Abstract
Background
Pulmonary arterial hypertension (PAH) is a fatal disease characterized by impaired regulation of pulmonary artery vascular growth and remodeling. Aberrant expression of miR-17 has been shown to be involved in the pathogenesis of PAH, but its underlying molecular mechanism has not been elucidated.
Material/Methods
Mitofusin 2 (MFN2) expression was determined by qRT-PCR. The protein expression levels of MFN2, proliferating cell nuclear antigen (PCNA), and pro-apoptotic protein cleaved Caspase-3 were measured using Western blot analysis. Cell proliferation and apoptosis were assessed by CellTiter-Glo reagent and flow cytometry, respectively. Caspase-3/7 activity was measured using an Apo-ONE Homogeneous Caspase-3/7 assay kit. The regulation of miR-17 on MFN2 expression was assessed using luciferase reporter assay system.
Results
miR-17 expression was upregulated in human pulmonary artery smooth muscle cells (hPASMCs) treated with hypoxia and lung tissues of PAH patients. Inhibition of miR-17 suppressed hypoxia-induced proliferation and promoted apoptosis in hPASMCs. miR-17 inhibited MFN2 expression by binding to its 3′-UTR. Decreased cell viability and increased apoptosis and Caspase-3 activity were observed in the anti-miR-17 + siNC group compared with the anti-miR-NC + siNC group. The expression of cleaved Caspase-3 was upregulated and the expression of PCNA was downregulated in the anti-miR-17 + siNC group. Moreover, these alterations were attenuated by knockdown of MFN2.
Conclusions
miR-17 regulates proliferation and apoptosis in hPASMCs through MFN2 modulation. We found that miR-17 acts as a potential regulator of proliferation and apoptosis of hPASMCs, and that it might be developed as a promising new strategy for the treatment of PAH.
MeSH Keywords: Apoptosis, Cell Proliferation, MicroRNAs, Mitochondrial Dynamics, Pulmonary Artery
Background
Pulmonary arterial hypertension (PAH) is a chronic and progressive disease, which is diagnosed by an increased mean pulmonary arterial pressure of >25 mmHg in resting state or 30 mmHg with exercise, measured by right heart catheterization [1]. Increased lung vascular resistance and pressure during severe PAH could result in right heart failure and death [2]. The 3- and 5-year survival rates of idiopathic PAH patients were 38% and 17%, respectively. Several types of specific drugs are used for the clinical treatment of PAH, including prostanoids, endothelin receptor antagonists, and phosphodiesterase type 5 inhibitors [3]. Various treatments have been used during the past few decades, but there is still no curative treatment for PAH [4]. The exact molecular mechanism of PAH is not clear, but several pathological changes of pulmonary vasculars have been found in PAH patients, which include pulmonary vascular remodeling, pulmonary vasoconstriction, and thickening of pulmonary artery walls. The smooth muscle layer plays a vital role in the pathogenesis of PAH [5]. Excessive proliferation of pulmonary artery smooth muscle cells (PASMCs) and apoptotic resistance contribute to irreversible pulmonary arterial remodeling, leading to decreased luminal diameters and, eventually, the blockage of resistance-level pulmonary arteries [6].
Apoptosis is an important physiological process in multicellular organisms and is essential for cell development and differentiation [7]. Intracellular zymogens termed caspases and cysteine-dependent aspartate specific protease are activated during apoptosis. Dysregulated apoptosis contributes to the development of many kinds of pathological conditions, including cancer and autoimmune and neurodegenerative diseases [8]. In apoptosis induced by multiple types of stimuli, mitochondria play a vital role in regulating Caspase activity through the production of cytochrome c [9]. Both PAEC apoptosis and endothelial dysfunction are considered to play a key role in the early phase of the pathogenesis of PAH. Excessive proliferation and migration of medial cells, including PASMC, fibroblasts, and PAEC, contribute to the pulmonary vascular remodeling [10].
microRNAs (miRNAs) are approximately 22 nucleotide-long, noncoding, small RNAs that regulate gene expression at the post-transcriptional level through translational repression or mRNA degradation. Aberrant expression of miRNAs has been found in cancer, neurodegenerative disease, immune system diseases, and cardiovascular disease. miR-182 facilitates clonal expansion of activated helper T lymphocytes and regulates IL-2-driven helper T cell-mediated immune responses in vitro and in vivo [11]. miR-21 negatively regulates the expression of tumor suppressor programmed cell death protein 4 and promotes invasion, intravasation, and metastasis in colorectal cancer [12]. In recent years, a large amount of evidence suggests that miRNAs are implicated in the pathologic process of PAH. A study has reported that miR-145 is overexpressed in lung tissues of patients with PAH when compared with health control individuals matched with the patients. Moreover, anti-miR-mediated downregulation of miR-145 prevents the development of PAH in mice exposed to hypoxia [13]. A previous study showed that miR-17 was transiently upregulated in the hypoxia-induced pulmonary hypertension mouse model. Moreover, inhibition of miR-17 improves heart and lung function in experimental pulmonary hypertension models by interfering with lung vascular and right ventricular remodeling [14]. Zhang et al. have reported that knockdown of MFN2 inhibits hypoxia-induced proliferation of PASMCs and the PI3K/Akt signaling pathway is critical for the promotive effect of MFN2 on PASMC proliferation. MFN2 contributes to cell cycle progression in the proliferation of PASMC, thus promoting pulmonary vascular remodeling [15]. Therefore, we speculated that downregulation of miR-17 might inhibit hPASMC proliferation and attenuate PAH, at least partially by targeting MFN2. In this study we demonstrated the crucial role of miR-17 in the pathologic process of PAH and investigated its underlying molecular mechanism.
Material and Methods
Acquisition of human lung tissues
Human lung tissues were acquired from idiopathic PAH patients undergoing lung transplantation (n=10) and from normal controls (normal lung tissues adjacent to benign lung tumors; n=10). This experiment was approved by the Ethics Committee of our hospital, and all the participants gave informed consent before the study.
Cell culture
Human pulmonary artery smooth muscle cells (hPASMCs; Cascade Biologics Inc., Portland, OR) were cultured in SmGM-2 BulletKit media (Lonza, Basel, Switzerland) containing 5% (volume/volume) heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA), 0.5 ng/ml human recombinant epidermal growth factor, 2 ng/ml human recombinant fibroblast growth factor, 5 μg/ml insulin, and 50 μg/ml gentamicin. HEK293 cells were maintained in DMEM supplemented with 10% heat-inactivated FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere containing 5% CO2. Cells at passages 6–8 were used for experiments. For induction of hypoxia, cells were transferred in a special hypoxia incubator (Thermo Scientific, model 3130, Rockford, IL) with 3% O2, 5% CO2, and balanced nitrogen. The O2 concentration inside the chamber was detected continuously by using an oxygen monitor (Hudson Ventronics Division, CA) to ensure that the O2 concentration was 3%.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated from human pulmonary artery smooth muscle cells with Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The purity of total RNAs was evaluated using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific) at 260 nm and 280 nm. cDNA was synthesized from 1 μg of total RNA by using a ImProm-II™ Reverse Transcription System (Promega, Madison, WI) in accordance with the manufacturer’s instructions. qPCR was conducted with a SensiFAST SYBR No-ROX kit (Bioline, Taunton, USA) in 7300 sequence detection system (Applied Biosystems, Foster City, CA). β-actin and U6 small nuclear RNA genes were used as internal controls for mitofusin 2 (MFN2) and miR-17 mRNAs expression, respectively. The primer sequences were as follows:
miR-17 forward: 5′-GCAGGAAAAAAGAGAACATCACC-3′,
miR-17 reverse: 5′-TGGCTTCCCGAGGCAG-3′;
U6 forward: 5′-CTCGCTTCGGCAGCACA-3′,
U6 reverse: 5′-AACGCTTCACGAATTTGCGT-3′;
MFN2 forward 5′-ATTCAGAAAGCCCAGGGCATG-3′,
MFN2 reverse 5′-GACCGTGTGCTGCTCAAACTTG-3′;
β-actin forward: 5′-TGAGAGGGAAATCGTGCGTGAC-3′,
β-actin reverse: 5′-AAGAAGGAAGGCTGGAAAAGAG-3′.
The relative expression levels were calculated using the 2−ΔΔCt method and normalized against the control gene. Each experiment was repeated 3 times.
Western blot analysis
At 48 h after transfection, cells were harvested and total proteins were isolated with RIPA reagents (Thermo Scientific). The protein concentration was detected using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Equal amount of proteins per sample were separated by SDS-polyacrylamide gel electrophoresis, and then transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dry milk powder in Tris-buffered saline containing 0.1% Tween-20 (Tris 20 mM, NaCl 150 mM, and Tween 20 0.1%, pH 7.6; TBST) for 1 h at room temperature, and then incubated with anti-MFN2 antibody (Abcam, Cambridge, MA), anti-PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cleaved Caspase-3 antibody (Abcam) or anti-β-actin antibody (Sigma-Aldrich, Louis, MO) overnight at 4°C. β-actin was used as an internal control. After 3 washes with TBST (10 min each), membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. After 3 washes with TBST (15 min each), blots were visualized with an enhanced chemiluminescence kit (Pierce, Rockford, IL) and then exposed to X-ray film. Protein bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Proliferation assay
Transfected hPASMCs (1×104) were plated into each well of 96-well plates, followed by incubation for 48 h in hypoxic conditions. Proliferation assay was performed using CellTiter-Glo reagent (Promega) according to the manufacturer’s protocol. The supernatant was removed, and 100 μl of SmGM-2 BulletKit media containing 20 μl of 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium, and inner salt (MTS)/phenazine methosulfate (PMS) mixture was added to each well for incubating another 4 h at 37°C in hypoxia. The optical density (OD) values were read at an absorption of 490 nm on a microplate scanner (BioTek, Winooski, VT).
Apoptosis assay
Transfected hPASMCs were re-plated in 96-well plates and cultured for 48 h in hypoxia. The apoptosis assay was carried out by using an Apo-ONE homogeneous kit (Promega). Caspase-3/7 activity was measured as a second parameter of apoptotic cell death. Fluorescence-activated cell sorter (FACS) was also used to measure the apoptosis rate by using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences Pharmingen, San Jose, CA). After 48 h of incubation in hypoxia, hPASMCs were harvested, washed twice with cold phosphate-buffered saline (PBS), and then stained with Annexin V-FITC and propidium iodide (PI) in the dark. Stained cells were identified and analyzed by a FACScan flow cytometer (Becton Dickinson, CA). The data analysis was performed using FACStation software (Becton Dickinson).
Luciferase assay
miR-17 mimics, anti-miR-17, siMFN2, and the luciferase reporter constructs fused with the 3′-UTR of human MFN2 were purchased from GenePharma (GenePharma Co., Ltd., Shanghai, China). hPASMCs were plated into a 24-well plate 1 day before transfection to reach approximately 80% confluence in the day of transfection. hPASMCs were co-transfected with 0.4 μg of wild-type or mutant MFN2 plasmid constructs and 50 nM miR-17 mimics or control miRNA using Lipofectamine 2000 (Invitrogen). At 48 h after transfection, firefly and Renilla luciferase activities were measured with the Dual-Luciferase Assay System (Promega) according to the manufacturer’s protocol.
Statistical analysis
Data are presented as the means ± standard deviation (SD). The statistical analysis was conducted by SPSS 16.0 software (SPSS Inc., Chicago, IL). The means were compared using student’s t-test between groups or one-way analysis of variance test among groups. Statistical significance was determined at a P<0.05 level.
Results
miR-17 is upregulated and MFN2 is downregulated in hPASMCs treated with hypoxia and lung tissues of PAH patients
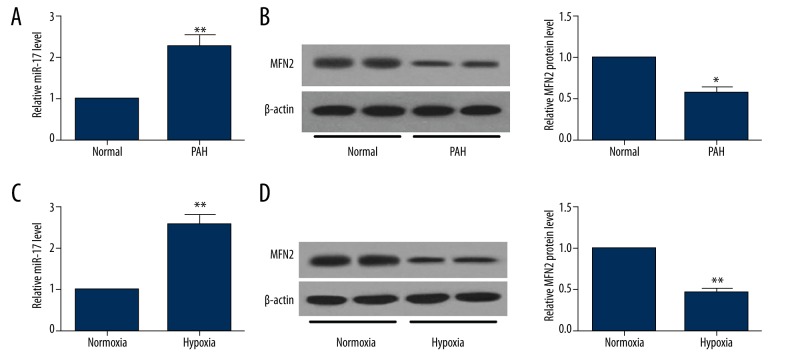
The expression levels of miR-17 and MFN2 in normal lung tissues and PAH tissues were determined by qRT-PCR and Western blot, respectively. miR-17 expression (Figure 1A) was upregulated and MFN2 protein (Figure 1B) was downregulated in PAH tissues compared with matched normal lung tissues. In addition, expression of miR-17 was also increased in hPASMCs exposed to hypoxia compared with that in normoxic controls (Figure 1C). A reduction of MFN2 protein level was observed in hPASMCs treated with hypoxia compared to controls (Figure 1D). These results suggest that downregulation of miR-17 and upregulation of MFN2 protein may be involved in the pathogenesis of PAH.
Figure 1.
The expression levels of miR-17 and MFN2 in lung tissues of PAH patients and hPASMCs under hypoxic condition. qRT-PCR and Western blot analysis were performed to detect the levels of miR-17 (A) and MFN2 protein (B) in lung homogenates from PAH patients (n=10) or normal controls (n=10). After hPASMCs were exposed to hypoxia for 48 h, cells were harvested to determine the expression of miR-17 (C) and MFN2 protein (D). Data are expressed as the mean ±SD. * P<0.05, ** P<0.01.
Inhibition of miR-17 suppresses hypoxia-induced hPASMC proliferation in vitro
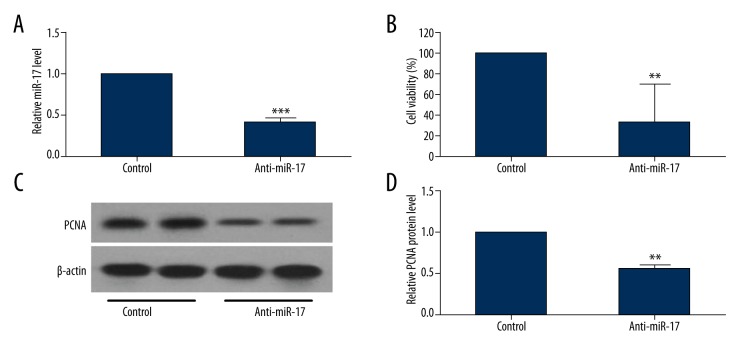
miR-17 expression was found to be upregulated in PAH tissues and hPASMCs, suggesting that miR-17 may function in regulating the proliferative phenotype of the pulmonary vasculature. To investigate the effect of miR-17 in hPASMC proliferation, hPASMCs were transfected with anti-miR-17 or control inhibitor (anti-miR-NC). The effect of miR-17 inhibitor was confirmed by using qRT-PCR method (Figure 2A). Proliferation of hPASMCs exposed to normoxia or hypoxia was determined by using the CellTiter-Glo Luminescent cell viability assay kit. As shown in Figure 2B, the number of viable cells was greater in the miR-17 inhibitor group as compared with the control group, indicating that inhibition of miR-17 suppressed hypoxia-induced hPASMC proliferation. Western blot assay showed that the protein expression level of the proliferation marker PCNA was decreased in hPASMCs transfected with anti-miR-17 compared with that in hPASMCs transfected with anti-miR-NC (Figure 2C, 2D).
Figure 2.
Inhibition of miR-17 downregulates hPASMC proliferation. hPASMCs were transfected with anti-miR-17 or control miRNAs. (A) At 48 h after transfection, miR-17 level was determined by using qRT-PCR. (B) Proliferation of hPASMCs transfected with anti-miR-17 was determined by using CellTiter-Glo Luminescent cell viability assay kit. (C, D) Western blot analysis was conducted to detect the protein expression level of the proliferation marker PCNA in hPASMCs transfected with anti-miR-17. Data are expressed as the mean ±SD (n=3). ** P<0.01, *** P<0.001.
Inhibition of miR-17 promotes apoptosis in hPASMCs
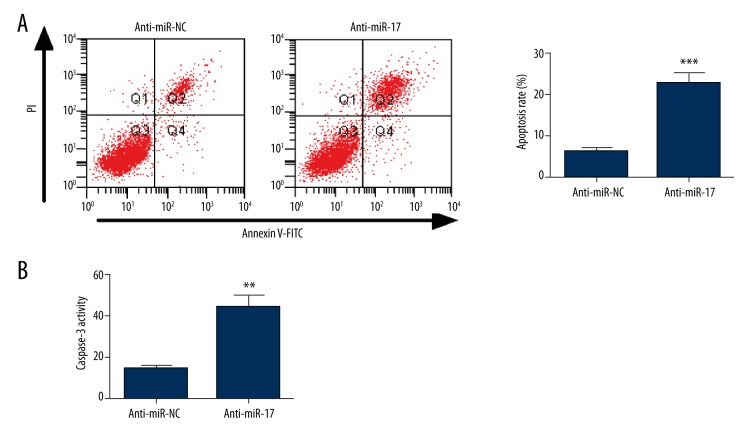
Decreased apoptotic activity has been found to exist in hPASMCs from PAH patients. To confirm the effect of miR-17 on hPASMC apoptosis, the apoptosis rate was measured by a flow cytometry. We found that the number of apoptotic hPASMCs was remarkably increased in the anti-miR-17 group compared with the control group (Figure 3A). Furthermore, cell apoptosis was evaluated using a Caspase 3/7 assay kit. The results showed that the Caspase-3 activity in hPASMCs transfected with miR-17 inhibitor was increased when compared with that in hPASMCs transfected with negative controls (Figure 3B). Taken together, these results suggest that inhibition of miR-17 contributes to apoptosis in hPASMCs.
Figure 3.
Inhibition of miR-17 increases apoptosis in hPASMCs. At 48 h post-transfection, flow cytometry and Caspase 3/7 activity assay were performed to assess cell apoptosis. (A) An increased cell apoptosis rate was observed in the anti-miR-17 group compared with the anti-miR-NC group. (B) Caspase 3 activity was remarkably increased in hPASMCs transfected with anti-miR-17 as compared with that in hPASMCs transfected with negative control. Data are expressed as the mean ±SD (n=3). ** P<0.01, *** P<0.001.
miR-17 directly inhibits expression of MFN2 via its 3′-UTR
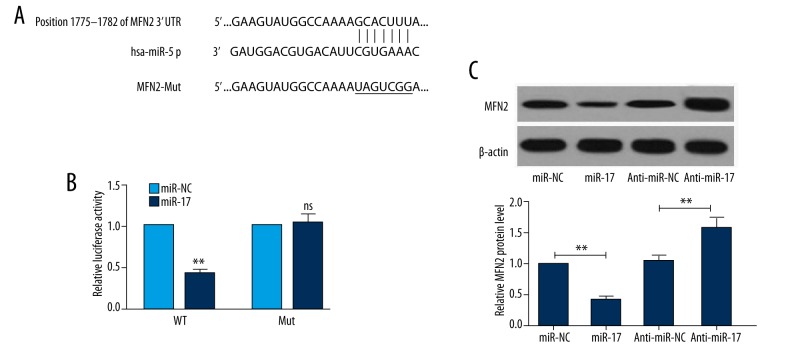
To identify whether miR-17 negatively regulates MFN2 at the post-transcriptional level, a dual-luciferase reporter assay was conducted at 48 h after transfection. The potential miR-17 target sites in MFN2 3′UTR region with or without mutation are shown in Figure 4A. A decrease in relative luciferase activity was observed when cells were co-transfected with miR-17 mimics and WT MFN 3′UTR, whereas no change was found in cells co-transfected with miR-17 and mutant MFN 3′UTR (Figure 4B). To determine whether miR-17 suppresses the expression of MFN2 protein, we conducted Western blot analysis to detect the expression in cells transfected with miR-NC, miR-17 mimics, anti-miR-17, or anti-miR-NC. As shown in Figure 4C, the protein expression level of MFN2 was higher in the miR-17 anti-miR-17 group than that in the anti-miR-NC group, indicating that anti-miR-17 upregulates MFN2 expression at the protein level.
Figure 4.
MFN2 is a target of miR-17. (A) Wild-type (WT) and mutant (Mut) 3′UTR binding sites are shown. The mutated bases are labeled with a horizontal line. (B) HEK293 cells were co-transfected with WT or Mut MFN2 3′-UTR and miR-17 mimic or miR-NC. The relative luciferase activity was detected 48 h after transfection. (C) At 48 h post-transfection, Western blot analysis was performed to assess the effects of miR-17 and aiti-miR-17 on expression of MFN2 protein. Data are expressed as the mean ±SD (n=3). * P<0.01, ** P<0.01; NS – no significant difference compared to control.
miR-17 regulates proliferation and apoptosis in hPASMCs through MFN2 modulation
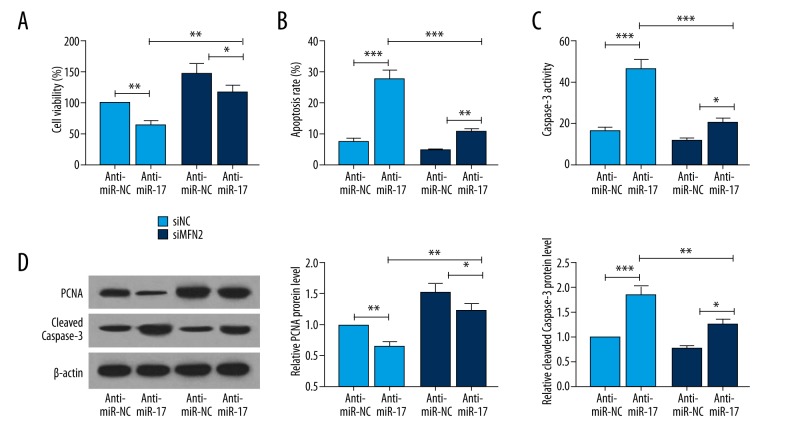
To investigate the underlying mechanism by which miR-17 regulates proliferation and apoptosis of hPASMCs under hypoxic conditions, we inhibited MFN2 expression in hPASMCs exposed to hypoxia. Decreased cell viability (Figure 5A) and increased apoptosis (Figure 5B) and Caspase-3 activity (Figure 5C) were observed in the anti-miR-17 + siMFN2 group as compared with the anti-miR-NC + siNC group. Moreover, upregulation of the pro-apoptotic protein cleaved Caspas-3 and downregulation of the proliferation marker PCNA were observed (Figure 5D). These data suggest that the pro-apoptotic and anti-proliferative effects may be attributed to MFN2 upregulation mediated by miR-17 inhibition.
Figure 5.
MFN2 is essential for the miR-17-mediated regulation of proliferation and apoptosis in hPASMCs. hPASMCs were co-transfected with anti-miR-17 or anti-miR-NC and with either siMFN2 or siNC, and then incubated for 48 h. (A) Proliferation assay was performed with CellTiter-Glo reagent. (B) The apoptosis rate was determined by FCM with Annexin V/PI staining. (C) The activity of Caspase-3 was measured using a Caspase-3 colorimetric assay kit. (D) To determine the protein expression of pro-apoptotic protein cleaved Caspase-3 and PCNA, cells were harvested 48 h post-transfection and then subjected to Western blot analyses. Data are expressed as the mean ±SD (n=3). * P<0.05, ** P<0.01, *** P<0.001.
Discussion
PAH is a cardiovascular disorder characterized by increased proliferation and suppressed apoptosis of PASMCs. Previous studies have shown that hypoxia induces PASMC proliferation and vascular remodeling, which eventually causes PAH [16,17]. Mitochondria are involved in the regulation of apoptosis through a range of mechanisms that are different in vertebrates and invertebrates. In vertebrates, mitochondria release cytochrome c from the intermembrane space to initiate Caspase activation in the cytosol during apoptosis [18]. In recent years, numerous studies have shown that several proteins involved in mitochondrial fission and fusion play a vital role in apoptosis induction [19]. MFN2, a mitochondrial transmembrane GTPase, localizes on the outer membrane mitochondria and is required for mitochondrial fusion. The MFN2 contains a GTPase domain in its NH2-terminal region, a coiled-coil domain, 2 transmembrane spans, and a coiled-coil domain in the carboxyl-terminal region [20]. MFN2 is abundantly expressed in many tissues and organs, including heart, skeletal muscle, liver, lung, brain, and kidney. MFN2 has been demonstrated to regulate cell proliferation, apoptosis, and differentiation of vascular smooth muscle cells and multiple cancer cell lines. The cell proliferation rate was obviously increased in MFN2-knockdown mouse embryonic fibroblast cells, but the proliferative effect was abolished by over-expression of MFN2 [21]. Here, we detected the expression levels of miR-17 and MFN2, and found that miR-17 is upregulated and MFN2 is downregulated in PAH tissues and hPASMCs treated with hypoxia. More importantly, the dual-luciferase reporter assay showed that MFN2 was a target gene of miR-17.
miRNAs are important regulators of gene expression in various cell types. A large number of miRNAs, aberrantly expressed in cancer cells, have been proved to be involved in modulating gene expressions, thereby regulating the malignant progression of cancer [22–24]. However, the roles of miRNAs in the pathogenesis of PAH remain unclear. A recent report showed that miR-214 was involved in the regulation of smooth muscle cell contractile phenotype in PAH. Hypoxia-induced hPASMC proliferation was suppressed by an antagonist of miR-214 [25]. The expression level of miR-140-5p was decreased in whole blood samples from patients with PAH and in the monocrotaline and Sugen5416 plus hypoxia models of PAH. Inhibition of endogenous miR-140-5p promoted PASMC proliferation and migration and downregulated the expression of SMURF1 [26]. Downregulation of miR-204 expression has been observed in PASMCs of PAH patients and rodent models of PAH. miR-204 reverses the pro-proliferative and antiapoptotic phenotypes of hPASMCs isolated from patients with PAH [27]. Caruso et al. found that miR-17-5p expression was upregulated in a monocrotaline model of PH in rats [28]. miR-17-5p expression was upregulated in hPASMC during hypoxia and inhibition of miR-17-5p decreased hypoxia-induced arginase II expression in hPASMC [29]. However, whether miR-17 is able to directly target MFN2 and thereby modulate the proliferation, differentiation, and apoptosis of PASMC in hypoxia has not been reported.
In this study, we conformed that the expression of miR-17 was enhanced in hPASMCs treated with hypoxia, which was consistent with the findings of previous research [30]. Moreover, endogenous miR-17 in hPASMCs were inhibited by its antagomir. We found that inhibition of miR-17 suppressed hypoxia-induced hPASMC proliferation and promoted apoptosis in hPASMCs in vitro. To further study the exact mechanism by which MFN2 functions in the pathogenesis of PAH, hPASMCs were transfected with siMFN2. We found that inhibition of miR-17 decreased cell viability and increased apoptosis and Caspase-3 activity. In addition, the pro-apoptotic protein cleaved Caspase-3 was increased and the proliferation marker PCNA was decreased in hPASMCs transfected with anti-miR-17 + siNC. However, the pro-apoptotic and anti-proliferative effects of anti-miR-17 were attenuated when hPASMCs were transfected with siMFN2, suggesting that MFN2 is required for miR-17-mediated regulation of hPASMC proliferation and apoptosis. Taken together, our data demonstrate that inhibition of miR-17 suppressed hypoxia-mediated hPASMC proliferation and promoted apoptosis, at least partly by modulating MFN2 expression, providing a theoretical foundation for the clinical application of miRNAs in therapy of PAH.
Conclusions
Our study reveals that miR-17 was overexpressed in hypoxia-mediated hPASMCs and in lung tissues from PAH patients. Aberrantly expressed miR-17 affected proliferation and apoptosis of hPASMCs, at least partially by targeting MFN2 expression, indicating that miR-17 plays a vital role in the development of PAH. Our findings suggest that downregulation of miR-17 could be a promising therapeutic strategy for the treatment of patients with PAH.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Montani D, Günther S, Dorfmüller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8:97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 4.Ghofrani H, Hoeper M, Humbert M, et al. Riociguat for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Real-life data from The expert registry. Hypertension. 2016;5(6):7. [Google Scholar]
- 5.Voelkel NF, Gomez-Arroyo J, Abbate A, et al. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40(6):1555–65. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122(12):4306–13. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7(4):313–19. doi: 10.1023/a:1016167228059. [DOI] [PubMed] [Google Scholar]
- 8.Fadeel B, Orrenius S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258(6):479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 9.Brentnall M, Rodriguez-Menocal L, De Guevara RL, et al. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrie A. The role of the osteoprotegerin/tumor necrosis factor related apoptosis-inducing ligand axis in the pathogenesis of pulmonary arterial hypertension. Vascul Pharmacol. 2014;63(3):114–17. doi: 10.1016/j.vph.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Stittrich AB, Haftmann C, Sgouroudis E, et al. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat Immunol. 2010;11(11):1057–62. doi: 10.1038/ni.1945. [DOI] [PubMed] [Google Scholar]
- 12.Asangani I, Rasheed S, Nikolova D, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 13.Caruso P, Dempsie Y, Stevens HC, et al. A role for miR-145 in pulmonary arterial hypertension evidence from mouse models and patient samples. Circ Res. 2012;111(3):290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 14.Pullamsetti SS, Doebele C, Fischer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185(4):409–19. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 15.Zhang D, Ma C, Li S, et al. Effect of Mitofusin 2 on smooth muscle cells proliferation in hypoxic pulmonary hypertension. Microvasc Res. 2012;84(3):286–96. doi: 10.1016/j.mvr.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Nara A, Nagai H, Shintaniishida K, et al. Pulmonary arterial hypertension in rats due to age-related arginase activation in intermittent hypoxia. Am J Respir Cell Mol Biol. 2015;53(2):184–92. doi: 10.1165/rcmb.2014-0163OC. [DOI] [PubMed] [Google Scholar]
- 17.Smith KA, Yuan JX. Hypoxia-inducible factor-1α in pulmonary arterial smooth muscle cells and hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2014;189(3):245–46. doi: 10.1164/rccm.201312-2148ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James DI, Martinou JC. Mitochondrial dynamics and apoptosis: A painful separation. Dev Cell. 2008;15(3):341–43. doi: 10.1016/j.devcel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol. 2007;176(4):405–14. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KH, Dasgupta A, Ding J, et al. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J. 2014;28(1):382–94. doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harel SA, Ben-Moshe NB, Aylon Y, et al. Reactivation of epigenetically silenced miR-512 and miR-373 sensitizes lung cancer cells to cisplatin and restricts tumor growth. Cell Death Differ. 2015;22(8):1328–40. doi: 10.1038/cdd.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo UG, Pong RC, Yang D, et al. Identification of a new mechanism of microRNA turnover from miR-106a-363 cluster leading to epithelial-to-mesenchymal transition in prostate cancer. Can Res. 2015;75(15 Suppl):2873–73. [Google Scholar]
- 24.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–33. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahoo S, Meijles DN, Al Ghouleh I, et al. MEF2C-MYOCD and Leiomodin1 suppression by miRNA-214 promotes smooth muscle cell phenotype switching in pulmonary arterial hypertension. PLoS One. 2016;11(5):e0153780. doi: 10.1371/journal.pone.0153780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman A, Arnold N, Pickworth J, et al. T5 MicroRNA-140-5p regulates disease phenotype in experimental pulmonary arterial hypertension via SMURF1. Thorax. 2015;70(Suppl 3):A3. [Google Scholar]
- 27.Courboulin A, Paulin R, Giguère NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–48. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caruso P, MacLean MR, Khanin R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30(4):716–23. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y, Jin Y, Chen B, et al. Arginase II is a target of miR-17-5p and regulates miR-17-5p expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L197–204. doi: 10.1152/ajplung.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2015;52(2):139–51. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]