Abstract
Metabolic cross-feeding interactions are ubiquitous in natural microbial communities. However, it remains generally unclear whether the production and exchange of metabolites incurs fitness costs to the producing cells and if so, which ecological mechanisms can facilitate a cooperative exchange of metabolites among unrelated individuals. We hypothesized that positive assortment within structured environments can maintain mutualistic cross-feeding. To test this, we engineered Acinetobacter baylyi and Escherichia coli to reciprocally exchange essential amino acids. Interspecific coculture experiments confirmed that non-cooperating types were selectively favoured in spatially unstructured (liquid culture), yet disfavoured in spatially structured environments (agar plates). Both an individual-based model and experiments with engineered genotypes indicated that a segregation of cross-feeders and non-cooperating auxotrophs stabilized cooperative cross-feeding in spatially structured environments. Chemical imaging confirmed that auxotrophs were spatially excluded from cooperative benefits. Together, these results demonstrate that cooperative cross-feeding between different bacterial species is favoured in structured environments such as bacterial biofilms, suggesting this type of interactions might be common in natural bacterial communities.
Introduction
Bacteria are ubiquitous and exist within surface-bound biofilm communities rather than as isolated, planktonic cells (Tolker-Nielsen and Molin, 2000). This strong propensity to attach to surfaces suggests a strong adaptive value of an aggregated lifestyle relative to a planktonic, free-living state. Reasons that may account for the inclination of bacteria to form biofilms include protection from predation, immune response, desiccation, or toxic chemicals (Costerton et al., 1999; Jefferson, 2004; Matz et al., 2005).
Surface-attached bacterial communities are commonly densely packed, thereby drastically affecting ecological interactions among co-residents (Hall-Stoodley et al., 2004). For example, a strong nutrient limitation can result in exploitative competition (Hansen et al., 2007) or benefit genotypes, which secrete compounds that are toxic to neighbouring cells (Lenski and Riley, 2002; Rendueles and Ghigo, 2012). On the other hand, an increasing number of cooperative behaviours are being discovered among microorganisms (Velicer, 2003) that can significantly affect the fitness of interacting genotypes (Kreft, 2004).
One important type of microbial cooperation involves the production of so-called ‘public goods'. These diffusible substances are released into the cell-external environment, thus benefiting not only the producer or its relatives, but potentially also other organisms in the vicinity. Examples involve digestive enzymes (Greig and Travisano, 2004), polymeric substances produced to form biofilms (van Gestel et al., 2014), or chelating compounds that aid nutrient acquisition (Luján et al., 2015).
Another type of interaction is metabolic cross-feeding or syntrophy (Schink, 2002; Sieuwerts et al., 2008; Morris et al., 2013), in which microorganisms release metabolites into the cell-external environment that are used by others as a nutrient or energy source (Honegger, 1998; Reeburgh, 2007; Morris et al., 2013; Pande et al., 2013; Udvardi and Poole, 2013). Although the production and utilization of by-products is likely incidental and not selected for, an active investment of one bacterial cell to benefit others is difficult to reconcile with natural selection. In particular, it is not clear how such interactions can resist the invasion of non-cooperating types, which reap the benefits without reciprocating (Herre et al., 1999; Strassmann et al., 2000; Sachs et al., 2004; Travisano and Velicer, 2004). Moreover, metabolic interactions are often obligatory (Morris et al., 2013). Thus, a cell that has lost its metabolic autonomy might face difficulties to encounter complementary genotypes to supply it with the metabolites that it requires to grow, thereby limiting the potential of cooperative cross-feeding to evolve in natural microbial communities (Oliveira et al., 2014).
Despite these predictions, an increasing number of cross-feeding interactions are being reported to have evolved in laboratory-based settings (Sieuwerts et al., 2008; Harcombe, 2010; Poltak and Cooper, 2011) or natural microbial communities (Schink, 2002; McCutcheon and Moran, 2007; Morris et al., 2013), in which the interacting partners seem to actively invest resources into their respective counterpart. One explanation to account for these observations could be a causal connection to the above-mentioned preference of bacteria to occur in surface-attached communities. Growth in spatially structured environments could enhance local feedbacks among cooperators and thus increase reciprocity (Sachs et al., 2004). As a consequence, the likelihood that cooperators interact with other cooperators might be increased over cooperator–non-cooperator interactions, and this so-called positive assortment (Fletcher and Doebeli, 2009) could help to maintain cooperative interactions (Nowak and May, 1992; Sachs et al., 2004; Kim et al., 2008; Nadell et al., 2010; Nahum et al., 2011; Estrela and Brown, 2013; Nadell et al., 2013; van Dyken et al., 2013).
Indeed, a recent study explored the dynamics of two engineered yeast populations (Saccharomyces cerevisiae) that reciprocally exchanged essential amino acids upon cell lysis (Shou et al., 2007). The authors provided compelling evidence that in their model system spatial assortment could indeed prevent non-cooperating types from overexploiting cooperative benefits (Momeni et al., 2013a). However, it remained unclear whether the observed positive assortment was a property that generally emerges when obligate cross-feeders interact in spatially structured environments, or if the finding was in any way specific to the focal model system.
So far, our understanding of the importance of spatial structure for promoting cooperation among microorganisms is almost exclusively based on intraspecific interactions (Rainey and Rainey, 2003; Shou et al., 2007; Nahum et al., 2011; van Dyken et al., 2013; Drescher et al., 2014; Müller et al., 2014; van Gestel et al., 2014). Moreover, spatial structure has also been shown to reduce levels of cooperation (Hauert and Doebeli, 2004), for example, by intensifying competition among cooperators (Taylor, 1992; Wilson et al., 1992) or by limiting the exchange of cooperative benefits (Verbruggen et al., 2012). Given that bacteria frequently live in species-rich, surface-attached communities, clarifying how growth in spatially structured environments affects the exchange of metabolites among obligately dependent genotypes and, thus, the potential of cooperative cross-feeding to evolve and be maintained within these communities, is a major outstanding problem.
Here we studied the effect of spatial structure on maintaining cooperative amino acid cross-feeding using synthetically engineered interactions between the two bacterial species Acinetobacter baylyi and Escherichia coli. We demonstrate that non-cooperative genotypes are selectively favoured in unstructured environments, whereas they are selected against in structured environments. By combining individual-based modelling with experimental work, we show that segregation of cooperators and non-cooperators within a single bacterial colony can result in a spatial isolation of non-cooperating types, thus limiting their impact on the cross-feeding consortium. Moreover, spatially resolved laser-assisted desorption/ionization time of flight mass spectrometry imaging (MALDI-TOF MSI) of bacterial colonies revealed increased amino acid concentrations in cooperator-rich regions, whereas amino acid concentrations were generally low in areas populated with non-cooperators. Together, our results illustrate that spatial structure can stabilize cooperative cross-feeding interactions between two bacterial species by spatial segregation of cooperating and non-cooperating genotypes, which limits the access of non-cooperators to cooperative benefits.
Materials and methods
Strain construction and plasmids used
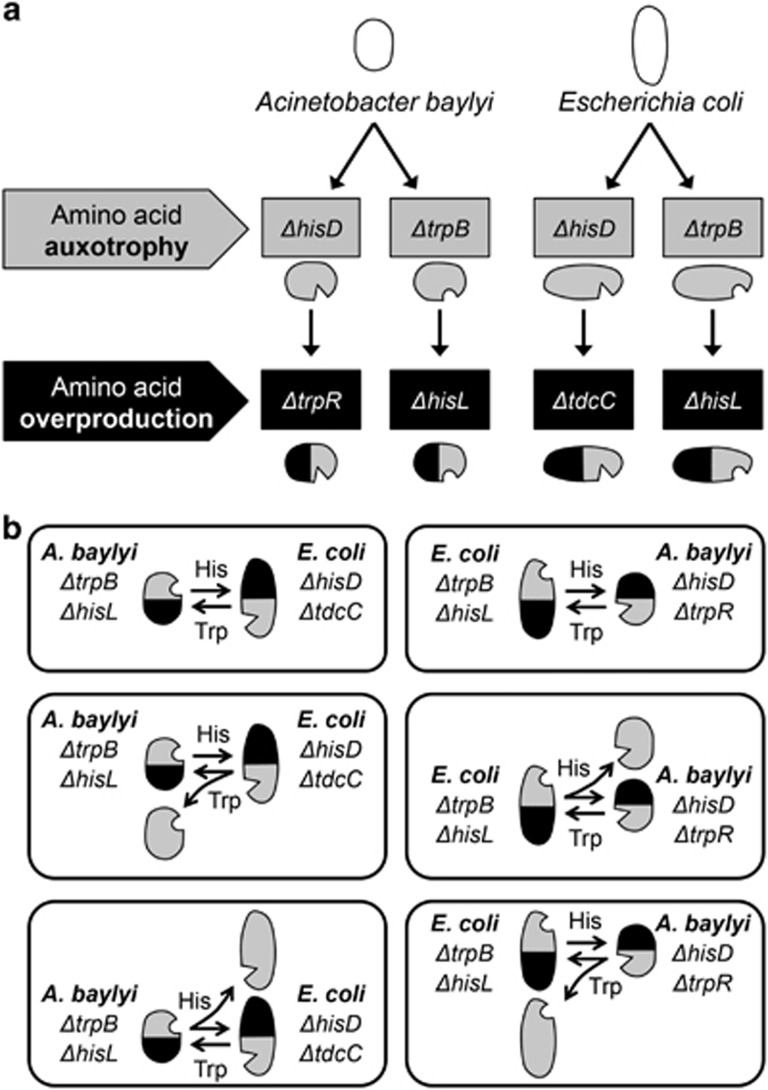
Genetic targets, which would lead to auxotrophies for the two amino acids histidine (His) and tryptophane (Trp) or increased production rates of His and Trp (that is, ‘overproducers' Figure 1a) upon deletion from the genomes of Acinetobacter baylyi ADP1 and Escherichia coli BW25113 were identified using the KEGG pathway database (Ogata et al., 1999). E. coli BW25113 (Baba et al., 2006) was used as wild type (WT), into which deletion alleles from existing strains (Baba et al., 2006) or the arabinose utilization locus (Ara+) from E. coli strain REL 607 (Lenski et al., 1991) were introduced by P1 phage transduction (Thomason et al., 2007). To construct double-deletion mutants, previously constructed auxotrophic genotypes were used as receiver and amino acid overproducers as donor genotype. For this, the kanamycin-resistance cassette was removed from the receiver's genome (Datsenko and Wanner, 2000). A. baylyi ADP1 deletion mutants were constructed as described (de Berardinis et al., 2008; Supplementary Methods).
Figure 1.
Design of synthetic cross-feeding interactions and consortia of cross-feeding and auxotrophic genotypes used. (a) Overview over the genes deleted in Acinetobacter baylyi and Escherichia coli wild type to yield four double-deletion mutants (that is, ‘cross-feeders'), in which a mutation causing overproduction of either histidine (His) or tryptophan (Trp) was combined with a mutation causing auxotrophy for the respective other amino acid. (b) Combinations of cross-feeders (cooperators) and auxotrophs (non-cooperators) used.
All double-deletion strains were transformed with the plasmid pJBA24-mCherry that expresses the red fluorescent protein mCherry, while auxotrophic strains were transformed with the plasmid pJBA24-EGFP that expresses the green fluorescent protein EGFP (Pande et al., 2015).
Culture conditions
Cultures were generally incubated at 30 °C and all the experiments were performed in minimal medium for Azospirillum brasilense (MMAB; Vanstockem et al., 1987) without biotin and using fructose (5 g l−1) instead of malate as carbon source. Pre-cultures were grown in MMAB medium supplemented with the focal amino acid (100 μm). Replicate pre-cultures were started from individual colonies growing on freshly streaked LB agar plates. Overnight pre-cultures were diluted to an optical density of 0.1 at 600 nm and 10 μl (~105 colony-forming units (CFUs)) of this dilution were used to inoculate subsequent experiments. If necessary, kanamycin (50 μg ml−1) or amino acids (100 μm His and/ or Trp) were supplemented to the growth medium.
Amino acid quantification using biosensors
Amino acid production of overproducing and WT strains of both species were estimated by coculturing the corresponding genotypes together with E. coli mutants auxotrophic for the corresponding amino acid (1:1 ratio) in MMAB medium (~105 CFU of each population). Growth of biosensors was estimated at 0 h and 24 h by plating on LB agar plates that did or did not contain kanamycin. Finally, the net cell count (CFU ml−1) of the biosensor was determined by subtracting the CFU count at 0 h from the value determined after 24 h. This experiment was replicated eight times.
Competitive fitness assays
The fitness of two genotypes was compared by co-inoculating both strains (1:1 ratio, ~105 CFU each) and determining their frequencies at 0 h and 24 h by dilution-plating the culture on LB agar plates with or without kanamycin. Relative fitness was calculated as the ratio of Malthusian parameters (Lenski et al., 1991). The cost of amino acid overproduction was determined by competing amino acid overproducers against the corresponding WT. Auxotrophs were competed against conspecific cross-feeders in the presence of the required amino acid (that is, His or Trp). These experiments were replicated eight times.
Effect of spatial structure
Two cross-feeders were co-inoculated (1:1 ratio, ~105 CFU each) in 1 ml MMAB medium or as a drop of 10 μl culture on a membrane filter (pore size 0.22 μm, Millipore GPWP0 1300, Merk KGaA, Darmstadt, Germany) and placed on MMAB agar in a 24-well flat-bottom microtiter plate (Nunc, Thermo Fisher Scientific, Schwerte, Germany). To ensure a random spatial distribution, all the genotypes were mixed in liquid medium before inoculation. The frequency of both cross-feeding strains was determined after 24 h by plating on tetrazolium arabinose indicator agar (Levin et al., 1977) with or without kanamycin. The effect of non-cooperators on a cross-feeding community was analysed by coculturing a proportion of 0%, 20%, and 60% of one of the four possible auxotrophic strains together with the cross-feeding community. After 0 h and 24 h, the population-level frequencies of the auxotroph and both cross-feeding mutants were determined using the arabinose utilization marker (Ara+/Ara−) by plating on tetrazolium arabinose indicator agar with and without kanamycin. This experiment was performed with all possible three-partite combinations of one auxotroph and two interspecific cross-feeders (Figure 1b).
To rule out a fitness disadvantage of auxotrophs growing on agar plates, interspecific cultures of two cross-feeding genotypes that contained initial population-level proportions of 0%, 20%, and 60% of one auxotroph were inoculated together on a membrane filter. This time, however, the filter was placed on MMAB agar, which was supplemented with both His and Trp. Finally, the population-level frequencies of auxotrophic and cross-feeding genotypes were determined as described above. All the experiments were replicated eight times.
Fluorescence microscopy
The spatial distribution of cross-feeders and auxotrophs was visualized by analysing colonies of EGFP-labelled auxotrophs and mCherry-labelled cross-feeders after 24 h of growth using fluorescence microscopy. For this, both cross-feeders (~105 CFUs of each strain) and auxotrophs (60% of the total population) were cocultured on a membrane filter as described above. After 24 h, the filter disc with the bacterial colony was transferred on a microscope slide. Samples were examined using an Axio Imager Z1 Zeiss microscope (Carl Zeiss, Jena, Germany). Images were analysed using the software AxioVision LE Rel. 4.4 (Carl Zeiss). Immediately after microscopic analysis, the colonies were subjected to MALDI MSI (Supplementary Methods).
The three-dimensional distribution of auxotrophs and cross-feeders in a colony were analysed using laser scanning confocal microscopy. For this, coculture colonies were grown for 24 h on filter membranes, which were subsequently transferred to a glass slide and scanned with a Zeiss LSM 710 NOL confocal microscope (Carl Zeiss) using a × 10 objective. Images were analysed using Zeiss LSM Image Browser (Carl Zeiss).
Results
Generation of synthetic cross-feeding interactions
To construct amino acid cross-feeding interactions between A. baylyi and E. coli, mutants of both species were created that were auxotrophic for either histidine (His) or tryptophan (Trp). For this, the terminal genes of the His (hisD) and Trp (trpB) biosynthetic pathway were deleted from the WT background of both species (Figure 1a). Subsequently, genotypes were generated that produce increased amounts of His and Trp (hereafter: overproducers, Figure 1a). In both species, histidine overproduction was achieved by deleting hisL (that is, the operon leader peptide) to eliminate negative transcriptional regulation of the His pathway. Tryptophan overproduction was realized by deleting trpR (that is, the tryptophan repressor protein) from the genome of A. baylyi WT and tdcC (that is, an amino acid transporter) from the E. coli WT. Combining an amino acid auxotrophy-causing mutation together with a mutation resulting in overproduction of the respective other amino acid in a single genetic background yielded two interspecific pairs of double-deletion mutants with complementary metabolic requirements (hereafter: cross-feeders, Figure 1a).
Characterization of synthetic cross-feeding interactions
The engineered cross-feeders and auxotrophs needed to fulfil two key requirements to serve as a model system for studying the maintenance of obligate interspecific metabolic cooperation. First, all cross-feeding mutants needed to produce increased amounts of the amino acids relative to non-producing auxotrophs. Second, amino acid overproduction should incur a fitness cost.
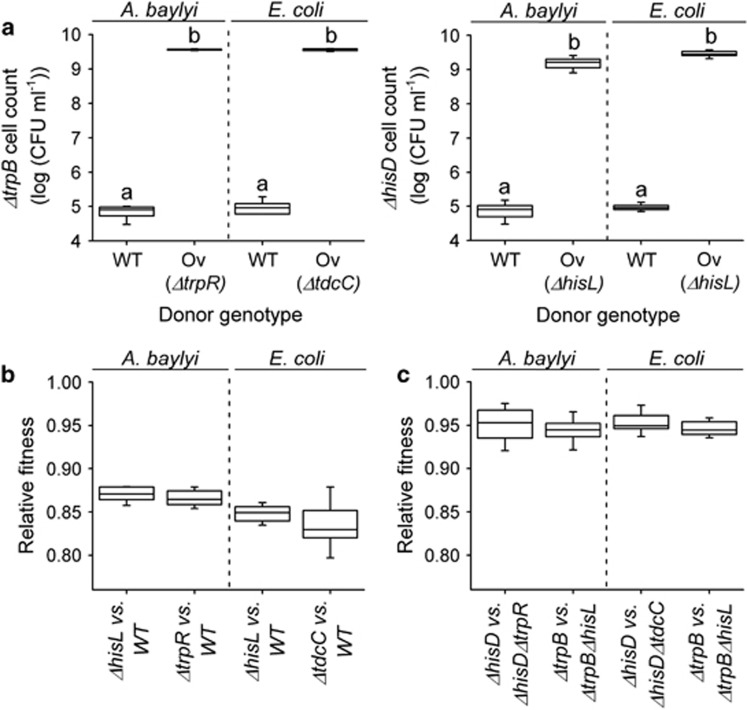
To verify the first condition, the amino acid overproducers of both species were individually cocultured with E. coli mutants auxotrophic for either His or Trp (initial ratio: 1:1). Growth of these auxotrophs during 24 h is indicative of the amino acids levels produced by the focal overproducers (Bertels et al., 2012). This experiment confirmed that overproducers of both species produced amino acid levels that were significantly increased over wild-type levels (least significant difference (LSD) post hoc test: P<0.05, n=8, Figure 2a) and which were sufficient to support the growth of cocultured auxotrophs.
Figure 2.
Amino acid production levels of overproducing genotypes and cost of amino acid production. (a) Amino acid production of wild-type (WT) and overproducing mutants (Ov) of A. baylyi (left) and E. coli (right) as determined by coculturing each overproduction mutant together with an E. coli biosensor auxotrophic for either histidine (ΔhisD) or tryptophan (ΔtrpR) (initial ratio: 1:1). Different letters indicate significant differences (LSD post hoc test: P<0.05, n=8). Shown is the log productivity of amino acid biosensors. (b) Competitive fitness of amino acid overproduction mutants relative to the respective WT. All strains tested were less fit than the corresponding WT (that is, below 1; one-sample t-test: P<0.01, n=8). (c) Competitive fitness of double-deletion mutants relative to the respective auxotrophic strain in minimal medium, which has been supplemented with the focal amino acid. The A. baylyi (left) and E. coli (right) double-deletion mutants were significantly less fit than the corresponding auxotrophs (that is, below 1; one-sample t-test: P<0.05, n=8).
Next, the prediction that amino acid overproduction incurs a fitness cost was tested in two ways. First, overproducers of both species were competed against the respective wild-type strains (initial ratio: 1:1) in minimal medium supplemented with the respective other amino acid. This experiment revealed that even in the presence of the cooperative benefit, overproducers were significantly less fit (10–20%) than the corresponding wild type (one-sample t-test: P<0.05, n=8, Figure 2b). Second, directly competing cross-feeding genotypes against the cognate auxotrophs (for example, ΔhisDΔtrpR versus ΔhisD) in minimal medium supplemented with the focal amino acid revealed a significantly reduced fitness (4–8%) of cross-feeders relative to auxotrophs (one-sample t-test: P<0.05, n=8, Figure 2c). Thus, both experiments indicated a substantial cost of amino acid overproduction. Together, these results confirm that the introduced overproduction alleles resulted in increased amino acid production levels that were costly to the producing cells. This library of mutants allowed assembling two interspecific consortia of cells that reciprocally exchanged His and Trp. By introducing one of the two possible auxotrophic genotypes to these consortia (Figure 1b), it was possible to determine how the presence of non-producing genotypes affects the focal cooperative interaction.
Spatial structure favours cooperative cross-feeding
Significant fitness costs of amino acid overproduction suggested a susceptibility of the generated cross-feeding interactions to non-cooperating auxotrophs. In spatially unstructured environments, the released amino acids should be equally available to both cross-feeders and auxotrophs. Under these conditions, the resulting social conflict should lead to a collapse of the cooperative cross-feeding interaction. In contrast, spatially structured environments are expected to favour cooperative cross-feeding (Nowak and May, 1992; Nowak et al., 1994; Sachs et al., 2004; Nadell et al., 2010; Momeni et al., 2013a).
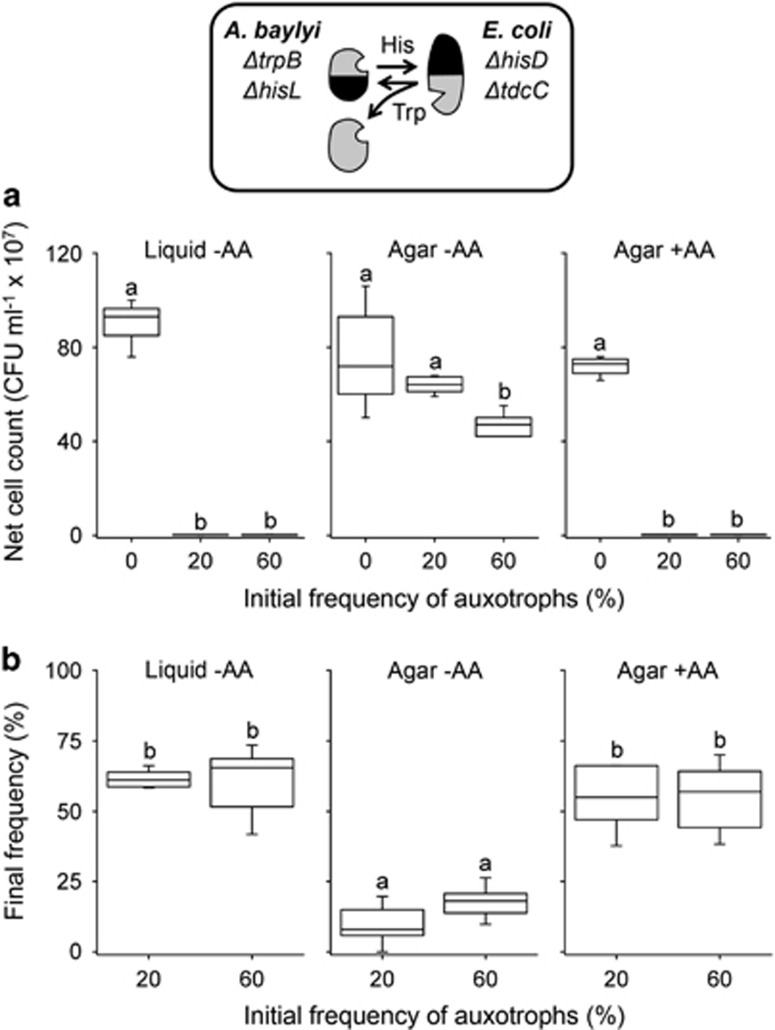
These predictions were verified by coculturing both cross-feeding consortia in the absence or presence of one of the two possible auxotrophs at different initial ratios (0%, 20%, or 60% of the total population) in either spatially unstructured (that is, shaken, liquid medium) or structured (that is, agar surface) environments. In line with expectations, these experiments confirmed that in unstructured environments, the presence of non-cooperating auxotrophs resulted in a significantly reduced productivity of the entire cross-feeding community (LSD post hoc test: P<0.05, n=8, Figure 3a), whereas the adverse effect of auxotrophs was limited in spatially structured environment (LSD post hoc test: P<0.05, n=8, Figure 3a). After 24 h, auxotrophs reached—independent of their initial proportion—a community-level frequency of ~60% in spatially unstructured environments, while their proportion in spatially structured environments did not exceed 10–25% (Figure 3b). This finding suggests that in contrast to expectations, the presence of non-cooperating auxotrophs did not result in a collapse of the cooperative system. Instead, the community-level frequency of auxotrophs was determined by negative frequency-dependent selection and stabilized at different proportions depending on the degree of spatial structuring in the environment.
Figure 3.
Effect of non-cooperating auxotrophs on consortia of cross-feeding genotypes. (a) Productivity of pairs of cross-feeding genotypes that were co-inoculated with 0%, 20%, or 60% of an initial frequency of auxotrophs. Consortia were cultured in liquid culture or on agar plates containing either unsupplemented minimal medium (−AA) or minimal medium, to which both amino acids (100 μm of histidine and tryptophan) have been added (+AA). Net cell count is the number of colony-forming units (CFUs) determined after 24 h minus the count at the beginning of the experiment (0 h). Different letters indicate significant differences (LSD post hoc test: P<0.05, n=8). (b) Frequency of auxotrophic genotypes after 24 h of coculture when initially inoculated at a frequency of 20% or 60% together with pairs of cross-feeding genotypes. Different letter indicate significant differences (LSD post hoc test: P<0.05, n=8). Shown are representative results of a combination of cross-feeding and auxotrophic genotypes (top panel). Qualitatively similar results were obtained from analysing the other combinations as well (Supplementary Figures 1 and 2).
An explanation for the limited effect of non-cooperating auxotrophs in spatially structured environments could simply be a reduced fitness of auxotrophic mutants when growing on solid media. To exclude this possibility, each cross-feeding consortium was cocultured with one of the two possible auxotrophs in a spatially structured environment that has been supplemented with both amino acids. By uncoupling the obligate interaction in this way, auxotrophs should increase in frequency, because they benefit from the available amino acids yet do not invest resources into their production. Indeed, amino acid supplementation significantly decreased the productivity of the entire cross-feeding consortia within 24 h when non-cooperating auxotrophs were present (LSD post hoc test: P<0.05, n=8, Figure 3a). Moreover, independent of their initial inoculum size, auxotrophs increased to a final community-level frequency of ~60% after 24 h, which was statistically indistinguishable from the proportion auxotrophs had reached in a spatially unstructured, liquid environment (LSD post hoc test: P<0.05, n=8, Figure 3b). These findings ruled out that non-cooperating auxotrophs were generally less fit when growing on solid agar surfaces. Furthermore, this result implied that alleviating the requirement for external amino acids provided non-producing auxotrophs with a selective advantage over producing types.
Repeating the same series of experiments with all other possible three-partite combinations of two amino acid cross-feeders and one auxotroph yielded qualitatively similar results in all the cases (Supplementary Figures 1 and 2).
Together, these experiments demonstrated that non-cooperating auxotrophs were selectively favoured in spatially unstructured environments, but selected against in structured environments. Furthermore, these results identified the obligate nature of the cross-feeding interaction as a key component of the ecological mechanism that hampered the invasion of amino acids auxotrophs in spatially structured environments.
Cross-feeding genotypes show intense population intermixing
An individual-based model was devised to identify the ecological mechanism that limited the exploitation of cooperative cross-feeding interactions by non-cooperating auxotrophs in spatially structured environments (Supplementary Methods). In parallel to the previous experiments, the model included three interacting types (two cross-feeders and one non-cooperating auxotroph), which were parameterized using experimentally determined parameter values. Simulations included a diffusion of released amino acids in a two-dimensional world and fitness of cell types depended on the local distribution of amino acids they required to grow.
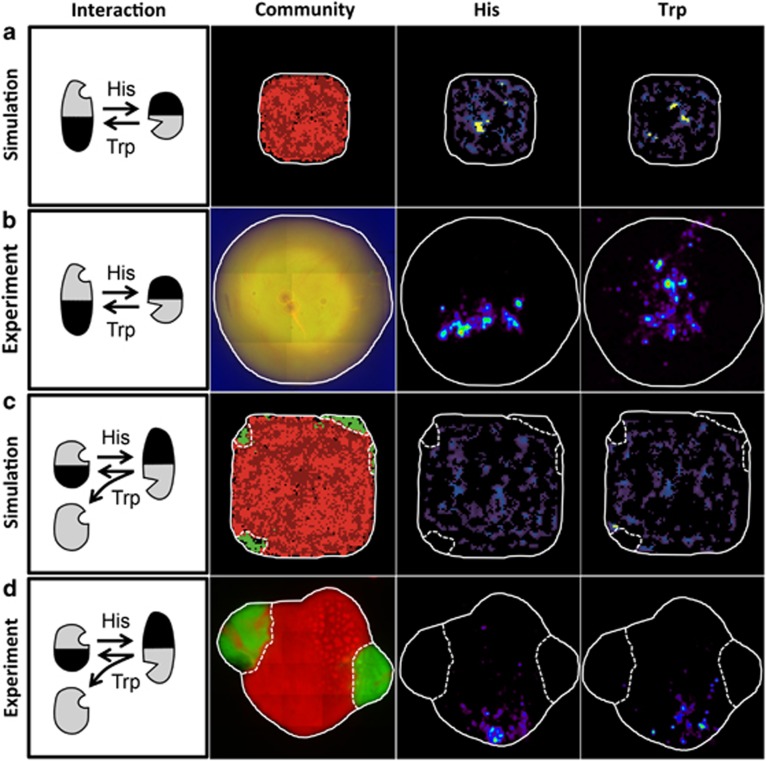
At first, replicate simulations were initiated with the two cross-feeding genotypes only. The results of these simulations revealed a high degree of intermixing between both partners (Figure 4a). To empirically validate these simulation results, both cross-feeders were individually labelled with mCherry (A. baylyi ΔhisDΔtrpR) and EFGP (E. coli ΔtrpBΔhisL) and the resulting interspecific colony was analysed using fluorescence microscopy. After 24 h, both cross-feeding types showed such a high degree of population intermixing that the resulting colonies appeared yellow under the fluorescence microscope (Figure 4b).
Figure 4.
Population intermixing and exclusion of non-cooperating genotypes is governed by the spatial distribution of available amino acids. Shown are sketches of the focal interactions (first column), the two- or three-partite bacterial communities consisting of cooperative cross-feeders (red colour) and non-cooperating auxotrophs (green colour) after 30 simulation steps or 24 h of growth in spatially structured environments (second column), as well as the spatial distribution of the two amino acids histidine (His) or tryptophan (Trp) within bacterial colonies at the end of the simulations or as experimentally determined by MALDI-TOF MS imaging (third and fourth column). Different intensities of blue (columns three and four) indicate different concentrations of both amino acids within a colony. White lines represent the boundary of the respective colony and the dashed lines delimit the spatial distribution of non-cooperating auxotrophs. (a, b) In the absence of non-cooperating auxotrophs, cross-feeding cells intermix to a high degree. (a) Simulation results after 30 time steps and (b) experimental result after 24 h of growth using the two cross-feeders A. baylyi (ΔhisDΔtrpR) and E. coli (ΔtrpBΔhisL). (c, d) Communities consisting of auxotrophic and cross-feeding genotypes show strong assortment during 24 h of growth in spatially structured environments. (c) Simulation results after 30 time steps and (d) experimental result after 24 h of growth using two cross-feeders of A. baylyi (ΔtrpBΔhisL) and E. coli (ΔhisDΔtdcC) as well as an auxotrophic A. baylyi strain (ΔtrpB). Qualitatively comparable results were obtained from other combinations of cross-feeding consortia and auxotrophs as well (Supplementary Figure 6).
Positive assortment of cross-feeders in spatially structured environments
Which ecological mechanism disfavoured non-cooperating auxotrophs in spatially structured environments? To answer this question, simulations were initiated in a spatially structured environment that involved two cooperating cross-feeders and one non-cooperating auxotroph. Multiple independent simulation runs revealed that without amino acids supplementation, non-cooperating auxotrophs of both types persisted exclusively at the edge of the expanding colony (Figure 4c, Supplementary Movie 1) and reached significantly lower densities than each of the two cross-feeding genotypes (Mann–Whitney U-test after 30 simulation steps: P<0.001, n=100, Supplementary Figure 3, Supplementary Movie 2). In contrast, when growth was not limited by the two focal amino acids, auxotrophic types intermixed with both cross-feeders and reached population densities that significantly exceeded the cross-feeding type that competed for the same amino acid (Mann–Whitney U-test after 30 simulation steps: P<0.001, n=100, Supplementary Figure 3). Thus, these simulations suggested the obligate relationship caused a spatial segregation of cross-feeders and non-producing auxotrophs, thereby resulting in a positive assortment among cross-feeding types.
To investigate whether these theoretical predictions were indeed causing the selective disadvantage non-cooperators experienced in spatially structured environments, two mCherry-labelled cross-feeding genotypes were cocultured with one of the two possible EGFP-labelled auxotrophs in a spatially structured environment and the resulting colonies analysed using fluorescence microscopy. In line with the above predictions, a top view of these colonies after 24 h of growth revealed that despite an initially random distribution of all genotypes, the growth of non-cooperating auxotrophs was restricted to small zones at the edge of cross-feeder-rich regions (Figure 4d). In contrast, non-cooperators swept through the entire population when His and Trp were supplemented (Supplementary Figure 4b). In fact, the mCherry-labelled cross-feeders and EGFP-labelled auxotrophs again intermixed to such a high degree that the resulting colony appeared yellow under the fluorescence microscope (Supplementary Figure 4b). To rule out that auxotrophs were located below cross-feeding genotypes, 24-h-old colonies of two cross-feeders and one auxotroph were analysed using laser scanning confocal microscopy. These experiments confirmed that cooperator-rich regions were indeed devoid of auxotrophic non-cooperators (Supplementary Figures 4a and c). Altogether, these results imply that in spatially structured environments, cross-feeding cells interacted preferentially with other cross-feeders, thereby spatially excluding non-cooperating auxotrophs.
Segregation is caused by the spatial distribution of amino acids
One explanation for the spatial exclusion of non-cooperating auxotrophs in spatially structured environments could be a limited access to free amino acids. If so, regions containing both cross-feeders should exhibit higher local amino acid concentrations relative to zones containing auxotrophs. To test this, the simulation runs in spatially structured environments were revisited, but this time, the spatial distributions of amino acids that caused the emergent community patterning were visualized. Interestingly, these results illustrated that regions occupied by cross-feeders were generally characterized by a patchy distribution of both amino acids (Figures 4a and c). This pattern materialized both in the absence (Figure 4a) and presence (Figure 4c) of non-cooperating auxotrophs. Comparing the concentrations of His and Trp in regions populated with cross-feeders to regions occupied by non-cooperating auxotrophs corroborated that amino acids were indeed less available to non-cooperating auxotrophs (Figure 4c). In addition, simulations revealed that when two cross-feeders interacted in spatially structured environments, the local concentrations of free amino acids available to cross-feeders increased over time (Supplementary Figure 5a). Interestingly, this pattern did not change when non-cooperating auxotrophs were present. Under these conditions, amino acids concentrations were again increased in regions populated by cross-feeders, yet reached only ~10% of these concentrations in areas occupied by auxotrophs (Mann–Whitney U-test after 30 simulation steps: P<0.001, n=100; Supplementary Figure 5b). Together, these simulation results revealed amino acids are patchily distributed in cross-feeder-rich regions, yet are significantly less available to non-cooperating auxotrophs.
To verify these predictions, cross-feeding consortia were cocultured in spatially structured environments in the absence or presence of one of the two possible auxotrophs and after 24 h of growth, the spatial distribution of His and Trp was visualized using MALDI MSI. In line with the simulation results, amino acids were heterogeneously distributed in areas where cross-feeders co-occurred (Figures 4b and d). In addition, regions occupied by non-cooperating auxotrophs were consistently devoid of detectable amino acids (Figure 4d, Supplementary Figure 6). To visualize the spatial distribution of His and Trp, colonies were dried using blotting paper and overnight desiccation. The colonies processed in this way showed in many instances the formation of crystalline deposits that occurred only in cross-feeder-rich regions. Analysing these surface crystals via FT-MS (Supplementary Methods) confirmed these crystals consisted indeed of His (m/z 154.0611 [(M-H)-]) and Trp (m/z 203.0820 [(M-H)-]). A perfect match of the high-resolution masses obtained from crystals and amino acid standards further corroborated this interpretation (Supplementary Figure 7). Altogether, these analyses established that a spatial exclusion of non-cooperating auxotrophs from cooperative benefits (here: amino acids) helped to maintain mutualistic cross-feeding interactions in spatially structured environments.
Discussion
Metabolic cross-feeding interactions that involve multiple bacterial species are common in nature (Schink, 2002; Morris et al., 2013). Evolutionary theory, however, predicts that interactions, in which the production and exchange of metabolites incurs fitness costs, should be prone to the invasion of non-cooperating individuals, which reap cooperative benefits without reciprocating (Herre et al., 1999; Sachs et al., 2004; Travisano and Velicer, 2004). The persistence of cooperative cross-feeding interactions hinges therefore on the existence of mechanisms that resolve these conflicts of interests. We hypothesized that spatially structured environments should enhance partner fidelity feedbacks within cooperator-rich patches (Trivers, 1971), possibly resulting in a positive assortment among cooperative individuals (Fletcher and Doebeli, 2009; Mitri et al., 2011; Estrela and Brown, 2013), which could maintain these interactions in the long run.
To test this hypothesis, we engineered an obligate cooperative cross-feeding interaction between A. baylyi and E. coli, in which growth of both partners obligately depended on a reciprocal exchange of His and Trp. Analysing the stability of this interaction, we find that spatial structure favoured cooperative cross-feeding over unstructured environments. Despite an initially random distribution of auxotrophs and cross-feeders, both populations self-organized into a spatially inhomogeneous distribution after 24 h, in which auxotrophs occurred exclusively in smaller patches at the edges of colonies that otherwise consisted of cross-feeding types. A chemical imaging technique (that is, MALDI MSI) indicated that this pattern was caused by an inhomogeneous distribution of amino acids, which were only present in cross-feeder-rich regions and therefore unavailable to auxotrophs. The resulting segregation of auxotrophic and cross-feeding genotypes in spatially structured environments limited the access of non-cooperators to cooperative benefits and thus maintained cooperative cross-feeding interactions in the focal bacterial communities.
Surface-attached biofilms are prime examples of spatially structured communities that can consist of multiple different bacterial species (Ley et al., 2006; Sudakaran et al., 2012). Strikingly, many biofilm communities are spatially stratified (Tolker-Nielsen and Molin, 2000; Stoodley et al., 2002) and several factors have been identified as possibly driving this patterning. First, physiological differentiation can result from environmental factors such as gradients of nutrients or oxygen (Serra and Hengge, 2014). For example, spatial stratification has been demonstrated in nitrifying biofilms (Okabe et al., 1999) or anaerobic granular sludge biofilms (Harmsen et al., 1996). Second, also ecological interactions among co-occurring bacteria can contribute to their spatial self-organization. Layered structures that physically separate cells of two species can emerge as a consequence of both synergistic and antagonistic interactions. Examples involve an exchange of metabolic by-products (Christensen et al., 2002) or competition for oxygen (Hansen et al., 2007), respectively.
Alternatively, strong metabolic interactions that involve an exchange of metabolites between different cells have been demonstrated to result in enhanced population intermixing (Thiele et al., 1988; Nielsen et al., 2000; Breugelmans et al., 2008; Estrela and Brown, 2013; Momeni et al., 2013b; Müller et al., 2014)—an observation that is consistent with the results of our study (Figures 4a and b). Notwithstanding the factors that caused the patterning in the first place, the results of our study suggest that when two organisms interact synergistically in a spatially structured environment, repeated interactions should reward mutants that increase their cooperative investment to benefit their respective partners. The automatic feedback that arises as a consequence of this process should intensify cooperative interactions in the long run. Whether and to which extent the synergistic metabolic interactions as well as the pronounced spatial heterogeneity in the concentrations of metabolites (Ramsing et al., 1993; Schramm et al., 1996) that are frequently observed within bacterial biofilms are a direct consequence of this process, however, is not known and should be subject to future studies.
Cooperation is expected to be evolutionary stable when cooperators are more likely to interact with other cooperators than with non-cooperating individuals (Fletcher and Doebeli, 2009). In the absence of derived mechanisms, with which cooperators can either recognize and thus preferentially interact with other cooperators (for example, ‘partner choice' Bull and Rice, 1991) or enforce cooperation by sanctioning non-cooperators (for example, ‘policing' Frank, 1995), alternative means are required to prevent non-cooperating individuals from overexploiting cooperative benefits (Travisano and Velicer, 2004). Evolutionary theory has suggested that spatial environments offer a possible solution to this dilemma (Mitri et al., 2011; Estrela and Brown, 2013): spatial self-organization can result in positive assortment of cooperators and thus help to maintain cooperative interactions.
Previous experimental work on this issue has largely focussed on intraspecific interactions. For example, Bacilus subtilis strains that produced extracellular polysaccharides (EPS, a substance that promotes biofilm expansion) and competed against EPS-deficient cells have been shown to segregate when growing on a two-dimensional surface—a mechanism that prevented EPS-non-producers from overexploiting the benefits of EPS production (van Gestel et al., 2014). Moreover, engineered yeast strains that reciprocally exchanged essential amino acids, showed positive assortment among cooperators when the interaction was staged in spatially structured environments (Momeni et al., 2013a). The authors of this study attributed the observed stabilizing effects to an asymmetric distribution of the released metabolites in the environment. A similar argument has been proposed by Drescher et al., 2014 to explain why thicker biofilms selectively benefited cooperative chitinase producers of Vibrio cholerae over conspecific non-producers. Most likely, a thicker biofilm matrix limited diffusion of the public good (that is, chitinase) and thus the availability of the chitin degradation products Vibrio needs to grow. These findings are in line with the main conclusion of our study, namely that limited diffusion of cooperative benefits in spatially structured environments confines their availability to non-producing individuals, which results in a positive assortment of cooperative producers. Building on these previous studies, our work is the first one to demonstrate experimentally that similar mechanisms also operate in reciprocal interspecific interactions and to causally link the exclusion of non-cooperators to the underlying distribution of the exchanged metabolites.
Conclusion
Our work has identified a powerful mechanism to maintain cooperative cross-feeding interactions between two bacterial species: a limited diffusion of released metabolites in spatially structured environments results in a positive assortment of genotypes that invest into the costly production of exchanged metabolites. As a consequence, non-producing cells are spatially excluded from cooperative benefits, which limits their impact on the population of cooperators. Given that bacteria commonly live in surface-bound polymicrobial communities, our results suggest obligate cooperative cross-feeding interactions within these communities might be more widespread than previously thought.
Acknowledgments
We thank Michael Reichelt for help with amino acid measurements, Wilhelm Boland for support as well as Bill S Hansson for providing access to the fluorescence microscope. We are grateful to the whole EEE group for helpful discussions. The project was funded by the Volkswagen Foundation and Jena School of Microbial Communication (JSMC).
Author contributions
SP and CK conceived and designed the study. SP performed all the experiments. SP and CK analysed and interpreted the results. SP, FK and AS performed the MALDI MSI, FT-MS experiments. SP and CK wrote the manuscript. All the co-authors amended the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M et al. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertels F, Merker H, Kost C. (2012). Design and characterization of auxotrophy-based amino acid biosensors. PLoS One 7: e41349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breugelmans P, Barken K, Tolker-Nielsen T, Hofkens J, Dejonghe W, Springael D. (2008). Architecture and spatial organization in a triple-species bacterial biofilm. FEMS Microbiol Ecol 64: 271–282. [DOI] [PubMed] [Google Scholar]
- Bull JJ, Rice WR. (1991). Distinguishing mechanisms for the evolution of cooperation. J Theor Biol 149: 63–74. [DOI] [PubMed] [Google Scholar]
- Christensen BB, Haagensen JAJ, Heydorn A, Molin S. (2002). Metabolic commensalism and competition in a two-species microbial consortium. Appl Environ Microbiol 68: 2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Datsenko K, Wanner B. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Berardinis V, Vallenet D, Castelli V, Besnard M, Pinet A, Cruaud C et al. (2008). A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol Syst Biol 4: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. (2014). Solutions to the public goods dilemma in bacterial biofilms. Curr Biol 24: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela S, Brown SP. (2013). Metabolic and demographic feedbacks shape the emergent spatial structure and function of microbial communities. PLoS Comput Biol 9: e1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JA, Doebeli M. (2009). A simple and general explanation for the evolution of altruism. Proc R Soc Lond B Biol Sci 276: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA. (1995). Mutual policing and repression of competition in the evolution of cooperative groups. Nature 377: 520–522. [DOI] [PubMed] [Google Scholar]
- Greig D, Travisano M. (2004). The prisoner's dilemma and polymorphism in yeast SUC genes. Proc R Soc Lond B Biol Sci 271(Suppl): S25–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2: 95–108. [DOI] [PubMed] [Google Scholar]
- Hansen SK, Rainey PB, Haagensen JAJ, Molin S. (2007). Evolution of species interactions in a biofilm community. Nature 445: 533–536. [DOI] [PubMed] [Google Scholar]
- Harcombe W. (2010). Novel cooperation experimentally evolved between species. Evolution 64: 2166–2172. [DOI] [PubMed] [Google Scholar]
- Harmsen HJM, Kengen HMP, Akkermans ADL, Stams AJM, de Vos WM. (1996). Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol 62: 1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauert C, Doebeli M. (2004). Spatial structure often inhibits the evolution of cooperation in the snowdrift game. Nature 428: 643–646. [DOI] [PubMed] [Google Scholar]
- Herre E, Knowlton N, Mueller U, Rehner S. (1999). The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14: 49–53. [DOI] [PubMed] [Google Scholar]
- Honegger R. (1998). The lichen symbiosis—What is so spectacular about it? Lichenologist 30: 193–212. [Google Scholar]
- Jefferson KK. (2004). What drives bacteria to produce a biofilm? FEMS Microbiol Lett 236: 163–173. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. (2008). Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA 105: 18188–18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft JU. (2004). Biofilms promote altruism. Microbiology 150: 2751–2760. [DOI] [PubMed] [Google Scholar]
- Lenski R, Rose M, Simpson S, Tadler S. (1991). Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat 138: 1315–1341. [Google Scholar]
- Lenski RE, Riley MA. (2002). Chemical warfare from an ecological perspective. Proc Natl Acad Sci USA 99: 556–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BR, Stewart FM, Chao L. (1977). Resource-limited growth, competition, and predation—A model and experimental studies with bacteria and bacteriophage. Am Nat 111: 3–24. [Google Scholar]
- Ley RE, Harris JK, Wilcox J, Spear JR, Miller SR, Bebout BM et al. (2006). Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl Environ Microbiol 72: 3685–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján AM, Gómez P, Buckling A. (2015). Siderophore cooperation of the bacterium Pseudomonas fluorescens in soil. Biol Lett 11: 20140934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz C, McDougald D, Moreno AM, Yung PY, Yildiz FH, Kjelleberg S. (2005). Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc Natl Acad Sci USA 102: 16819–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. (2007). Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci USA 104: 19392–19397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri S, Xavier JB, Foster KR. (2011). Social evolution in multispecies biofilms. Proc Natl Acad Sci USA 108: 10839–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni B, Brileya KA, Fields MW, Shou WY. (2013. b). Strong inter-population cooperation leads to partner intermixing in microbial communities. Elife 2: e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni B, Waite A, Shou W. (2013. a). Spatial self-organization favors heterotypic cooperation over cheating. Elife 2: e00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B, Henneberger R, Huber H, Moissl-Eichinger C. (2013). Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37: 384–406. [DOI] [PubMed] [Google Scholar]
- Müller MJI, Neugeboren BI, Nelson DR, Murray AW. (2014). Genetic drift opposes mutualism during spatial population expansion. Proc Natl Acad Sci USA 111: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadell C, Bucci V, Drescher K, Levin SA, Bassle BL, Xavier JB. (2013). Cutting through complexity of cell collectives. Proc R Soc Lond B Biol Sci 280: 20122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadell C, Foster K, Xavier J. (2010). Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol 6: e1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum JR, Harding BN, Kerr B. (2011). Evolution of restraint in a structured rock-paper-scissors community. Proc Natl Acad Sci USA 108: 10831–10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AT, Tolker-Nielsen T, Barken KB, Molin S. (2000). Role of commensal relationships on the spatial structure of a surface-attached microbial consortium. Environ Microbiol 2: 59–68. [DOI] [PubMed] [Google Scholar]
- Nowak M, Bonhoeffer S, May R. (1994). Spatial games and the maintenance of cooperation. Proc Natl Acad Sci USA 91: 4877–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M, May R. (1992). Evolutionary games and spatial chaos. Nature 359: 826–829. [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. (1999). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 27: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Satoh H, Watanabe Y. (1999). In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 65: 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira NM, Niehus R, Foster KR. (2014). Evolutionary limits to cooperation in microbial communities. Proc Natl Acad Sci USA 111: 17941–17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S, Merker H, Bohl K, Reichelt M, Schuster S, de Figueiredo LF et al. (2013). Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J 8: 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S, Shitut S, Freund L, Westermann M, Bertels F, Colesie C et al. (2015). Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat Commun 6: 6238. [DOI] [PubMed] [Google Scholar]
- Poltak S, Cooper V. (2011). Ecological succession in long-term experimentally evolved biofilms produces synergistic communities. ISME J 5: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey P, Rainey K. (2003). Evolution of cooperation and conflict in experimental bacterial populations. Nature 425: 72–74. [DOI] [PubMed] [Google Scholar]
- Ramsing N, Kuhl M, Jorgensen B. (1993). Distribution of sulfate-reducing bacteria, O2, and H2S in photosynthetic biofilms determined by oligonucleotide probes and microelectrodes. Appl Environ Microbiol 59: 3840–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeburgh W. (2007). Oceanic methane biogeochemistry. Chem Rev 107: 486–513. [DOI] [PubMed] [Google Scholar]
- Rendueles O, Ghigo JM. (2012). Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol Rev 36: 972–989. [DOI] [PubMed] [Google Scholar]
- Sachs J, Mueller U, Wilcox T, Bull J. (2004). The evolution of cooperation. Q Rev Biol 79: 135–160. [DOI] [PubMed] [Google Scholar]
- Schink B. (2002). Synergistic interactions in the microbial world. Antonie Leeuwenhoek 81: 257–261. [DOI] [PubMed] [Google Scholar]
- Schramm A, Larsen L, Revsbech N, Ramsing N, Amann R, Schleifer K. (1996). Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 62: 4641–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra DO, Hengge R. (2014). Stress responses go three dimensional—The spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ Microbiol 16: 1455–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W, Ram S, Vilar JM. (2007). Synthetic cooperation in engineered yeast populations. Proc Natl Acad Sci USA 104: 1877–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieuwerts S, de Bok FAM, Hugenholtz J, van Hylckama Vlieg JET. (2008). Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl Environ Microbiol 74: 4997–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies D, Costerton J. (2002). Biofilms as complex differentiated communities. Annu Rev Microbiol 56: 187–209. [DOI] [PubMed] [Google Scholar]
- Strassmann J, Zhu Y, Queller D. (2000). Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 408: 965–967. [DOI] [PubMed] [Google Scholar]
- Sudakaran S, Salem H, Kost C, Kaltenpoth M. (2012). Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Mol Ecol 21: 6134–6151. [DOI] [PubMed] [Google Scholar]
- Taylor PD. (1992). Altruism in viscous populations—An inclusive fitness model. Evol Ecol 6: 352–356. [Google Scholar]
- Thiele JH, Chartrain M, Zeikus JG. (1988). Control of interspecies electron flow during anaerobic digestion: role of floc formation in syntrophic methanogenesis. Appl Environ Microbiol 54: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason LC, Costantino N, Court DL. (2007). Escherichia coli genome manipulation by P1 transduction. Curr Protoc Mol Biol 1: 1 17. [DOI] [PubMed] [Google Scholar]
- Tolker-Nielsen T, Molin S. (2000). Spatial organization of microbial biofilm communities. Microb Ecol 40: 75–84. [DOI] [PubMed] [Google Scholar]
- Travisano M, Velicer GJ. (2004). Strategies of microbial cheater control. Trends Microbiol 12: 72–78. [DOI] [PubMed] [Google Scholar]
- Trivers RL. (1971). Evolution of reciprocal altruism. Q Rev Biol 46: 35–57. [Google Scholar]
- Udvardi M, Poole P. (2013). Transport and metabolism in legume-rhizobia symbioses. Annu Rev Plant Biol 64: 781–805. [DOI] [PubMed] [Google Scholar]
- van Dyken JD, Müller MJI, Mack KML, Desai MM. (2013). Spatial population expansion promotes the evolution of cooperation in an experimental prisoner's dilemma. Curr Biol 23: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel J, Weissing FJ, Kuipers OP, Kovacs AT. (2014). Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISMEJ 8: 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstockem M, Michiels K, Vanderleyden J, Vangool A. (1987). Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl Environ Microbiol 53: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer GJ. (2003). Social strife in the microbial world. Trends Microbiol 11: 330–337. [DOI] [PubMed] [Google Scholar]
- Verbruggen E, El Mouden C, Jansa J, Akkermans G, Bucking H, West SA et al. (2012). Spatial structure and interspecific cooperation: theory and an empirical test using the mycorrhizal mutualism. Am Nat 179: E133–E146. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Pollock GB, Dugatkin LA. (1992). Can altruism evolve in purely viscous populations. Evol Ecol 6: 331–341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.