion with a recipient cell and transfer of its content into a target was reported on the genome [22], proteome [36], and lipidome levels [37]. Although this paracrine diffusion of exosomes is limited to acidic pH, as cell and exosome membrane exhibit the same fluidity at pH 5, there is profound evidence that microenvironment of cancer is characterized by lower pH favoring this type of communication [36]. The other way of exosome internalization is phagocytosis, where the exosome content, as well as its membrane, is immersed in a target cell inside an additional vesicle known as phagosome [38].
Beyond the communication-related functions, exosomes secreted from normal cells have been associated with initiation of an immunomodulatory activity. Promotion of immune responses usually takes place through T-cell activation and exosome-derived MHC-peptide complexes binding to their cognate T-cell receptor, leading to activation of primed CD4+/CD8+ T lymphocytes [32, 39]. Their ability to activate immune response is related to the fact that exosomes carry their membrane antigens to APCs and that they bear signals that may promote the transformation of the acceptor cells into immunogenic APCs [40]. Additionally, the strength of the activating effect seems to depend on the physiological state of the cell, since mature DC were proven to release exosomes more efficient at inducing T-cell activation than those from immature DCs [41]. On the other hand, exosome vesicles secreted by certain tumors may also carry immunosuppressive peptide complexes on their membranes. It was already shown that they are able to slow down proliferation of natural killer (NK) cells [42] or CD4+/CD8+ T-cells [43] and to promote differentiation of myeloid cells [44] and regulatory T-cells [45], which are regarded as immunosuppressive cells. Exosomes inducing antigen-specific tolerance are also called “tolerosomes”. In normal cells they were shown to participate in increasing the tolerance to food antigens in intestines [46], and in enhancing an immune tolerance during pregnancy [47].
Another function of exosomes released from tumor cells is spread of oncogenes. In particular, the oncogenic mutant epidermal growth factor receptor type III (EGFRvIII) expressed by a subset of glioma cells is associated with cancer aggressiveness and transferred in vesicles to another tumor cells missing this mutant receptor [48]. This transfer resulted in lunching of downstream anti-apoptotic and angiogenic mediators such as vascular endothelial growth factor (VEGF) in the recipient cells, leading to morphological transformation, and increasing the anchorage-independent growth capacity of cells. Additional studies have also proven the angiogenic potential of cancer-derived exosomes by carrying angiogenic mRNA, which was translated into proteins in recipient cells [49,50].
Exosomes are also suggested to play an important role in spreading pathogens like viruses or prions between cells. Hijacking of exosome pathways was shown in case of miRNA released from cells infected by Epstein Barr virus and transported by exosomes to cells uninfected by virus [51]. Selected types of cells, such as lymphocytes, monocytes, macrophages, and dendritic cells, infected with HIV-1 can release both virions and exosomes that are difficult to distinguish [52]. Additionally, HIV-1 was demonstrated to exploit the exosome antigen-dissemination pathway intrinsic to mature DCs [53].
5. INFLUENCE OF IONIZING RADIATION ON EXOSOME SECRETION
Growing evidence supports the observation of ionizing radiation-induced increased exosome release in both dose and time dependent manners. This phenomenon occurs due to activation of additional stress-inducible pathways of exosome secretion (Fig. 1). One pathway requires increased expression of TSAP6 (transmembrane protein tumor suppressor-activated pathway 6), which is stimulated by DNA damage-activated p53 transcription factor; this was first noted in human epithelial lung H460 cell line exposed to ionizing radiation [9]. Increased rate of exosome secretion following irradiation of p53-competent cells was confirmed in aneuploid immortal keratinocyte HaCaT [54], human breast adenocarcinoma MCF7 [8], and human prostate cancer cell lines [55], where this effect was additionally related to senescence. This data emphasize the importance of the p53 status in cells used as exosome models, as p53 overexpression typical for cancer cells results in abnormally high rate of radiation-induced exosome release. This relationship was shown in human glioblastoma cell lines [7], where a higher relative increase in exosome release following irradiation was observed in p53-mutated/overexpressed cell lines (LN18 and U251) in comparison to U87 cell line with wild-type p53.
Figure 1.
Schematic representation of exosome formation, secretion and uptake by recipient cells. Exosomes are formed and secreted via a constitutive pathway involving the Golgi Network and an inducible pathway, which can be activated by ionizing radiation. Exosomes are released by a donor cell and accepted by a recipient cell through phagocytosis or membrane fusion leading to activation of specific processes. Processes upregulated in the target cell due to uptake of “radiation-activated” exosomes are marked in bold.
6. INFLUENCE OF IONIZING RADIATION ON EXOSOME COMPOSITION
The content of an exosome primarily depends on the type and the state of a donor cell, but it may also be influenced by stress conditions on which the cell is exposed. One of the major environmental factors inducing cellular stress is ionizing radiation. Radiation, next to drug therapy, is currently the main therapeutic tool for various types of diseases, especially cancer. Radiation-induced ionizations may act directly on the cellular component molecules or indirectly on water molecules, causing water-derived radicals. Radicals react with adjacent molecules in a very short time, which results in breakage of chemical bonds or oxidation of the affected molecules. Although the most critical effect in cells is damage of DNA, other cellular effects are also observed, such as increased levels of reactive oxygen species (ROS), nitric oxide (NO), cytokines, and disturbed calcium transport.
Literature data regarding radiation-induced changes in exosome composition is very limited and refer mainly to its proteome (Table 1). First reports came from irradiated prostate cancer cells producing in vitro exosomes with elevated levels of B7-H3 (CD276), which was later identified as diagnostic marker of prostate cancer [55]. Importantly, authors of this report pointed out that radiation-induced changes in exosome composition and release were accompanied by induction of senescence in these cells. The same cancer model was also studied by another group using serum samples and showing radiotherapy-related increased levels of Hsp72, which generally protects cells from cellular stress [56]. Exosomes from exposed glioblastoma cells had abnormally elevated connective tissue growth factor (CTGF) mRNA and insulin-like growth factor binding protein 2 (IGFBP2) protein level, which are responsible for migration and invasion of different cancer types [7]. Interestingly, when considering a 1.33-fold change cutoff many mRNA levels changed (Crt-derived vs IR-derived exosomes) 24 h (1308 mRNAs) and 48 h (209 mRNAs) after IR. In contrast to mRNA, levels of only a few miRNAs were changed. Additionally, the combined mRNA and protein array data were analyzed using functional networks showing cellular movement as a top associated network function as well as the top molecular and cellular function. This observation further confirmed the influence of IR-derived exosomes on recipient cell migration. A recent study on a head and neck cancer cell model revealed that exosomes from irradiated cells had substantially increased levels of proteins involved in transcription, translation, cell division, and cell signaling as well as decreased levels of apolipoproteins and immunoglobulins [57]. A long list of transcription/translation (e.g. EIFs, PSMs, RPLs and RPSs) proteins present exclusively in IR-treated samples may evidence an intense adaptation mechanisms to radiation stress by for example removing redundant components in the form of exosomes. The number of such components increase in cells affected by IR due to cell cycle arrest, which blocks transcription and consequently translation and cell division. For more detailed information about identified proteins in this study please see the supplementary file of the paper [57]. Although the data regarding the influence of ionizing radiation on the released exosome composition are based on different cellular models and modes of exposure to ionizing radiation, they collectively point out that exosomal cargo indeed reflects specific changes induced by ionizing radiation.
Table 1.
Exosomal components significantly changed after donor cell exposure to ionizing radiation.
| Biological source of exosomes | Dose of ionizing radiation | Time from IR to exosome isolation | Changed components in exosomes released from treated cells | Refs. |
|---|---|---|---|---|
| Human epithelial prostate cell carcinoma (22Rv1) | 4 Gy | 96 h | Increased level of B7-H3 (CD276) | [55] |
| Human serum | Radiotherapy treatment | No information | Increased level of HSP72 protein | [56] |
| Human glioblastoma multiforme (U87MG) | 4 Gy | 24-48 h | Abnormally increased levels of CTGF mRNA and IGFBP2 protein | [7] |
| Human head and neck squamous cell carcinoma (FaDu) | 2 Gy | 18 h | Increased levels of proteins involved in transcription/translation (EIFs, PSMs, RPLs and RPSs), cell cycle/division (chaperones, ubiquitination-related factors and proteasome components) and cell signaling (ARFs, RABs and RASs proteins); Decreased levels of apolipoproteins and immunoglobulins |
[57] |
7. INFLUENCE OF IONIZING RADIATION ON EXOSOME-MEDIATED INTERCELLULAR COMMUNICATION
The most recognizable effect indicating radiation-related intercellular communication is the Radiation-Induced Bystander Effect. This phenomenon arises in cells that were not exposed to radiation themselves. Among major factors transmitting the RIBE are cytokines: IL-8 [58], TGF-β [59], and TNF-α [60]. However, there are also reports pointing at calcium fluxes, NO [61], ROS [62], and plasma membrane-bound lipid rafts [63]. The major postulated mechanism of RIBE assumes an involvement of either gap junction intercellular communication (GJIC), where signaling molecules are flowing between neighboring cells through connecting channels, or paracrine activity of molecules released by exposed cells into the extracellular matrix and then endocytosed by unexposed recipient cells.
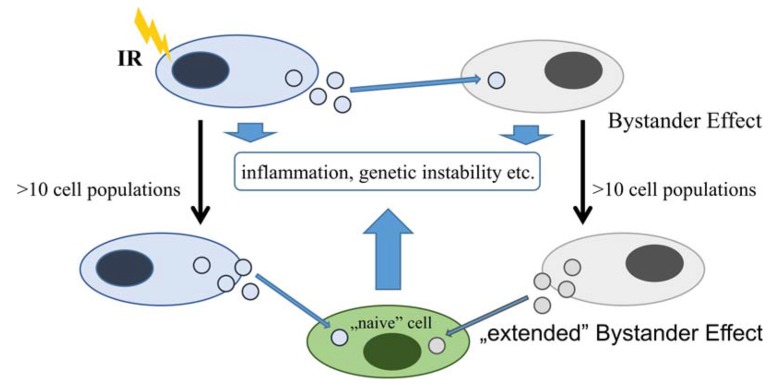
Recent interesting reports on exosome involvement in initiation of RIBE resulted from a few in vitro human studies (comparison in Table 2) on breast adenocarcinoma [6,8] and aneuploid immortal keratinocyte cell lines [54]. The suggested key transmitting factors are exosome protein and RNA molecules. In case of proteins, cytokines were shown to be present in exosomes released from fibroblast cells [64] inducing inflammation in receiving cells. Another report showed that exosomes released from Caco-2 epithelial colorectal adenocarcinoma cells carried HMGB1, which is also a cytokine-like proinflammatory protein [65]. Regarding RNAs it was suggested that miRNA play an indirect function in RIBE [66] initiating the so-called delayed Bystander Effect through epigenetic changes [67] and apoptosis [68]. Recent work performed on MCF7 cells [8] confirmed that RNA or protein components of exosomes are able to initiate RIBE demonstrating the synergistic effect of both RNA and protein signals in inducing RIBE. Additionally, this research showed that delayed responses, such as GI and inflammation, are caused not only by exosomes released by directly irradiated cells, but also by exosomes secreted from bystander cells, as well as by the progeny of directly irradiated and bystander cells (Figure 2). This observation suggests a strong influence of even a single exposed cell in a microenvironment through exosomes from its progeny and from the progeny of bystander cells. Therefore, further studies should be carried out to test the longevity of this effect and key factors involved.
Table 2.
Examples of RIBEs perpetuated by exosomes in recipient cells.
| Donor cells | Recipient cells |
Dose of ionizing
radiation |
Time from IR to exosome isolation | Bystander effects in recipient cells | Refs. |
|---|---|---|---|---|---|
| Human breast cancer cell line (MCF-7) | MCF-7 | 2 Gy | 4 h | DNA (comet assay) and chromosomal (metaphases analysis) damage after 24 h of incubation | [6] |
| Human keratinocyte cells (HaCaT) | HaCaT | 0.005 Gy; 0.05 Gy ; 0.5 Gy | 1 h | Cell death (the alamarBlue® assay) after 72 h of incubation | [54] |
| Human breast cancer cell line (MCF-7) | MCF-7 | 2 Gy | 4 h | DNA (comet assay) and chromosomal (metaphases analysis damage) after 24 h of incubation up to 20 cell-doublings | [8] |
Figure 2.
Schematic representation of possible exosome-mediated delayed radiation-induced bystander effect. Effect of ionizing radiation mediated by exosomes involves bystander cells and additionally cells that uptake exosomes from progeny of irradiated cells as well as progeny of bystander cells (based on data presented by [8]).
Another interesting manifestation of the influence of ionizing radiation on the intercellular communication via exosomes is the enhancement of recipient cells migration. In a glioblastoma cell line model, radiation-derived exosomes enhance the migration of recipient cells [7]. These exosomes contained more CTGF mRNA and IGFBP2 protein, which are involved in the migration/invasion of cancer cell through activation of TrkA and FAK signaling.
Ionizing radiation not only impacts the intercellular communication through exosomes released from exposed donor cells, but also influences the exosome uptake by the exposed recipient cells. Exposure of MSC human bone marrow-derived cells to ionizing radiation was shown to increase the cellular uptake of exosomes through CD29/CD81 complex formation, however it was independent from ROS generation, and p38 MAPK-dependent endocytosis and pinocytosis [69].
CONCLUSION
Exosomes are nano-sized vesicles that may deliver bioactive cargos including lipids, growth factors and their receptors, proteases, signaling molecules, mRNA, and non-coding RNA, released from one cell (origin) to recipient cells. Cancer derived exosomes are important players in the formation of the tumor microenvironment by (a) enabling the escape of tumor cells from immunity check and enhancing inflammatory response, (b) affecting differentiation of fibroblasts and mesenchymal cells into myofibroblasts, (c) triggering the angiogenic process, and (d) enhancing the metastatic evolution of the tumor by promoting epithelial-to-mesenchymal transformation and by preparing the tumor niche in the new anatomical location. Hence, understanding exosome-mediated signaling between tumor and their microenvironment, and identifying factors affecting such communication seems critical for better diagnosis and treatment of human malignancies. Unfortunately, the role of ionizing radiation as a modulating factor of exosome secretion is poorly understood yet. The effects of radiation on exosome-mediated signaling have been studied in very few in vitro models. Nevertheless, available reports evidenced that radiation affected composition and secretion rate of exosomes released from donor cells, as well as functions of recipient cell receiving exosomes from irradiated cells. Hence, these early observations clearly indicated importance of described phenomena for mechanisms involved in response of cancer cells to ionizing radiation, also in the context of cancer radiotherapy.
ACKNOWLEDGEMENTS
This work was supported by the National Science Centre, Poland, Grant 2013/11/B/NZ7/01512.
LIST OF ABBREVIATIONS
- APC
Antigen-Presenting Cell
- CDE
Cancer Derived Exosomes
- CTGF
Connective Tissue Growth Factor
- DC
Dendritic Cells
- EGFRvIII
Epidermal Growth Factor Receptor Type III
- ESCRT
Endosomal Sorting Complex Required for Transport
- GJIC
Gap Junction Intercellular Communication
- GM3
Monosialoganglioside
- ICAM1
Intercellular Adhesion Molecule 1
- IGFBP2
Insulin-like Growth Factor Binding Protein 2
- ILV
Intraluminal Vesicle
- LAMP
Lysosomal-Associated Membrane Protein
- LBPA
Lysobisphosphatidic Acid
- MHC
Major Histocompatibility Complex
- MVB
Multivesicular Bodies
- NK
Natural Killer
- NO
Nitric Oxide
- PC
Phosphatydilcholine
- PI
Phosphatidylinositol
- RIBE
Radiation-Induced Bystander Effect
- ROS
Reactive Oxygen Species
- SM
Sphingomyelin
- SNARE
Soluble N-ethylmaleimide-sensitive Factor Attachment Protein Receptors
- TNFR1
Tumor Necrosis Factor Receptor 1
- TSAP6
Tumor Suppressor-Activated Pathway 6
- VEGF
Vascular Endothelial Growth Factor
CONFLICT OF INTEREST
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- 1.György B., Szabó T.G., Pásztói M., Pál Z., Misják P., Aradi B., László V., Pállinger E., Pap E., Kittel A., Nagy G., Falus A., Buzás E.I. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012;44(11):2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miksa M., Wu R., Dong W., Komura H., Amin D., Ji Y., Wang Z., Wang H., Ravikumar T.S., Tracey K.J., Wang P. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J. Immunol. 2009;183(9):5983–5990. doi: 10.4049/jimmunol.0802994. [corrected]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mayah A.H., Irons S.L., Pink R.C., Carter D.R., Kadhim M.A. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat. Res. 2012;177(5):539–545. doi: 10.1667/RR2868.1. [DOI] [PubMed] [Google Scholar]
- 7.Arscott W.T., Tandle A.T., Zhao S., Shabason J.E., Gordon I.K., Schlaff C.D., Zhang G., Tofilon P.J., Camphausen K.A. Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl. Oncol. 2013;6(6):638–648. doi: 10.1593/tlo.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mayah A., Bright S., Chapman K., Irons S., Luo P., Carter D., Goodwin E., Kadhim M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. 2015;772:38–45. doi: 10.1016/j.mrfmmm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Yu X., Harris S.L., Levine A.J. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 10.Safaei R., Larson B.J., Cheng T.C., Gibson M.A., Otani S., Naerdemann W., Howell S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005;4(10):1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 11.Iero M., Valenti R., Huber V., Filipazzi P., Parmiani G., Fais S., Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 12.Henne W.M., Stenmark H., Emr S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 2013;5(9):a016766. doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carayon K., Chaoui K., Ronzier E., Lazar I., Bertrand-Michel J., Roques V., Balor S., Terce F., Lopez A., Salomé L., Joly E. Proteolipidic composition of exosomes changes during reticulocyte maturation. J. Biol. Chem. 2011;286(39):34426–34439. doi: 10.1074/jbc.M111.257444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M.A., Bakhti M., Grønborg M., Möbius W., Rhee J., Barr F.A., Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., Goud B., Benaroch P., Hacohen N., Fukuda M., Desnos C., Seabra M.C., Darchen F., Amigorena S., Moita L.F., Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. 2010. [DOI] [PubMed]
- 17.Chaineau M., Danglot L., Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583(23):3817–3826. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Laulagnier K., Grand D., Dujardin A., Hamdi S., Vincent-Schneider H., Lankar D., Salles J.P., Bonnerot C., Perret B., Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572(1-3):11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 19.Laulagnier K., Vincent-Schneider H., Hamdi S., Subra C., Lankar D., Record M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol. Dis. 2005;35(2):116–121. doi: 10.1016/j.bcmd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Alonso R., Mazzeo C., Rodriguez M.C., Marsh M., Fraile-Ramos A., Calvo V., Avila-Flores A., Merida I., Izquierdo M. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18(7):1161–1173. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 23.Gibbings D.J., Ciaudo C., Erhardt M., Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 2009;11(9):1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 24.Iguchi H., Kosaka N., Ochiya T. Secretory microRNAs as a versatile communication tool. Commun. Integr. Biol. 2010;3(5):478–481. doi: 10.4161/cib.3.5.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonyak M.A., Li B., Boroughs L.K., Johnson J.L., Druso J.E., Bryant K.L., Holowka D.A., Cerione R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA. 2011;108(12):4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subra C., Laulagnier K., Perret B., Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89(2):205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J.F., Kobayashi T., Salles J.P., Perret B., Bonnerot C., Record M. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004;380(Pt 1):161–171. doi: 10.1042/bj20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone R.M., Mathew A., Mason A.B., Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell. Physiol. 1991;147(1):27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006;36(2):315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig A.K., Giebel B. Exosomes: small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012;44(1):11–15. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Nolte-’t Hoen E.N., Buschow S.I., Anderton S.M., Stoorvogel W., Wauben M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113(9):1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 33.Segura E., Guérin C., Hogg N., Amigorena S., Théry C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J. Immunol. 2007;179(3):1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 34.Hawari F.I., Rouhani F.N., Cui X., Yu Z.X., Buckley C., Kaler M., Levine S.J. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc. Natl. Acad. Sci. USA. 2004;101(5):1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakulinen J., Junnikkala S., Sorsa T., Meri S. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur. J. Immunol. 2004;34(9):2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- 36.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., Colone M., Tatti M., Sargiacomo M., Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., Poirot M., Record M. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010;51(8):2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng D., Zhao W.L., Ye Y.Y., Bai X.C., Liu R.Q., Chang L.F., Zhou Q., Sui S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 39.Admyre C., Johansson S.M., Paulie S., Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur. J. Immunol. 2006;36(7):1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 40.Bhatnagar S., Shinagawa K., Castellino F.J., Schorey J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 42.Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J.C., Barnes S., Kimberly R.P., Grizzle W.E., Zhang H.G. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176(3):1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 43.Clayton A., Mitchell J.P., Court J., Mason M.D., Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 44.Valenti R., Huber V., Filipazzi P., Pilla L., Sovena G., Villa A., Corbelli A., Fais S., Parmiani G., Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 45.Szajnik M., Czystowska M., Szczepanski M.J., Mandapathil M., Whiteside T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson M., Lundin S., Dahlgren U., Kahu H., Pettersson I., Telemo E. “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 2001;31(10):2892–2900. doi: 10.1002/1521-4141(2001010)31:10<2892::AID-IMMU2892>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 47.Taylor D.D., Akyol S., Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J. Immunol. 2006;176(3):1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 48.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 49.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr, Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong B.S., Cho J.H., Kim H., Choi E.J., Rho S., Kim J., Kim J.H., Choi D.S., Kim Y.K., Hwang D., Gho Y.S. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Würdinger T., Middeldorp J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chertova E., Chertov O., Coren L.V., Roser J.D., Trubey C.M., Bess J.W., Jr, Sowder R.C., II, Barsov E., Hood B.L., Fisher R.J., Nagashima K., Conrads T.P., Veenstra T.D., Lifson J.D., Ott D.E. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 2006;80(18):9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izquierdo-Useros N., Naranjo-Gómez M., Archer J., Hatch S.C., Erkizia I., Blanco J., Borràs F.E., Puertas M.C., Connor J.H., Fernández-Figueras M.T., Moore L., Clotet B., Gummuluru S., Martinez-Picado J. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113(12):2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jella K.K., Rani S., O’Driscoll L., McClean B., Byrne H.J., Lyng F.M. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014;181(2):138–145. doi: 10.1667/RR13337.1. [DOI] [PubMed] [Google Scholar]
- 55.Lehmann B.D., Paine M.S., Brooks A.M., McCubrey J.A., Renegar R.H., Wang R., Terrian D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68(19):7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurwitz M.D., Kaur P., Nagaraja G.M., Bausero M.A., Manola J., Asea A. Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer. Radiother. Oncol. 2010;95(3):350–358. doi: 10.1016/j.radonc.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jelonek K., Wojakowska A., Marczak L., Muer A., Tinhofer-Keilholz I., Lysek-Gladysinska M., Widlak P., Pietrowska M. Ionizing radiation affects protein composition of exosomes secreted in vitro from head and neck squamous cell carcinoma. Acta Biochim. Pol. 2015;62(2):265–272. doi: 10.18388/abp.2015_970. [DOI] [PubMed] [Google Scholar]
- 58.Facoetti A., Ballarini F., Cherubini R., Gerardi S., Nano R., Ottolenghi A., Prise K.M., Trott K.R., Zilio C. Gamma ray-induced bystander effect in tumour glioblastoma cells: a specific study on cell survival, cytokine release and cytokine receptors. Radiat. Prot. Dosimetry. 2006;122(1-4):271–274. doi: 10.1093/rpd/ncl431. [DOI] [PubMed] [Google Scholar]
- 59.Burr K.L., Robinson J.I., Rastogi S., Boylan M.T., Coates P.J., Lorimore S.A., Wright E.G. Radiation-induced delayed bystander-type effects mediated by hemopoietic cells. Radiat. Res. 2010;173(6):760–768. doi: 10.1667/RR1937.1. [DOI] [PubMed] [Google Scholar]
- 60.Moore S.R., Marsden S., Macdonald D., Mitchell S., Folkard M., Michael B., Goodhead D.T., Prise K.M., Kadhim M.A. Genomic instability in human lymphocytes irradiated with individual charged particles: involvement of tumor necrosis factor alpha in irradiated cells but not bystander cells. Radiat. Res. 2005;163(2):183–190. doi: 10.1667/RR3298. [DOI] [PubMed] [Google Scholar]
- 61.Shao C., Prise K.M., Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat. Res. 2008;638(1-2):139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto H., Hamada N., Takahashi A., Kobayashi Y., Ohnishi T. Vanguards of paradigm shift in radiation biology: radiation-induced adaptive and bystander responses. J. Radiat. Res. (Tokyo) 2007;48(2):97–106. doi: 10.1269/jrr.06090. [DOI] [PubMed] [Google Scholar]
- 63.Hamada N., Matsumoto H., Hara T., Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J. Radiat. Res. (Tokyo) 2007;48(2):87–95. doi: 10.1269/jrr.06084. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y., Xiang X., Zhuang X., Zhang S., Liu C., Cheng Z., Michalek S., Grizzle W., Zhang H.G. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 2010;176(5):2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S., Stolz D.B., Sappington P.L., Macias C.A., Killeen M.E., Tenhunen J.J., Delude R.L., Fink M.P. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Cell Physiol. 2006;290(4):C990–C999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 66.Dickey J.S., Zemp F.J., Martin O.A., Kovalchuk O. The role of miRNA in the direct and indirect effects of ionizing radiation. Radiat. Environ. Biophys. 2011;50(4):491–499. doi: 10.1007/s00411-011-0386-5. [DOI] [PubMed] [Google Scholar]
- 67.Koturbash I., Boyko A., Rodriguez-Juarez R., McDonald R.J., Tryndyak V.P., Kovalchuk I., Pogribny I.P., Kovalchuk O. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis. 2007;28(8):1831–1838. doi: 10.1093/carcin/bgm053. [DOI] [PubMed] [Google Scholar]
- 68.Kovalchuk O., Zemp F.J., Filkowski J.N., Altamirano A.M., Dickey J.S., Jenkins-Baker G., Marino S.A., Brenner D.J., Bonner W.M., Sedelnikova O.A. microRNAome changes in bystander three-dimensional human tissue models suggest priming of apoptotic pathways. Carcinogenesis. 2010;31(10):1882–1888. doi: 10.1093/carcin/bgq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazawa M., Tomiyama K., Saotome-Nakamura A., Obara C., Yasuda T., Gotoh T., Tanaka I., Yakumaru H., Ishihara H., Tajima K. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem. Biophys. Res. Commun. 2014;446(4):1165–1171. doi: 10.1016/j.bbrc.2014.03.067. [DOI] [PubMed] [Google Scholar]