Abstract
Background
This study aimed to investigate the inhibitory effect of blueberry anthocyanin (BBA) on Angiotensin II (Ang II)-induced apoptosis of human umbilical vein endothelial cells (HUVECs), and its regulation mechanisms involving Bax and Caspase 3.
Material/Methods
HUVECs were first treated by different concentrations of Ang II (10−9, 10−8, 10−7, 10−6, 10−5, and 10−4 mol/L) and BBA (80, 40, 20, 10, 5, and 2.5 μg/ml). After 24 h and 48 h of treatment, MTT was performed to detect the viability of HUVECs. Then, HUVECs were randomly divided into the Ang II group (10−6 mol/L Ang II) and Ang II + BBA group (10−6 mol/L Ang II and 20 μg/ml BBA), and the apoptosis rate was detected by flow cytometry. Western blot analysis was performed to detect the expression of Bax and Caspase 3 in these 2 groups. During the whole process, HUVECs without any treatments served as the control group.
Results
The cell viability of HUVECs was significantly reduced by Ang II in a time- and concentration-dependent manner (P<0.05), while BBA significantly elevated the cell viability of HUVECs until a peak of 20.0 μg/ml. The apoptosis rate of HUVECs was significantly increased by Ang II (P<0.01) and reduced by the BBA intervention (P<0.05). Ang II significantly elevated the expression of Bax and Caspase 3 in HUVECs, but their expression was significantly inhibited by BBA.
Conclusions
BBA increased cell viability and reduced apoptosis rate of HUVECs induced by Ang II through Bax- and Caspase 3-dependent pathways.
MeSH Keywords: Angiotensin II, Blueberry Plant, Caspase 3, Human Umbilical Vein Endothelial Cells
Background
As the basic component of vascular endothelium, endothelial cells are important in various aspects of vascular biology, including barrier function, blood clotting, vasoconstriction, and vasodilation [1]. Endothelial dysfunction is a common form of damage in endothelial cells, and generally results from various inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematous [2]. It is reported that endothelial dysfunction is a hallmark of vascular diseases, such as coronary artery disease [3], hypertension [4], and thrombosis [5]. Thus, endothelial dysfunction is considered to be a serious problem in clinical practice and urgently needs to be solved.
Endothelium-derived relaxing and contracting factors (EDRF/EDCF) are necessary in the regulation of vascular tone and blood flow distribution of organs [6]. Imbalance of these factors leads to endothelial damage and dysfunction [7]. Various EDCFs have been identified, including endothelin (ET), urotensin II (UTII), and angiotensin II (Ang II) [6]. Among these factors, Ang II is a peptide hormone involved in the renin-angiotensin-aldosterone system, which is extremely important in vasoconstriction and subsequent increased blood pressure [8,9]. Low concentrations of Ang II in vivo maintain normal structure and tension of the blood vessels, while increased Ang II leads to arterial hypertension and even vascular endothelial damage [10]. In addition, excessive Ang II is shown to be correlated with enhanced inflammation [11], calcium overload [12], and serious oxidative stress [13], which promote apoptosis of endothelial cells [14].
Flavonoid compounds have been reported to protect the cardiovascular system [15]. Blueberry anthocyanin (BBA) is a newly discovered flavonoid compound extracted from the fruit of the blueberry plant. BBA has been reported to have anti-inflammatory effects on endothelial cells through the nuclear factor-kappa B pathway, which could prevent chronic inflammations in various diseases [16]. Intake of BBA improved vascular function in healthy men in a time- and intake-dependent manner [17]. BBA was shown to be efficacious against senescence and light-induced damage in retinal pigment epithelium cells [18]. BBA is therefore considered to be effective in protection of endothelial cells via its antioxidant and anti-inflammation effects. However, little research has been conducted on the effects of BBA on Ang II-induced endothelial dysfunction and related mechanisms.
Human umbilical vein endothelial cells (HUVECs) are derived from the endothelium of veins in the umbilical cord, and are a popular laboratory model for research on the function and pathology of endothelial cells [19]. In the present study, endothelial damage in HUVECs was induced by Ang II, and the effects of BBA on cell viability and apoptosis of HUVECs were evaluated. We also analyzed the related protection mechanism of BBA with Bax and Caspase 3. Our findings may provide new insights for clinical treatment of cardiovascular diseases induced by endothelial dysfunction.
Material and Methods
Cultivation and identification of HUVECs
HUVECs (preserved from the pharmacology laboratory in the College of Basic Medical Sciences, Jilin University) were resuscitated by culturing in DMEM medium with high glucose (Gibco, USA) at 37oC. These cells were then removed to DMEM medium containing 10% FBS in an incubator (SANYO, Japan) at 37oC with 5% CO2. When 90% confluence was reached (3–5 days), these cells were digested with 0.25% trypsin and passaged.
Detection of cell viability
HUVECs in logarithmic growth phase were treated by different concentrations of Ang II (Sigma, USA) (10−9, 10−8, 10−7, 10−6, 10−5, and 10−4 mol/L) and BBA (preserved in our laboratory) (80, 40, 20, 10, 5, and 2.5 μg/ml). After 24 h and 48 h of treatment, tetrazolium (MTT) was used to detect the viability of HUVECs. We added 100 μg of MTT (Sigma, USA) into HUVECs seeded at a density of 0.5×103/well in 96-well plates. After 4 h of culturing, 150 μl of DMSO was added into each well, and optical density (OD) at 490 nm was detected by using an ultraviolet spectrophotometer (Bio-Rad, USA).
Detection of apoptosis rate
HUVECs in logarithmic growth phase were randomly divided into the Ang II group (treated with 10−6 mol/L Ang II for 2 h) and the Ang II + BBA group (sequentially treated by 10−6 mol/L Ang II for 2 h and 20 μg/ml BBA for 24 h). HUVECs without any treatments served as controls. The apoptosis rates of HUVECs in these 3 groups were detected by flow cytometry. Cells were first resuspended in 400 μl 1× binding buffer at a density of 1×106/ml. Then, 5 μl of Annexin V-FITC (Sigma, USA) was added and cells were incubated for 15 min in the dark at room temperature. After 5 min of incubation with 10 μl of Propidium iodide (PI), the fluorescence intensity of cells in each group was detected by flow cytometry (Bio-Rad, USA).
Western blot
Western blot analysis was performed to detect the expression of Bax and Caspase 3 in HUVECs treated by Ang II and Ang II + BBA. Cellular lysates from HUVECs in each group were first isolated by RIPA lysis buffer. Then, total proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on 12% polyacrylamide gels and transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was blocked with 5% skim milk in TBST for 2 h, and specific diluted primary antibody (anti-Bax, anti-Caspase 3, Sigma, USA) was added. After incubation overnight at 4°C, the membrane was washed with TBST 3 times and incubated for another 2 h at 25°C with FITC-conjugated secondary antibody (Sigma, USA). Finally, the samples were washed with TBST, and semi-quantitative gel images were analyzed by use of an automatic gel imager using Quantity One software (Bio-Rad, USA). During this process, GAPHD was used as the internal control.
Statistical analyses
All data are expressed as mean ± standard deviation (SD). Comparison between different groups was performed using the t-test (LSD-t), and a P-value less than 0.05 was considered to be significantly different. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL).
Results
The effects of Ang II and BBA on cell viability of HUVECs
To reveal the effects of Ang II and BBA on the HUVECs viability, MTT was conducted. As a result, the cell viability of HUVECs was gradually decreased with increased concentration of Ang II (P<0.05), and obvious inhibition emerged at the concentration of 10−6 mol/L (P<0.01). When Ang II concentration reached 10−4 mol/L, the inhibition rate of cell viability was 44% at 24 h and 50% at 48 h. Increased treatment time with Ang II were also found to reduce the cell viability of HUVECs (P<0.05). In contrast, BBA at relatively lower concentrations (until a peak concentration of 20.0 μg/ml) significantly elevated the cell viability of HUVECs, while higher concentrations reduced the cell viability (P<0.05). The prolonged intervention time with BBA also reduced the cell viability (P<0.05). However, the cell viability was significantly higher at 48 h than at 24 h at concentrations of 10.0 and 20.0 μg/ml (P<0.05) (Table 1).
Table 1.
Cell viability of human umbilical vein endothelial cells (HUVECs) treated by different concentrations of Ang II and blueberry anthocyanin (BBA) (n=5).
| Concentrations of Ang II | Cell viability (OD/%) | Concentrations of BBA | Cell viability (OD/%) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| 0 mol/L | 1.11±0.03 (100%) | 1.09±0.01 (100%) | 0 μg/ml | 1.15±0.02 (100%) | 1.18±0.02 (100%) |
| 10−9 mol/L | 0. 98±0.02 (90%)* | 0.98±0.01 (89%)*,# | 2.5 μg/ml | 1.23±0.04 (107%)* | 1.23±0.04 (104%)*,# |
| 10−8 mol/L | 0.93±0.01 (84%)* | 0.89±0.02 (81%)*,# | 5.0 μg/ml | 1.25±0.04 (109%)* | 1.26±0.03 (106%)*,# |
| 10−7 mol/L | 0.84±0.02 (76%)* | 0.80±0.02 (73%)*,# | 10.0 μg/ml | 1.27±0.01 (111%)* | 1.32±0.03 (112%)* |
| 10−6 mol/L | 0.80±0.01 (73%)** | 0.74±0.01 (68%)**,# | 20.0 μg/ml | 1.31±0.01 (114%)* | 1.39±0.01 (117%)**,# |
| 10−5 mol/L | 0.71±0.03 (66%)** | 0.66±0.03 (60%)**,# | 40.0 μg/ml | 1.22±0.01 (107%)* | 1.19±0.04 (102%)*,# |
| 10−4 mol/L | 0.62±0.03 (56%)** | 0.55±0.03 (50%)**,# | 80.0 μg/ml | 1.01±0.03 (89%)** | 0.99±0.07 (84%)**,# |
Represent significantly different at P<0.05 and P<0.01 when compared with HUVECs without treatment, respectively.
Represent significantly different at P<0.05 when compared with 24 h of treatment.
The effects of Ang II and BBA on apoptosis of HUVECs
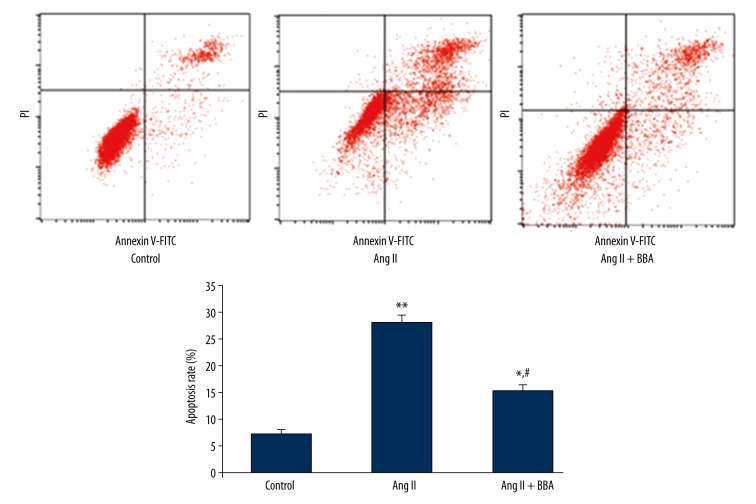
To evaluate the effects of Ang II and BBA on HUVECs apoptosis, Ang II (10−6 mol/L) and BBA (20.0 μg/ml) at optimum concentration were used to treat HUVECs. As shown in Figure 1, the apoptosis rate of HUVECs was significantly increased in the Ang II group (P<0.01). In the Ang II + BBA group, however, the apoptosis rate of HUVECs was significantly reduced, although the rate was still higher than that of the control group (P<0.05) (Figure 1).
Figure 1.
Apoptosis of human umbilical vein endothelial cells (HUVECs) treated by 10−6 mol/L Ang II and 20.0 μg/ml blueberry anthocyanin (BBA). *, ** Represent significantly different at P<0.05 and P<0.01, respectively, when compared with HUVECs without treatment (Control). # Represents significantly different at P<0.05 when compared with Ang II group.
Changed expression of Bax and Caspase 3 in HUVECs
To evaluate the anti-apoptosis mechanisms of BBA in HUVECs, the expression level of Bax and Caspase 3 was detected. As shown in Fig. 2, Ang II significantly elevated the expression of Bax and Caspase 3 in HUVECs. However, a significantly lower level of Bax and Caspase 3 was found in the Ang II + BBA group compared with that of the Ang II group. The expression level of Bax and Caspase 3 in the Ang II + BBA group was slightly higher than that of the control group (Figure 2).
Figure 2.
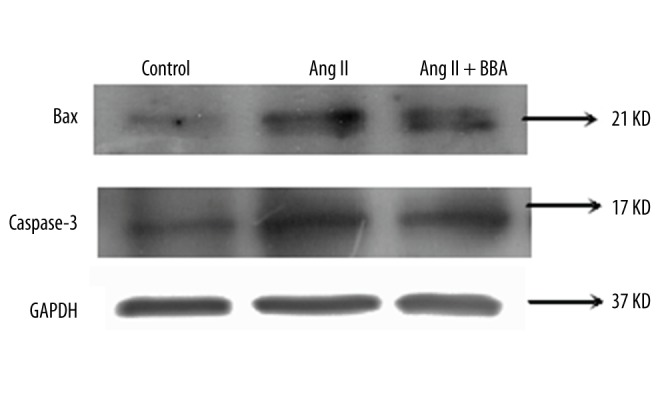
The expression of Bax and Caspase 3 in human umbilical vein endothelial cells (HUVECs) treated by 10−6 mol/L Ang II and 20.0 μg/ml blueberry anthocyanin (BBA). GAPHD was used as internal control.
Discussion
Endothelial dysfunction is defined as a systemic pathological state of the endothelium, which is closely related to cardiovascular diseases, including hypertension, hypercholesterolemia, and thrombosis [20]. The imbalance of EDRF/EDCF produced by endothelium is considered to be the main cause of endothelial dysfunction, and damage and apoptosis of vascular endothelial cells are always the starting point of these vascular diseases [21]. In the present study, EDCF Ang II was used to induce endothelial dysfunction in HUVECs, and an effective protective role of BBA was demonstrated in endothelial cells.
Previous studies have identified relationships between Ang II and endothelial dysfunction [10,22,23]. It has been reported that Ang II is associated with vascular oxidative stress and endothelial dysfunction, which can induce various vascular diseases [24,25]. Ang II was also shown to be able to progressively induce autophagy, senescence, and apoptosis in HUVECs [26]. In the present study, the cell viability of HUVECs was significantly reduced by Ang II in a time- and concentration-dependent manner, and the apoptosis rate was increased by Ang II. Our results agree with previous studies and further illustrate the role of Ang II in endothelial dysfunction. In special mechanisms of this process, Ang II may increase the permeability of blood vessels, induce uptake and oxidation of LDL, promote generation of ROS and inactivation of NO, and finally lead to apoptosis of endothelial cells [27,28].
BBA, a newly discovered flavonoid compound extracted from blueberries, is effective in protection of endothelial cells [18,29]. In addition, the antioxidant and anti-inflammation roles of BBA were reported to be able to significantly protect and improve vascular functions [17,30]. In the present study, BBA at an optimum concentration of 20.0 μg/ml significantly increased the cell viability and reduced the apoptosis rate of HUVECs. This phenomenon is consistent with the special antioxidant and anti-inflammation characteristics of BBA, and illustrate that BBA benefits HUVECs. Our results also demonstrate the significantly decreased expression of Bax and Caspase 3 after intervention with BBA. Bax is a member of the BCL2 family and acts as a pro-apoptotic regulator in a variety of cellular activities [31]. In healthy mammalian cells, Bax inactive monomer mainly is distributed in cytosol. When apoptotic signaling emerges, the molecular structure of Bax shifts, then permeability transition pores of mitochondria open via interaction with a voltage-dependent anion channel, and the mitochondrial membrane is finally destroyed [32]. Activated BAX induces the release of cytochrome c and other pro-apoptotic factors [33]. Caspase 3 is a member of the cysteine-aspartic acid protease family and plays a central role in cell apoptosis [34]. In apoptotic cells, active Caspase 3 leads to chromatin condensation, nuclear destruction, mRNA degradation, and enzyme inactivation [35]. To sum up, the decreased expression of Bax and Caspase 3 indicates that the potential inhibition mechanisms of BBA on apoptosis of HUVECs may be related to Bax- and Caspase 3-dependent pathways.
Conclusions
In conclusion, BBA can recover the reduced cell viability and inhibit apoptosis of HUVECs induced by Ang II through Bax- and Caspase 3-dependent pathways. The ability of BBA to protect vascular endothelial cells illustrates that BBA should be considered as a drug candidate in the treatment of cardiovascular diseases resulting from endothelial dysfunction.
Footnotes
Statement
The authors declare that they have no competing interests.
Source of support: This work was supported by the International Cooperation Project of Jilin Science and Technology Department (No. 140402GH010315031), the China Jilin Forest Industry Group (No. 2010220101002181), and the Development and Reform Commission of Jilin Province (No. 2013C0302)
References
- 1.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 2.Steyers CM, III, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–49. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veerasamy M, Bagnall A, Neely D, et al. Endothelial dysfunction and coronary artery disease: a state of the art review. Cardiol Rev. 2015;23:119–29. doi: 10.1097/CRD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 4.Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep. 2015;17:85. doi: 10.1007/s11906-015-0596-3. [DOI] [PubMed] [Google Scholar]
- 5.Loscalzo J. Oxidative stress in endothelial cell dysfunction and thrombosis. Pathophysiol Haemost Thromb. 2002;32:359–60. doi: 10.1159/000073600. [DOI] [PubMed] [Google Scholar]
- 6.Rubanyi GM. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991;46:27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- 7.Kimura S. Endothelium-derived contracting and relaxing factors. Kokyu To Junkan. 1992;40:1043–49. [PubMed] [Google Scholar]
- 8.Kang G, Lee YR, Joo HK, et al. Trichostatin a modulates angiotensin II-induced vasoconstriction and blood pressure via inhibition of p66shc activation. Korean J Physiol Pharmacol. 2015;19:467–72. doi: 10.4196/kjpp.2015.19.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becher UM, Endtmann C, Tiyerili V, et al. Endothelial damage and regeneration: The role of the renin-angiotensin-aldosterone system. Curr Hypertens Rep. 2011;13:86–92. doi: 10.1007/s11906-010-0171-x. [DOI] [PubMed] [Google Scholar]
- 10.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 11.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–77. [PubMed] [Google Scholar]
- 12.Alvin Z, Laurence GG, Coleman BR, et al. Regulation of L-type inward calcium channel activity by captopril and angiotensin II via the phosphatidyl inositol 3-kinase pathway in cardiomyocytes from volume-overload hypertrophied rat hearts. Can J Physiol Pharmacol. 2011;89:206–15. doi: 10.1139/Y11-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pueyo ME, Gonzalez W, Nicoletti A, et al. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 14.Yang HY, Bian YF, Zhang HP, et al. Angiotensin-(1–7) treatment ameliorates angiotensin II-induced apoptosis of human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol. 2012;39:1004–10. doi: 10.1111/1440-1681.12016. [DOI] [PubMed] [Google Scholar]
- 15.Tenorio Lopez FA, del Valle Mondragon L, Pastelin Hernandez G. Flavonoids and the cardiovascular system: can they be a therapeutic alternative? Arch Cardiol Mex. 2006;76(Suppl 4):S33–45. [PubMed] [Google Scholar]
- 16.Huang WY, Liu YM, Wang J, et al. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules. 2014;19:12827–41. doi: 10.3390/molecules190812827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, et al. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr. 2013;98:1179–91. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Song X, Zhang D, et al. Blueberry anthocyanins: Protection against ageing and light-induced damage in retinal pigment epithelial cells. Br J Nutr. 2012;108:16–27. doi: 10.1017/S000711451100523X. [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Zhang Y, Georgescu SP, et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2:93–102. doi: 10.1007/s12015-006-0015-x. [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Yang CB, Gao L, Zhao JJ. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review) Exp Ther Med. 2015;9:3–10. doi: 10.3892/etm.2014.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luscher TF. Vascular protection: Current possibilities and future perspectives. Int J Clin Pract Suppl. 2001;(117):3–6. [PubMed] [Google Scholar]
- 22.Shatanawi A, Romero MJ, Iddings JA, et al. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol. 2011;300:C1181–92. doi: 10.1152/ajpcell.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomolak JR, Didion SP. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol. 2014;5:396. doi: 10.3389/fphys.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, Zhang C, Ma X, et al. Angiotensin-(1–7) counteracts angiotensin II-induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp Cell Res. 2015;336:58–65. doi: 10.1016/j.yexcr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane MO, Etienne-Selloum N, Madeira SV, et al. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: preventive effect of red wine polyphenols. Pflugers Arch. 2010;459:671–79. doi: 10.1007/s00424-009-0759-7. [DOI] [PubMed] [Google Scholar]
- 26.Shan H, Guo D, Li X, et al. From autophagy to senescence and apoptosis in Angiotensin II-treated vascular endothelial cells. APMIS. 2014;122:985–92. doi: 10.1111/apm.12242. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Li S, Wu H, et al. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int J Mol Med. 2015;36(5):1223–32. doi: 10.3892/ijmm.2015.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C, Liu Z, Liu K, Yang P. Mechanisms of Ghrelin anti-heart failure: inhibition of Ang II-induced cardiomyocyte apoptosis by down-regulating AT1R expression. PLoS One. 2014;9:e85785. doi: 10.1371/journal.pone.0085785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang D, Liu Y, et al. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J Sci Food Agric. 2015;95:936–44. doi: 10.1002/jsfa.6765. [DOI] [PubMed] [Google Scholar]
- 30.Riso P, Klimis-Zacas D, Del Bo C, et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013;52:949–61. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 31.Barclay LA, Wales TE, Garner TP, et al. Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism. Mol Cell. 2015;57:873–86. doi: 10.1016/j.molcel.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan KO, Fu NY, Sukumaran SK, et al. MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci USA. 2005;102:14623–28. doi: 10.1073/pnas.0503524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng C, Li Y, Xu D, et al. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- 34.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 35.Coudray AM, Louvet C, Kornprobst M, et al. Increased anticancer activity of the thymidylate synthase inhibitor BGC9331 combined with the topoisomerase I inhibitor SN-38 in human colorectal and breast cancer cells: Induction of apoptosis and ROCK cleavage through caspase-3-dependent and -independent mechanisms. Int J Oncol. 2005;27:553–61. [PubMed] [Google Scholar]