Abstract
Chemolithotrophic ammonia-oxidizing bacteria and Thaumarchaeota are central players in the global nitrogen cycle. Obligate ammonia chemolithotrophy has been characterized for bacteria; however, large gaps remain in the Thaumarchaeotal pathway. Using batch growth experiments and instantaneous microrespirometry measurements of resting biomass, we show that the terrestrial Thaumarchaeon Nitrososphaera viennensis EN76T exhibits tight control over production and consumption of nitric oxide (NO) during ammonia catabolism, unlike the ammonia-oxidizing bacterium Nitrosospira multiformis ATCC 25196T. In particular, pulses of hydroxylamine into a microelectrode chamber as the sole substrate for N. viennensis resulted in iterative production and consumption of NO followed by conversion of hydroxylamine to nitrite. In support of these observations, oxidation of ammonia in growing cultures of N. viennensis, but not of N. multiformis, was inhibited by the NO-scavenger PTIO. When based on the marginal nitrous oxide (N2O) levels detected in cell-free media controls, the higher levels produced by N. multiformis were explained by enzyme activity, whereas N2O in N. viennensis cultures was attributed to abiotic reactions of released N-oxide intermediates with media components. Our results are conceptualized in a pathway for ammonia-dependent chemolithotrophy in Thaumarchaea, which identifies NO as an essential intermediate in the pathway and implements known biochemistry to be executed by a proposed but still elusive copper enzyme. Taken together, this work identifies differences in ammonia-dependent chemolithotrophy between bacteria and the Thaumarchaeota, advances a central catabolic role of NO only in the Thaumarchaeotal pathway and reveals stark differences in how the two microbial cohorts contribute to N2O emissions.
Introduction
Ammonia-oxidizing archaea, in the phylum Thaumarchaeota, and ammonia-oxidizing bacteria are abundant and diverse microorganisms that control the oxidation of ammonia (NH3) to nitrite (NO2−) in the global biogeochemical nitrogen cycle. Through many decades of research, the biochemical pathway for chemolithotrophic growth of ammonia-oxidizing bacteria has been principally elucidated (Sayavedra-Soto and Arp, 2011); however, this pathway has yet to be characterized in the more recently discovered thaumarchaeotal ammonia-oxidizers. This lesser understanding is largely due to the difficulty of growing reliable and sufficient biomass from pure cultures for performing physiological experiments, thus making identification of the genetic inventory that supports chemolithotrophic growth of the thaumarchaeotal ammonia-oxidizers a challenge. In contrast, the pathways for the autotrophic assimilation of carbon have been identified in both cohorts (Arp et al., 2007; Könneke et al., 2014).
Previous experiments with the marine isolate Nitrosopumilus maritimus SCM1 indicated that ammonia oxidation is dependent on the activity of the ammonia monooxygenase enzyme and (an) unknown enzyme(s) that convert(s) hydroxylamine (NH2OH) to NO2− and provide electrons for energy conservation (Vajrala et al., 2012). In ammonia-oxidizing bacteria, this second step is performed by hydroxylamine dehydrogenase (EC 1.7.2.6); however, no homologues of hydroxylamine dehydrogenase-encoding genes have been identified in genome sequences obtained from any pure or enrichment culture of Thaumarchaea (Walker et al., 2010; Kim et al., 2011; Tourna et al., 2011; Spang et al., 2012). In addition to NH2OH, there is also evidence that nitric oxide (NO) plays an important role in the Thaumarchaeotal but not in the bacterial ammonia oxidation pathway (Shen et al., 2013; Martens-Habbena et al., 2015). Martens-Habbena et al. (2015) demonstrated that NO accumulated in N. maritimus SCM1 cultures during active oxidation of NH4Cl in a closed microrespirometry chamber, and was released at higher levels under saturating versus non-saturating availability of NH4Cl. Exposure to increasing concentrations of an NO-scavenging compound over a 24 h period resulted in decreased levels of nitrite production in batch cultures of ammonia-oxidizing Thaumarchaea, but not bacteria (Martens-Habbena et al., 2015). The authors concluded that NO was either released as a free intermediate during ammonia oxidation by N. maritimus, or it could serve a functional role as an electron delivery mechanism to ammonia monooxygenase, an idea that has been proposed previously (Schleper and Nicol, 2010).
Although the detection of nitrous oxide (N2O) has been reported for both enrichments and pure cultures of Thaumarchaea engaged in ammonia oxidation (Santoro et al., 2011; Loscher et al., 2012; Jung et al., 2014; Stieglmeier et al., 2014b), the isotope data reported by Stieglmeier et al. (2014b) revealed that ammonia-oxidizing Thaumarchaea cannot enzymatically reduce NO2− to N2O via NO in the pathway known as ‘nitrifier denitrification'. Several publications have suggested that ammonia-oxidizing Thaumarchaea are a major source of N2O to the environment based on their relative abundance in oxic environments, the isotopic signature of the detected N2O, and that the authors failed to detect known bacterial denitrification genes and pertinent activities (Santoro et al., 2011; Loscher et al., 2012; Jung et al., 2014). Yet, control experiments to verify or falsify chemical formation of N2O facilitated by interaction of Thaumarchaeotal metabolites with components of the cultivation or incubation media or assay solutions remain absent from the literature. It should be noted that interactions of ammonia oxidation intermediates with iron, manganese, and organic compounds could generate substantial amounts of N2O under environmentally relevant conditions (Zhu-Barker et al., 2015).
The present study addresses critical ecophysiological questions about how two different cohorts of microorganisms, simultaneously involved in the biogeochemical nitrogen cycle through ammonia-oxidation, vary in their contributions, particularly to production of nitrous oxide. This study also furthers the observation of NO as an intermediate for ammonia chemolithotrophy in the terrestrial Thaumarchaeon Nitrososphaera viennensis strain EN76T (Stieglmeier et al., 2014a) by examining its complete profile of NO production and consumption during substrate oxidation at oxic conditions and the transition into an extended period of anoxia. In contrast to the above referenced studies of ‘N2O production' by ammonia-oxidizing Thaumarchaea, our results do not support any scenario in which N. viennensis enzymatically reduces NO to N2O through a denitrification pathway. Instead, the results support that N2O was formed abiotically from NO by interaction with media components or with debris in killed cell controls. We further demonstrated that NO is an active and necessary intermediate during the oxidation of NH2OH to NO2− in ammonia-oxidizing Thaumarchaea rather than participating directly in the oxidation of NH3 to NH2OH as suggested previously (Schleper and Nicol, 2010). Based on these results, a new pathway for obligate ammonia-dependent chemolithotrophy for ammonia-oxidizing Thaumarchaea is proposed that implicates a novel copper enzyme to perform a biochemistry known to occur in ammonia-oxidizing bacteria facilitated by heme-containing cytochrome c.
Materials and methods
Strains and cultivation
N. viennensis strain EN76T was maintained at 37 °C in 50 ml freshwater medium (FWM) supplemented with 2 mM NH4Cl, 0.5 mM sodium pyruvate and 50 μg/ml carbenicillin and buffered with HEPES (Tourna et al., 2011; Stieglmeier et al., 2014a) and inoculated at 4% v/v. Cultures were grown in Wheaton bottles (150 ml) sealed with caps inlaid with grey butyl rubber stoppers. Nitrosospira multiformis ATCC 25196T was maintained at 28 °C in 100 ml HEPES-buffered HK medium (HKM) (Krümmel and Harms, 1982) containing 3 mM ammonium and phenol red as pH indicator (pH of 7.5–8) and inoculated at 5% v/v into 250 ml Wheaton bottles. The pH of N. multiformis cultures was maintained with regular additions of 10% NaHCO3.
Growth experiments with NO-scavenger PTIO
For monitoring activity in the presence of an NO-scavenging compound, N. viennensis was cultivated in 20 ml FWM. N. multiformis was cultivated in 20 ml phosphate-buffered mineral medium (Skinner and Walker, 1961) amended with 1 mM NH4Cl and pH was adjusted regularly with 5% Na2CO3. In early to mid-exponential phase of growth, 150 μM of 2-phenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl 3-oxide (PTIO; Sigma-Aldrich, Vienna, Austria), a chemical that scavenges NO (Goldstein et al., 2003) was injected into the cultures. Ammonium consumption and nitrite production were measured over a period of 6–8 days using standard colorimetric assays (Clesceri et al., 1998) and N2O was measured via GC (AGILENT 6890N, Vienna, Austria; injector: 120 °C, detector: 350 °C, oven: 35 °C, carrier gas: N2) in connection with an automatic sample-injection system (DANI HSS 86.50, Head-space-Sampler, Sprockhövel, Germany). Detailed sampling and sample preparation has been described previously (Stieglmeier et al., 2014a).
Instantaneous measurement of NO and N2O during oxidation of ammonia
In preparation for experiments measuring instantaneous O2 consumption and either NO or N2O production, N. viennensis was inoculated at 4% v/v into 2 l of HEPES-buffered FWM and N. multiformis was inoculated at 5% v/v into 250 ml HKM. Cells were harvested at late exponential phase (N. viennensis, 1–1.5 mM NO2−; N. multiformis, 2–2.5 mM NO2−) by filtration on Supor200 0.2 μm filters (Pall, Ann Arbor, WI, USA) and rinsed three times with substrate-free media (N. viennensis, FWM; N. multiformis, HKM). Washed cells (N. viennensis, ca. 1 × 1011 total cells; N. multiformis, ca. 1 × 1010 total cells) were re-suspended into 10 ml of substrate-free growth medium for each strain in a 10 ml two-port microrespiratory (MR) chamber with fitted injection lids (Unisense, Aarhus, Denmark). Cell concentrations for microrespirometry experiments were chosen on the basis of comparable oxygen consumption rates between the two strains. O2 concentration was measured using an OX-MR 500 μm tip diameter MR oxygen electrode (Unisense), N2O concentration was measured using an N2O-500 N2O minisensor electrode with 500 μm tip diameter (Unisense), and NO was measured using an ami-600 NO sensor with 600 μm tip diameter (Innovative Instruments Inc., Tampa, FL, USA). The availability of O2 in the MR chamber, a closed system, corresponded to either ca. 207 μM O2 (FWM) or ca. 243 μM O2 (HKM) respectively, based on equilibrium O2 concentration at operating temperatures and medium salinities. For microrespirometry experiments involving ammonia oxidation, cells were provided 2 mM NH4Cl. The microrespirometry chamber was maintained at 37 °C and 28 °C for measurements with N. vienennsis and N. multiformis cells, respectively, reflecting their optimal growth temperatures.
Instantaneous measurement of NO from N. viennensis during oxidation of NH2OH
For experiments measuring the oxidation of NH2OH (99.999% purity, Sigma-Aldrich, St Louis, MO, USA), N. viennensis was provided with multiple additions of 200 μM NH2OH (based on chamber volume) to maintain a steady rate of O2 consumption. NO production was measured until O2 was undetectable in the chamber. Samples were taken post-experiment for NO2− measurements.
Instantaneous ammonia and hydroxylamine oxidation by N. viennensis in the presence of PTIO
Microrespirometry experiments with the NO-scavenger PTIO were performed with N. viennensis cells harvested as described above; cells were incubated with 200 μM PTIO in the dark with shaking at 37 °C for 1 h prior to adding the cells to a 2 ml 1-port MR chamber at 37 °C for the measurement of NH4+- and NH2OH-dependent O2 consumption (Supplementary Figure S1). Confirmation of the NO-scavenging activity of PTIO was confirmed chemically by addition of 1 μl PAPA NONOate ((Z)-1-[N-(3-aminopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate; Cayman Chemical, Ann Arbor, MI, USA; half-life of 15 min at 37 °C liberating 2 moles of NO per mole of parent compound) to FWM in the 2 ml MR chamber with the NO sensor at 37 °C. Once the rate of NO release from PAPA NONOate slowed, 200 μM PTIO was added to the chamber and NO disappearance was immediately measured. After ~7 min of NO-chelation by PTIO, another 1 μl of PAPA NONOate was added but NO levels remained below detection levels (Supplementary Figure S2).
Instantaneous measurement of N2O from media and killed-cell controls
To measure the abiotic production of N2O from either FWM or HKM, 10 ml of cell-free media was added to the 10 ml MR chamber. The NO-donor MAHMA NONOate (6-(2-Hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-hexanamine, NOC-9; Cayman Chemical) was added to the MR chamber in increasing additions of 20–100 μl, which is equivalent to the release of ca. 1.1–5.5 μM NO, or in a single addition of 100 μl (ca. 5.5 μM NO). The half-life of MAHMA NONOate at pH 7.4 is 1 min and 3 min at 37 °C and 22–25 °C, respectively. N2O production was measured using the N2O microelectrode during the decay of 1 mol MAHMA NONOate into 2 moles NO in either FWM or HKM. Experiments were performed at 37 °C and 28 °C for FWM and HKM, respectively, after sparging to ca. 0–3% O2 saturation with N2 (Praxair) as determined by O2 electrode. Chemical controls to confirm that FWM alone did not react with NH2OH to form measureable NO involved addition of 200, 400 and 600 μM NH2OH to sparged (ca. 0–3% O2) FWM or FWM+200 μM NO2− to reflect maximum concentration available once cells depleted MR chamber O2 (Supplementary Figures S3a and b). Control experiments with heat killed N. viennensis (ca. 1 × 1011 cells) were performed in sparged FWM+NaNO2 (200 μM) with measurement of NO and N2O upon addition of 200 μM NH2OH (Supplementary Figure S4) to determine whether NH2OH interacts with cellular debris.
Results
Effects of the NO-scavenger PTIO on N2O levels measured in cultures of N. viennensis and N. multiformis
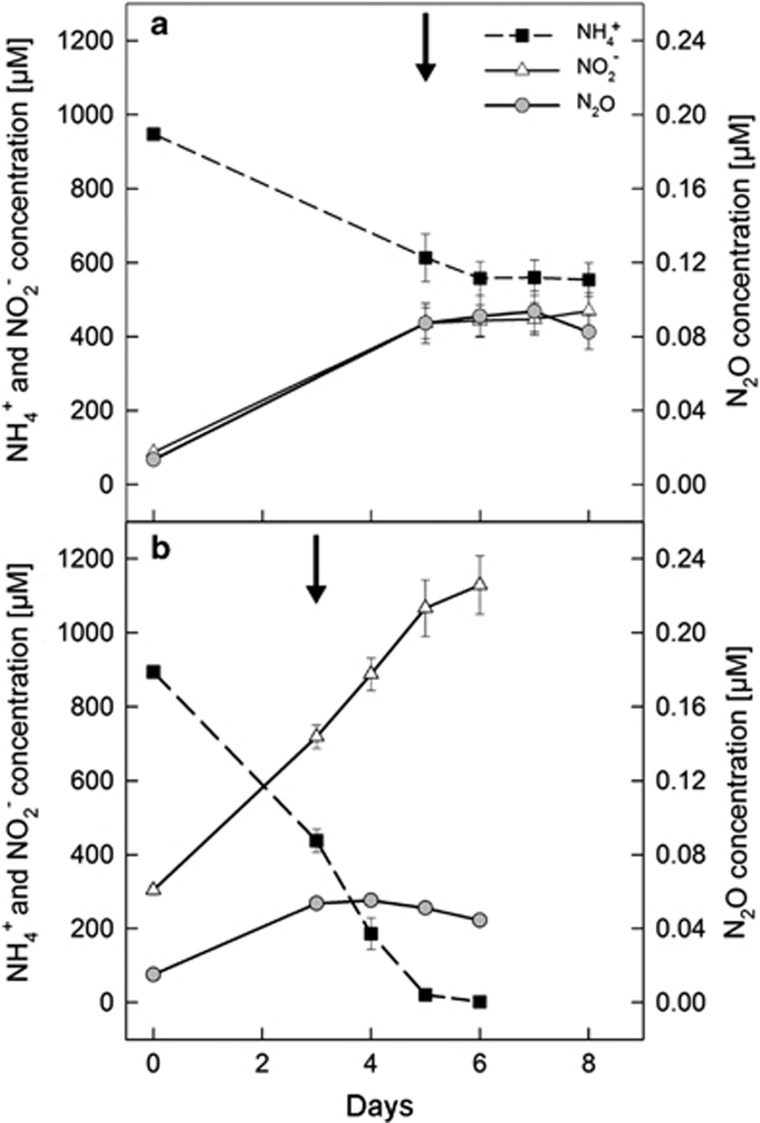
To investigate the role of NO in chemolithotrophic oxidation of ammonia to nitrite and generation of N2O in these two organisms, batch cultures of N. viennensis or N. multiformis were grown to mid-log phase, at which point PTIO (150 μM) was added (Figure 1). Addition of PTIO resulted in the immediate saturation of N2O levels in either culture as would be expected in the absence or decreasing levels of NO intermediates. However, in cultures of N. viennensis, PTIO also caused an inhibition of both ammonium consumption and nitrite production (Figure 1a), whereas in cultures of N. multiformis, ammonia oxidation and nitrite production continued at the same rate as before PTIO addition (Figure 1b). These results indicated that NO is an essential, dynamic, intermediate in the process of ammonia oxidation to nitrite and thus ammonia-dependent chemolithotrophy for N. viennensis, but not for N. multiformis.
Figure 1.
Inhibition of N. viennensis (a) and N. multiformis (b) with the NO-scavenger PTIO. Ammonium consumption (black squares, dotted line) and nitrite production (white triangles, solid line) as well as N2O production (gray circles, solid line) are plotted. The black arrow indicates the time point of PTIO addition (150 μgml−1) to the cultures. Mean values of five-fold replicated experiments with standard deviations are shown.
Dynamics of NO and N2O production during and following ammonia oxidation
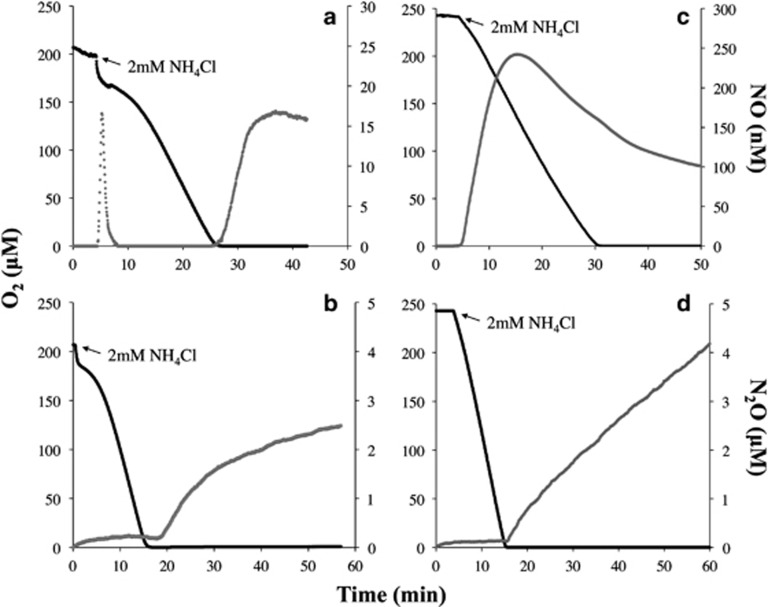
The effect of PTIO on growing cultures of N. viennensis and N. multiformis indicated different requirements for NO during ammonia oxidation. Using a MR chamber, the dynamics of NO production and consumption were measured during and after ammonia oxidation (2 mM NH4Cl) by N. viennensis or N. multiformis as determined by O2 consumption profiles (Figures 2a and c). To achieve an equivalent rate of O2 consumption for rate comparison, 10 times more N. viennensis than N. multiformis cells were required in the MR chamber. Whereas N. multiformis showed a linear rate of O2 consumption during ammonia oxidation (Figure 2c), the initial rate of O2 consumption by N. viennensis was quite rapid, followed by a slower, linear rate (Figures 2a and b). N. viennensis produced a maximum of ca. 1.41 nM of NO per 1 × 1010 cells (n=4) at the beginning of substrate oxidation, concomitant with the initial rapid rate of O2 consumption. The NO was immediately re-consumed as the cells achieved the slower, linear rate of O2 consumption (Figure 2a). After ca. 3 min from the point at which O2 became undetectable, N. viennensis cells began to release NO reaching a maximum of ca. 1.39 nM per 1x1010 cells (n=4). None of the NO released by N. viennensis following O2 depletion was re-consumed. In contrast, N. multiformis produced a maximum of ca. 92.15 nM NO per 1 × 1010 cells (n=4) and re-consumption of NO began once ca. 50% of the available O2 was consumed (Figure 2c).
Figure 2.
Instantaneous measurements of O2 (black line), NO (a and c) and N2O (b and d) (gray dots) after addition of 2 mM NH4Cl in liquid phase suspensions of N. viennensis (a and b) and N. multiformis (c and d) cells. Panels are single representative measurements of reproducible results (n=4). Note that y-axes for NO are on different scales for N. viennensis versus N. multiformis. N. viennensis cell concentration was 1011 cells per ml, whereas N. multiformis cell concentration was 1010 cells per ml to achieve equivalent rates of O2 consumption by the two strains.
Levels of N2O were measured during and following ammonia oxidation; however, no N2O was detectable during ammonia oxidation by cells of either microbe (Figures 2b and d). Assays including N. viennensis cells contained measurable N2O levels increasing at a non-linear rate after ca. 5 min following depletion of O2, yielding an average maximum at ca. 40 min of 0.19 μM per 1 × 1010 cells (Figure 2b; n=4). In contrast, assays including N. multiformis cells contained measurable N2O levels immediately upon O2 depletion increasing at a linear rate to an average maximum of 5.6 μM per 1 × 1010 cells at ca. 40 min (Figure 2d; n=4).
Dynamics of NO production and consumption during NH2OH oxidation by N. viennensis EN76T
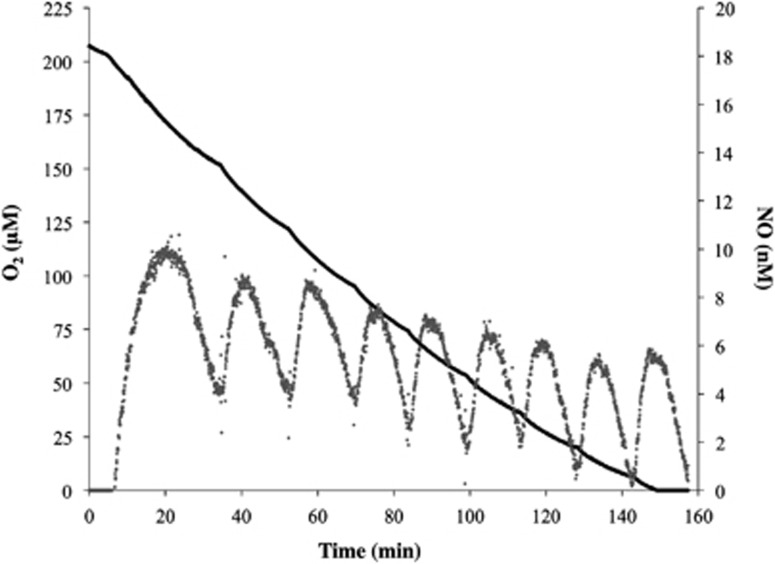
Although the experiments described above indicated that N. viennensis cultures produce and consume NO during ammonia oxidation to nitrite, it was not clear whether NO acted as an intermediate in the ammonia- or hydroxylamine-oxidizing step of the pathway. Therefore, we examined production and consumption of NO by N. viennensis cells when fed with NH2OH instead of ammonium. NH2OH was added in 200 μM pulses to the MR chamber containing N. viennensis cells to support linear O2 consumption until all of the available O2 was consumed (Figure 3). Each subsequent addition of equal aliquots of NH2OH led to the production of ca. 5 nM NO per 1 × 1010 cells (n=3), followed by an immediate re-consumption of NO and O2 until the next addition of NH2OH (Figure 3). NO2− accumulated to ca. 206 μM (n=3) in the culture medium, which matched the ca. 207 μM O2 consumed during the time course of the experiment. Importantly, free conversion of NH2OH to NO in the absence of cells was stochastic and insignificant (Supplementary Figure S3a). Addition of NH2OH to FWM containing 200 μM NaNO2 resulted in the production of ca. 4 nM NO but only once a concentration of 1.2 mM NH2OH was reached in the MR chamber (Supplementary Figure S3b).
Figure 3.
Instantaneous measurements of O2 consumption (black line) and NO production (grey dots) from 200 μM pulses of NH2OH in liquid phase incubations of N. viennensis in the absence of NH4+. Plot is a single representative of replicable experiments (n=4).
In an effort to demonstrate the requirement of NO by N. viennensis for the oxidation of either NH3 or NH2OH, washed N. viennensis cells were incubated with 200 μM PTIO for 1 h prior to measurement of NH4+- or NH2OH-dependent O2 consumption. The presence of PTIO did not prevent substrate-dependent O2 consumption of either substrate by N. viennensis cells (Supplementary Figure S1), although PTIO was able to effectively scavenge NO in cell-free FWM containing the NO-donating compound, PAPA-NONOate (Supplementary Figure S2).
Detection of abiotic N2O in growth media without viable cells
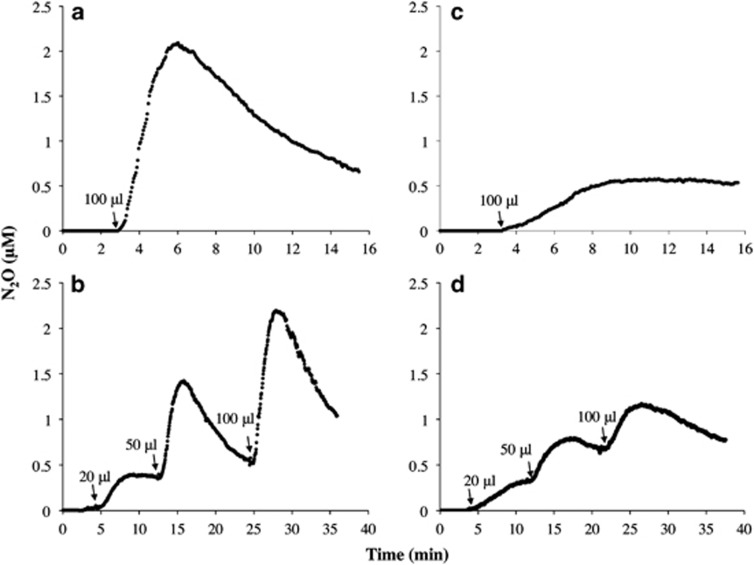
Inspired by the observed differences in N2O production profiles between cultures of N. viennensis and N. multiformis, we performed abiotic experiments in the MR chamber using cell-free FWM or HKM and the NO-donating compound, MAHMA NONOate. Addition of MAHMA NONOate to FWM released ca. 5.5 μM NO, 70% of which was converted to N2O (Figure 4a). In contrast, addition of an equal aliquot of MAHMA NONOate to HKM resulted in only a 20% conversion of the released NO to N2O (Figure 4c). Continuous addition of MAHMA NONOate to produce 1.1–5.5 μM NO in FWM or HKM resulted in a sustained high-efficiency conversion of released NO to N2O only in FWM, but not HKM (Figures 4b and d). Reactivity of NH2OH in FWM+heat-killed N. viennensis cells was also explored (Supplementary Figure S4). When NH2OH was introduced into the MR chamber containing heat-killed cells, accumulation of NO reached ca. 110 nM NO over 5 min (Supplementary Figure S4a). After 30 min, N2O accumulated to levels of ca. 90 μM, demonstrating that NH2OH was eventually converted to N2O in the absence of physiologically active cells (Supplementary Figure S4b).
Figure 4.
Abiotic production of N2O from the NO-donor MAHMA NONOate in either FWM (a and b) or HKM (c and d). Panels are single representative measurements of reproducible results (n=3). The addition of varying concentrations of MAHMA NONOate is indicated by arrows.
Discussion
The NO-scavenger, PTIO, stops ammonia-dependent chemolithotrophy of N. viennensis
The measured cessation of ammonium consumption and nitrite production upon PTIO addition to growing cultures of N. viennensis demonstrates the requirement of free NO for ammonia chemolithotrophy that was not observed for N. multiformis. These results confirm prior growth experiments with enrichment cultures (Jung et al., 2014) and reported effects on nitrite production and activity by ammonia-oxidizing Thaumarchaea and bacteria incubated with PTIO (Shen et al., 2013; Martens-Habbena et al., 2015). For both N. viennensis and N. multiformis, PTIO addition abolished N2O production, suggesting that the presence of the enzyme-generated free NO intermediate is required for formation of N2O production by both strains, regardless of whether NO is reduced biotically by enzyme activity or abiotically.
NO is produced and immediately consumed during active ammonia oxidation by N. viennensis EN76T
The initial, rapid production of NO followed by its equally rapid consumption during ammonia-dependent O2 consumption by N. viennensis differed from results in similar experiments with N. maritimus SCM1 (Martens-Habbena et al., 2015). In this prior study, N. maritimus SCM1 produced NO at a steady-state level prior to its consumption once NH4+ was depleted or its partial consumption at saturating concentrations of NH4+. A major difference in the two profiles observed for both cultures was that O2 levels remained quite high in assays with N. maritimus SCM1 such that complete consumption of NO was not observed as a function of time and O2 consumption as observed for N. viennensis EN76T. Even so, experiments with both N. viennensis and N. maritimus confirm that NO is being produced and consumed during ammonia oxidation. In addition, the present experiments demonstrate that NO is being released at the onset of anoxia. A likely fate of released NO at anoxia was its conversion to N2O, because 1000 times more N2O than NO was measured once the microrespirometry chamber reached anoxia, suggesting rapid conversion of released NO to N2O (Figures 2a and c). Another contributor to N2O levels measured in anoxic assays with N. viennensis cells could be the reactivity of cell components with released NH2OH as heat-killed cells showed a rapid conversion of exogenous NH2OH to measureable NO and N2O (Supplementary figure S4).
NO dynamics during ammonium-dependent O2 consumption by N. multiformis showed a vastly different profile compared to that of either N. viennensis or N. maritimus, revealing ca. 10 times more NO released from the cells, some of which was slowly re-consumed during ammonia oxidation and through anoxia. The comparison of NOx profiles in microrespirometry measurements with cells of N. multiformis and the ammonia-oxidizing Thaumarchaea reveals an intriguing difference in how NOx is metabolized during ammonia oxidation by bacteria and Thaumarchaea, which requires further investigation. Unlike N. viennensis, N2O production by N. multiformis during anoxia was linear and 10 times more N2O was produced per number of cells. This is a confirmatory evidence that ammonia-oxidizing bacteria, but not N. viennensis, are capable of producing N2O enzymatically via nitrifier denitrification (Stieglmeier et al., 2014b).
NO is produced and consumed during NH2OH oxidation to NO2− in N. viennensis
The rapid production and consumption of NO during NH2OH oxidation by N. viennensis along with the stoichiometric production of NO2− with O2 consumption suggest that NO is directly participating in the dehydrogenation of NH2OH. Recent models have postulated that NO is involved in providing reductant to ammonia monooxygenase (Schleper and Nicol, 2010; Stahl and de la Torre, 2012); however, if this were the case then the rapid production/consumption cycle of NO during NH2OH oxidation would not be observed. Experiments to demonstrate the role of NO in NH2OH oxidation by pre-incubating washed cells with PTIO with the goal to observe quenching of NH3 or NH2OH-dependent O2 consumption were inconclusive. It is possible that PTIO is only effective during active growth of N. viennensis (which was observed; Figure 1), or perhaps PTIO was ineffective at chelating rapidly cycling NO at the high cell densities used in the MR chamber.
N2O in N. viennensis cultures originates from the abiotic reaction of biotic N-oxide intermediates with medium or cellular components
Previous studies measuring N2O in pure and enrichments cultures of ammonia-oxidizing Thaumarchaea suggested an enzymatic origin of measured N2O (Santoro et al., 2011; Loscher et al., 2012; Jung et al., 2014); however, control experiments to test for abiotic reduction of NO to N2O, including by medium components, were not performed. We observed a high rate of NO reduction to N2O in FWM both in the presence of an NO-donating molecule and in the presence of NH2OH plus heat-killed cells. Based on the difference in metal content of both media, we propose that reduction of NO to N2O in FWM is facilitated by iron, which is present in FWM at a relatively high final concentration of 7.5 uMl−1 in the form of FeNaEDTA but absent from HKM. Under anoxic conditions, in which the metal components of the medium are reduced, the Fe(II) and reduced trace metals act as chemical catalysts for NO reduction to N2O. This ‘chemodenitrification' process has been implicated by hypothesis in contributing to abiotic N2O production in reduced environments where Fe(II) is abundant (Samarkin et al., 2010; Kampschreur et al., 2011; Jones et al., 2015).
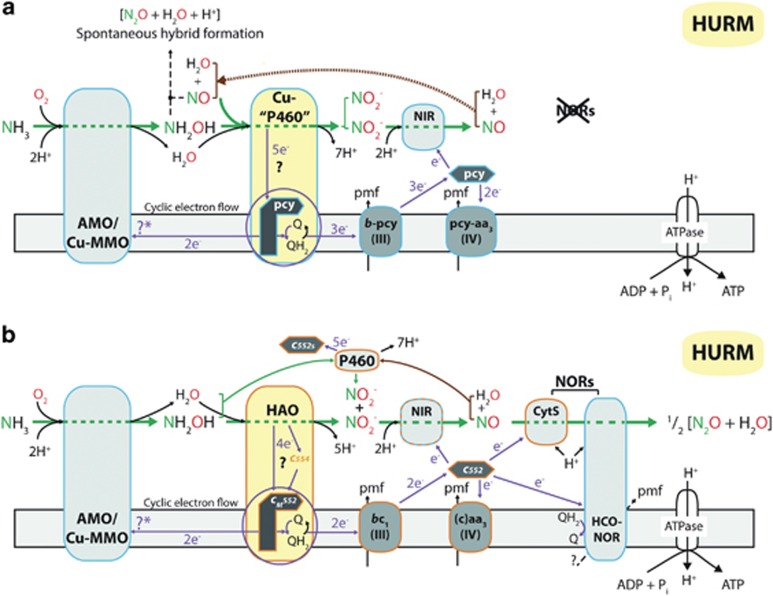
Proposed pathway for ammonia chemolithotrophy in ammonia-oxidizing Thaumarchaea in which NO facilitates NH2OH oxidation
Our revised model of ammonia-dependent chemolithotrophy of the Thaumarchaeota places NO as a necessary co-reactant for the oxidation of NH2OH to NO2− (Figure 5a). This NO-dependent dehydrogenation of NH2OH to NO2− is not based on novel chemistry because ammonia-oxidizing bacteria, and others such as aerobic methane-oxidizing bacteria, utilize the heme-containing cytochrome P460 enzyme to facilitate this reaction (Figure 5b; Simon and Klotz, 2013). Instead, the central reaction in the Thaumarchaeotal nitrification pathway is based on a proposed novel copper enzyme capable of performing known P460 activity. This model achieves the proper substrate stoichiometry and reductant flow. In addition, the modelled rapid cycling of NO (and, concomitantly electrons) to support NH2OH oxidation would logically preclude any enzymology for NO reduction to N2O. In agreement with this requirement, none of the sequenced genomes of ammonia-oxidizing Thaumarchaeota revealed the presence of canonical and alternate inventory for NO reduction to N2O. Nitrite reductase (nirK) is encoded in the genomes of all published sequences of ammonia-oxidizing Thaumarchaea (Bartossek et al., 2010, 2012) and nirK transcripts have been detected at very high steady-state levels in environmental metatranscriptomes (Hollibaugh et al., 2011; Radax et al., 2012), which makes this enzyme the most parsimonious source of the NO needed to support ammonia-dependent chemolithotrophy. The proposal that NO2− reduction and not NH2OH oxidation is the more likely source of the NO required for the oxidation of NH2OH to NO2− is supported by the following logic and reasoning:
Figure 5.
Proposed pathway for ammonia-dependent chemolithotrophy in the ammonia-oxidizing Thaumarchaea (a) compared with known pathways of N-oxide transformation in ammonia-oxidizing bacteria (b). The model presents a central role of NO in the oxidation of NH2OH, and its contribution to hybrid formation of N2O as proposed by Stieglmeier et al. (2014b). Due to the lack of heme proteins including HAO and quinone-reactive proteins such as cM552 (CycB), redox processes in ammonia-oxidizing archaea are likely mediated by Cu protein complexes (Walker et al., 2010; Stahl and de la Torre, 2012). The present literature suggests that NH3 is monooxygenated to NH2OH by ammonia monooxygenase (AMO) and that NH2OH is dehydrogenated to NO2− by activities of a number of unknown enzymes (Walker et al., 2010; Stahl and de la Torre, 2012; Vajrala et al., 2012). Based on existing chemistry facilitated by heme proteins in ammonia-oxidizing bacteria (b), the model in (a) proposes that the oxidation of NH2OH to NO2− and subsequent extraction of five electrons results from a reaction of NH2OH with NO and H2O facilitated by a novel Cu-containing enzyme. This could be one of the multi-copper oxidases encoded in all genomes of ammonia-oxidizing Thaumarchaeota (Bartossek et al., 2010, 2012; Walker et al., 2010). NO is provided by the Cu-containing NirK, which enzymatically reduces one NO2− per NH3 oxidized to NO. A fraction of the enzyme-produced NO and NH2OH could react to form N2O by hybrid formation. The figure was adapted from Simon and Klotz (2013). AMO/Cu-MMO, ammonia monooxygenase; c552, cytochrome c redox carrier; CytS: cytochrome c'-beta (see Simon and Klotz, 2013, and references therein); HAO, hydroxylamine dehydrogenase; HCO, heme-copper oxidase; HURM, hydroxylamine:ubiquinone redox module (see Simon and Klotz, 2013, and references therein); NirK, Cu-containing NO-forming nitrite reductase; NOR, nitric oxide reductase; P460, tetraheme cytochrome c protein P460 (CytL; see Simon and Klotz, 2013, and references therein); pcy, plastocyanin; pmf, proton-motive force; Q/QH2, quinone/quinol pool.
(1) A two-step oxidation of NH2OH to NO2− via a NO intermediate would require the operation of two enzyme complexes that feed extracted electrons (3+1) via two redox shuttles to two quinone-reactive enzymes. In addition to requiring additional unknown inventory, such a pathway would also not generate enough electrons needed to provide for effective linear electron flow (4−2−1=1). In contrast, the proposed one-step model provides for effective linear electron flow (5−2−1=2; Figure 5). The bioenergetics contrast stands in the context that an observed active NirK activity would draw one electron per reduced NO2− in both scenarios in addition to that the two-step model would include two linearly connected sources of NO production in a genomic background not encoding identifiable NO detoxification inventory and a scenario that should not lead to stoichiometric conversion of N-NH3 to N-NO2−.
(2) Isotopic measurements of 15N2O produced by N. viennensis suggest a ‘hybrid signature' in that one N atom would originate from NH3 (contributed as NH2OH) and one atom would originate from NO2− (contributed as NO) (Stieglmeier et al., 2014b). This finding also contradicts a two-step model and supports the model shown in Figure 5.
The proposed one-step model is parsimonious in that it requires the innovation of only one enzyme in ammonia-oxidizing Thaumarchaea. Based on existing knowledge, this novel enzyme is copper-based and facilitates known redox chemistry in context with known enzyme complexes such as the NH2OH-producing ammonia monooxygenase, NO-producing NirK, plastocyanin redox carriers and a quinone-reactive membrane protein, all of which are copper proteins and have been identified in all sequenced genomes of ammonia-oxidizing Thaumarchaea (Walker et al., 2010; Bartossek et al., 2012; Stahl and de la Torre, 2012).
We propose that the model of catabolic electron flow presented here (Figure 5a) applies to all obligate chemolithotrophic ammonia-oxidizing Thaumarchaea because it is based on and supported by results from above referenced experiments with marine ammonia-oxidizing Thaumarchaea including N. maritimus SCM1 and experiments with terrestrial ammonia-oxidizing Thaumarchaeota including the data presented here for N. viennensis EN76T.
Conclusion
The present study establishes that both ammonia-oxidizing Thaumarchaea and bacteria contribute to the production of N2O, although the mechanisms by which they do so are distinct. Whereas the ammonia-oxidizing bacteria produce N2O enzymatically through nitrifier denitrification, the ammonia-oxidizing Thaumarchaea release intermediates (NO and/or NH2OH), which are then reduced non-enzymatically to N2O in anoxic microenvironments (Zhu-Barker et al., 2015). Due to the relatively high abundance and activity of Thaumarchaea across terrestrial, freshwater, and marine environments (Zhang et al., 2010; Pratscher et al., 2011; French et al., 2012; Berg et al., 2015) and their established tolerance of low ammonium and oxygen environments (Martens-Habbena et al., 2009), their contributions to NOx emissions is likely of high global significance (Babbin et al., 2015). For instance, marine Thaumarchaea may be essential in providing a substantial concentration of NO to denitrifying microorganisms within oxygen minimum zones, and in return, the denitrifiers could provide organic carbon to the Thaumarchaeota to establish a nitrifying-denitrifying consortium (Karner et al., 2001; Beman et al., 2012; Ganesh et al., 2015). The present study also supports that both ammonia-oxidizing Thaumarchaea and bacterial ammonia-oxidizers likely contribute to chemodenitrification in terrestrial environments through the release and subsequent transformation of metabolites (NH2OH, NO and NO2−) either abiotically or via denitrifying consortia (Jones et al., 2015), which dominate in less oligotrophic environments. The elucidation of NO as an essential pathway intermediate and released metabolite of the ammonia-oxidizing Thaumarchaea in the absence of a nitrifier denitrificaiton pathway will allow refinement of the relative contributions of ammonia-oxidizing microorganisms to global N2O production.
Acknowledgments
JK was supported by graduate fellowship funds from Alberta Innovates Technology Futures. LYS was supported by a discovery grant from NSERC (RGPIN-2014-03745). Work in CS laboratory was supported by the Austrian Science Fund grant P25369. MGK was supported by NSF grant MCD1202648.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Arp DJ, Chain P, Klotz MG. (2007). The impact of genome analyses on our understanding of ammonia-oxidizing bacteria. Annu Rev Microbiol. 61: 503–528. [DOI] [PubMed] [Google Scholar]
- Babbin AR, Bianchi D, Jayakumar A, Ward BB. (2015). Nitrogen cycling. Rapid nitrous oxide cycling in the suboxic ocean. Science 348: 1127–1129. [DOI] [PubMed] [Google Scholar]
- Bartossek R, Nicol GW, Lanzen A, Klenk HP, Schleper C. (2010). Homologues of nitrite reductases in ammonia-oxidizing archaea: diversity and genomic context. Environ Microbiol 12: 1075–1088. [DOI] [PubMed] [Google Scholar]
- Bartossek R, Spang A, Weidler G, Lanzen A, Schleper C. (2012). Metagenomic analysis of ammonia-oxidizing archaea affiliated with the soil group. Front Microbiol 3: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beman JM, Popp BN, Alford SE. (2012). Quantification of ammonia oxidation rates and ammonia-oxidizing archaea and bacteria at high resolution in the Gulf of California and eastern tropical North Pacific Ocean. Limnol Oceanogr 57: 711–726. [Google Scholar]
- Berg C, Vandieken V, Thamdrup B, Jürgens K. (2015). Significance of archaeal nitrification in hypoxic waters of the Baltic Sea. ISME J 9: 1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clesceri LS, Greenberg AE, Eaton AD. (1998) Standard Methods for the Examination of Water and Wastewater. 20th edn, American Public Health Assoc: Washington, DC, USA. [Google Scholar]
- French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A. (2012). Ecophysiological characterization of ammonia-oxidizing archaea and bacteria from freshwater. Appl Environ Microbiol 78: 5773–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh S, Bristow LA, Larsen M, Sarode N, Thamdrup B, Stewart FJ. (2015). Size-fraction partitioning of community gene transcription and nitrogen metabolism in a marine oxygen minimum zone. ISME J 9: 2682–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Russo A, Samuni A. (2003). Reactions of PTIO and carboxy-PTIO with NO, NO2, and O2−. J Biol Chem 278: 50949–50955. [DOI] [PubMed] [Google Scholar]
- Hollibaugh JT, Gifford S, Sharma S, Bano N, Moran MA. (2011). Metatranscriptomic analysis of ammonia-oxidizing organisms in an estuarine bacterioplankton assemblage. ISME J 5: 866–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LC, Peters B, Pacheco JSL, Casciotti KL, Fendorf S. (2015). Stable isotopes and iron oxide mineral products as markers of chemodenitrification. Environ Sci Technol 49: 3444–3452. [DOI] [PubMed] [Google Scholar]
- Jung MY, Well R, Min D, Giesemann A, Park SJ, Kim JG et al. (2014). Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J 8: 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschreur MJ, Kleerebezem R, de Vet WWJM, van Loosdrecht MCM. (2011). Reduced iron induced nitric oxide and nitrous oxide emission. Water Res 45: 5945–5952. [DOI] [PubMed] [Google Scholar]
- Karner MB, DeLong EF, Karl DM. (2001). Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409: 507–510. [DOI] [PubMed] [Google Scholar]
- Kim BK, Jung M-Y, Yu DS, Park S-J, Oh TK, Rhee S-K et al. (2011). Genome sequence of an ammonia-oxidizing soil archaeon, ‘Candidatus Nitrosoarchaeum koreensis' MY1. J Bacteriol 193: 5539–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T et al. (2014). Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc Natl Acad Sci 111: 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krümmel A, Harms H. (1982). Effect of organic matter on growth and cell yield of ammonia-oxidizing bacteria. Arch Microbiol 133: 50–54. [Google Scholar]
- Loscher CR, Kock A, Konneke M, LaRoche J, Bange HW, Schmitz RA. (2012). Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences 9: 2419–2429. [Google Scholar]
- Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. (2009). Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979. [DOI] [PubMed] [Google Scholar]
- Martens-Habbena W, Qin W, Horak REA, Urakawa H, Schauer AJ, Moffett JW et al. (2015). The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ Microbiol. 17: 2261–2274. [DOI] [PubMed] [Google Scholar]
- Pratscher J, Dumont MG, Conrad R. (2011). Ammonia oxidation coupled to CO2 fixation by archaea and bacteria in an agricultural soil. Proc Natl Acad Sci 108: 4170–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radax R, Rattei T, Lanzen A, Bayer C, Rapp HT, Urich T, Schleper C. (2012). Metatranscriptomics of the marine sponge Geodia barretti: tackling phylogeny and function of its microbial community. Environ Microbiol 14: 1308–1324. [DOI] [PubMed] [Google Scholar]
- Samarkin VA, Madigan MT, Bowles MW, Casciotti KL, Priscu JC, McKay CP et al. (2010). Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nature Geosci 3: 341–344. [Google Scholar]
- Santoro AE, Buchwald C, McIlvin MR, Casciotti KL. (2011). Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333: 1282–1285. [DOI] [PubMed] [Google Scholar]
- Sayavedra-Soto L, Arp DJ. (2011) Ammonia-oxidizing bacteria: their biochemistry and molecular biology. In: Ward BB, Arp DJ, Klotz MG (eds), Nitrification. ASM Press: Washington, DC, USA, pp 11–37. [Google Scholar]
- Schleper C, Nicol GW. (2010). Ammonia-oxidising archaea– physiology, ecology and evolution. Adv Microb Physiol 57: 1–41. [DOI] [PubMed] [Google Scholar]
- Shen T, Stieglmeier M, Dai J, Urich T, Schleper C. (2013). Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrificiation inhibitors. FEMS Microbiol Lett 344: 121–129. [DOI] [PubMed] [Google Scholar]
- Simon J, Klotz MG. (2013). Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. Biochim Biophys Acta 1827: 114–135. [DOI] [PubMed] [Google Scholar]
- Skinner FA, Walker N. (1961). Growth of Nitrosomonas europaea in batch and continuous culture. Arch Microbiol 38: 339–349. [Google Scholar]
- Spang A, Poehlein A, Offre P, Zumbrägel S, Haider S, Rychlik N et al. (2012). The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: insights into metabolic versatility and environmental adaptations. Environ Microbiol 14: 3122–3145. [DOI] [PubMed] [Google Scholar]
- Stahl DA, de la Torre JR. (2012). Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol 66: 83–101. [DOI] [PubMed] [Google Scholar]
- Stieglmeier M, Klingl A, Alves RJE, Rittmann SK-MR, Melcher M, Leisch N et al. (2014. a). Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int J Syst Evol Microbiol 64: 2738–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A et al. (2014. b). Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T et al. (2011). Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci 108: 8420–8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA et al. (2012). Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci 110: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci 107: 8818–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-M, Offre PR, He J-Z, Verhamme DT, Nicol GW, Prosser JI. (2010). Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci 107: 17240–17245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB. (2015). The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126: 251–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.