Abstract
Here we explore strategies of resource utilization and allocation of algal versus terrestrially derived carbon (C) by lake bacterioplankton. We quantified the consumption of terrestrial and algal dissolved organic carbon, and the subsequent allocation of these pools to bacterial growth and respiration, based on the δ13C isotopic signatures of bacterial biomass and respiratory carbon dioxide (CO2). Our results confirm that bacterial communities preferentially remove algal C from the terrestrially dominated organic C pool of lakes, but contrary to current assumptions, selectively allocate this autochthonous substrate to respiration, whereas terrestrial C was preferentially allocated to biosynthesis. The results provide further evidence of a mechanism whereby inputs of labile, algal-derived organic C may stimulate the incorporation of a more recalcitrant, terrestrial C pool. This mechanism resulted in a counterintuitive pattern of high and relatively constant levels of allochthony (~76%) in bacterial biomass across lakes that otherwise differ greatly in productivity and external inputs.
Introduction
The bulk dissolved organic carbon (DOC) pool of freshwater ecosystems is particularly diverse and complex owing to its multiple aquatic (for example, benthic and pelagic algae, macrophytes) and terrestrial (for example, vascular plants, soils) origins (Aitkenhead-Peterson et al., 2003; Bertilsson and Jones, 2003), and thus represents a major challenge in terms of resource utilization for aquatic bacterial communities. Past experimental work suggests that, faced with such highly heterogeneous substrates, bacteria may develop different strategies of resource utilization. For example, culture studies have shown that bacteria may selectively consume specific substrates within simple mixtures (Mateles and Chian, 1969; Sundh, 1992). In addition, bacteria may differentially allocate specific substrates to growth or respiration depending on the chemical properties and accessibility of the consumed substrate (Russell, 2007), and on the energy and stoichiometric requirements of cells (Vallino et al., 1996). Despite extensive culture evidence, however, we do not know the extent to which these strategies of bacterial resource utilization actually occur in natural aquatic ecosystems.
A widespread view in aquatic ecology and carbon (C) biogeochemistry is that bacterial communities exposed to a mix of algal and terrestrial compounds should develop a strategy of resource utilization whereby algal C is preferentially consumed and incorporated into biomass over terrestrial C owing to its greater accessibility and nutritional quality (Hobbie, 1988; Bianchi, 2011; Guillemette et al., 2013). However, the preferential consumption of algal C and its subsequent allocation to growth rather than to respiration remain to be empirically tested, as past studies have never simultaneously quantified the sources of DOC that support these different metabolic pathways. Further, the lack of concurrent measurements of the sources of C supporting bacterial biomass production (BBP) and respiration has hampered the exploration of potential interactions between the utilization of the terrestrial and algal C pools. For example, it was recently hypothesized that the utilization of labile, algal-derived C by bacteria may increase the overall utilization of terrestrial C by bacterial communities through a priming effect (Guenet et al., 2010). However, we do not know if the preferential consumption of algal C may also enhance its incorporation into bacterial biomass, thus increasing the overall importance of terrestrial subsidies (or allochthony) for aquatic bacterial communities.
Assessing these resource utilization strategies is important not only from a bacterial bioenergetics perspective, but also critical to our understanding of the functioning of aquatic ecosystems as a whole. There is an ongoing debate concerning the importance of terrestrial subsidies to aquatic food webs (Brett et al., 2009; Cole et al., 2011), partly originating from the fact that the pathways of delivery and transfer of these subsidies are still not well understood (Cole et al., 2006; Berggren et al., 2010b). Because they repackage a portion of the terrestrial DOC entering aquatic ecosystems in the form of bacterial biomass (Kritzberg et al., 2004), bacteria are likely to be central for the transfer of this allochthonous substrate to lake food webs (Tranvik, 1992; Berggren et al., 2010b). In addition, there is also direct evidence for the respiration of terrestrial C by bacteria (Karlsson et al., 2007; McCallister and del Giorgio, 2008), and that this process may contribute to the widespread net heterotrophy (gross primary production: ecosystem respiration <1) that characterizes the vast majority of inland waters (Duarte and Prairie, 2005). The lack of quantitative assessments of bacterial resource utilization strategies hinder an accurate assessment of the true level of allochthony both at the base of aquatic food webs, and in terms of whole-ecosystem metabolism, because the metabolic fate of the terrestrial vs algal C is still poorly known.
In this study we have explicitly tested two key hypotheses related to the strategies of resource utilization of algal and terrestrial sources of organic carbon by lake bacterioplankton: (1) that freshwater bacterioplankton preferentially consume organic C of algal origin within a complex ambient DOC pool and (2) that this algal C is preferentially allocated to growth rather than to respiration. We directly measured the δ13C isotopic signature of bacterial biomass in lake water incubations, and combined these to our own previously published measurements of the δ13C isotopic signature of respiratory carbon dioxide (CO2) determined in the same incubations (McCallister and del Giorgio, 2008) to quantify the proportion of terrestrial DOC either in BBP or respired (generically referred to as ‘allochthony' hereafter). We further combined these latter estimates with the actual rates of bacterial respiration (BR) and BBP to: (1) determine the actual flow of terrestrial and algal C into biomass and respiration, (2) assess possible interactions between these processes and (3) reconstruct the overall proportion of terrestrial C consumed by bacteria. The results presented herein confirm the selective consumption of specific DOC sources by bacteria, and further suggest a differential allocation of these sources to catabolic and anabolic pathways.
Materials and methods
Study sites and sample collection
We sampled eight lakes in the Eastern Townships region of Southern Québec, Canada (45.50°N, 73.58°W) during the summer period of 2004 and revisited four a month later. These lakes are located within the same drainage basin, which is dominated by temperate mixed wood forest, and cover a wide range in trophic status, DOC concentrations and morphometry (Supplementary Table S1). Lake water samples (60 l) were collected at a depth of 1.0 m using a diaphragm pump, stored in acid-washed polycarbonate bottles, and kept cool in the dark until return to the laboratory (<3 h). Further pumping of lake water (200 l) through a 50-μm mesh size net allowed the collection of zooplankton samples, which were placed at 4 °C overnight to empty gut contents. Over 100 individual cladocerans or copepods, used to estimate the δ13C isotopic signature of the algal endmember (see below), were then collected in smooth-walled tin capsules, acidified with 10% HCl and dried (45 °C) before isotopic analysis. Eight liters of unprocessed water was taken for the spectrophotometric determination of chlorophyll a (Chl a), and the colorimetric analysis of total phosphorus and nitrogen (Cattaneo and Prairie, 1995). The remaining water sample was filtered through a combusted (525 °C for 4 h) AE glass fiber filter (1.0 μm; Millipore, Billerica, MA, USA) to remove bacterial grazers (>90%), and to conduct bacterial metabolism experiments (see below). The 1.0-μm filtered water was subsequently passed through a Gelman filter capsule (0.2 μm; Pall, Port Washington, NY, USA) to collect DOC samples (poisoned with 5 N sulfuric acid), to determine water color at 440 nm (a440 in m−1; Naperian units) with a Ultrospec 2100 (Biochrom, Cambridge, UK) ultraviolet-visible spectrometer and to carry out the Respiratory C Recovery System (ReCReS) experiments.
Bacterial metabolism
Short-term BR incubations detailed in del Giorgio et al., 2006 were carried out in parallel with the ReCReS system experiments described below. In brief, BR was determined over 6 h as change in oxygen (O2) in 1.0-μm filtered water incubations kept in the dark at room temperature (20 °C). A membrane-inlet mass spectrometer was used to determine O2 concentrations at three time points in triplicate (Kana et al., 1994), and rates of O2 consumption were converted into C units using a respiratory quotient of 1.2 (Berggren et al., 2012). In parallel, bacterial biomass production was measured using the 3H-leucine incorporation technique (Kirchman, 1993) three times during the experiments (triplicate samples at each time point), and we used the average values to estimate BBP. Bacterial growth efficiency, defined as the share of the C consumed used for growth, was calculated as BBP/(BBP+BR), and total bacterial C consumption as the sum of BBP and BR.
Bacterial respiratory CO2 and biomass collection
The quantitative recovery of bacterial respiratory CO2 and biomass was achieved with the ReCReS system (McCallister et al., 2006). The system allows the collection of the CO2 derived from BR in freshwater samples while keeping background dissolved inorganic carbon values to a minimum (<2%). An airtight incubation system (20 l), in which a 0.2-μm filtered water sample is inoculated with a concentrate of lake bacteria (1.0 μm filtered) for 4 days, is coupled to a harvest system to capture the respiratory CO2 produced during these incubations. Following harvesting, the CO2-containing traps were mounted to a vacuum extraction line to strip the respiratory CO2 from residual moisture prior to transfer to break seals pending isotopic analysis. Potential methodological contaminations of the ReCReS system and fractionation artifacts are assumed to be marginal after prior testing (McCallister et al., 2006).
At the end of the incubation, bacterial cells present in the 20 l water sample were concentrated using tangential flow ultra-filtration (1000 kDa membrane; Millipore), and the bacterial biomass was recovered on a precombusted (550 °C for 4 h) 0.2 μm Anodisc 47 filter (Whatman, Springfield Mill, UK). All filters containing bacterial biomass were acid fumed overnight with HCl and dried at 45 °C prior to transfer into tin capsules and isotopic analysis.
Isotopic analysis
Within 3 days of collection, the respiratory CO2 breakseals were analyzed for δ13C with a continuous flow GasBench peripheral (Thermo Finnigan, Bremen, Germany) interfaced to an Isotope Ratio Mass Spectrometer Delta XP (Thermo Finnigan) with an analytical precision of 0.10‰ (G.G. Hatch Lab, University of Ottawa, Canada). Stable carbon isotope ratios for bacterial biomass and zooplankton were measured using a FinniganMAT Deltaplus dual-inlet continuous flow isotope ratio mass spectrometer (Thermo Finnigan) with on-line sample combustion with a VarioEL III (Elementar Analysensysteme GmbH, Hanau, Germany) elemental analyser. A few zooplankton samples were assessed for analytical precision and run in duplicate (relative s.d. <0.3‰). Finally, DOC concentration and δ13C determination were performed on a modified 1010 TIC TOC analyzer (O.I. Analytical, College Station, TX, USA) connected to a Finnigan MAT DeltaPlus IRMS with a Conflo III continuous flow interface (Thermo Finnigan) as described in St-Jean, 2003. Stable isotopes values are reported in standard δ notation as:
where R is 13C:12C.
Apportioning terrestrial and algal C contribution
We determined the relative contribution of terrigenous and algal sources to bulk DOC, bacterial respiratory CO2 and biomass based on a two-source mixing model described by the following equations:
where δ13CC-Comp. corresponds to the isotopic signature of a given C component, ƒ1 and and ƒ2 are the relative contributions of terrestrial (CTerrestrial) and algal (CAlgal) sources to this component, respectively. Uncertainties (expressed as±s.e.) in the relative contribution of terrestrial and algal C sources to the bulk DOC pool, bacterial biomass and respiratory CO2 were constrained using IsoError (Phillips and Gregg, 2001). In addition, the model accounts for variations in the δ13C of the algal and terrestrial endmembers, as well as the analytical error of the mixtures (±0.2‰ for all isotopic analyses, that is, bulk DOC, bacterial biomass and respiratory CO2). The terrestrial endmember was set to the commonly accepted value of −27.0‰ typically found for terrestrial C3 plants (Lajtha and Marshall, 1994; Boschker and Middelburg, 2002), and we further introduced a ±0.5‰ error in the model in line with the results of two DOC samples collected in a typical forested stream from the surrounding watershed of these lakes, with a δ13C of −27.2‰±0.1. The algal δ13C end point was constrained using the zooplankton isotopic signature, and we took whichever zooplankton fraction had the most depleted δ13C isotopic signature (McCallister and del Giorgio, 2008). This approach was shown to yield algal δ13C estimates that compare well with other approaches (Karlsson et al., 2007; Marty and Planas, 2008). There is clear evidence that zooplankton biomass may also contain terrestrial C (Cole et al., 2011; Karlsson et al., 2012), and to account for this we further assumed a 16% contribution of terrestrial C to zooplankton biomass, based on the average terrestrial C content reported by Mohamed and Taylor, 2009 for other Canadian temperate lakes (N=25), and in accordance with the level of allochthony previously observed in several of the lakes sampled in this study (del Giorgio and France, 1996). We further used the range of zooplankton allochthony 9–23% (±1 s.d.) reported by Mohamed and Taylor, 2009, which also covers the range found in the boreal region of Northern Québec (Berggren et al., 2014), as a measure of uncertainty of the algal endmember. Finally, we used a similar approach to that of Karlsson et al. (2007) and Attermeyer et al. (2014), and corrected the isotopic signature of respiratory CO2 for a +0.5‰ fractionation during respiration according to Hullar et al., 1996. Similarly, the biomass δ13C values were corrected for a +0.6‰ fractionation during biosynthesis based on the average of published measurements made in other aquatic settings (see Supplementary Table S2 for details).
Proportion of terrestrial DOC consumed by bacteria
We estimated the relative proportion of terrestrial DOC sustaining total bacterial carbon consumption by combining our estimates of terrestrial C in bacterial biomass and respiratory CO2 with the short-term rates of bacterial metabolism measured in the parallel metabolic incubations. We previously employed a similar approach to estimate the rates of BR supported by terrestrial DOC (McCallister and del Giorgio, 2008), and we applied this same approach here for bacterial biomass. Further, we used a mass balance to ascertain the proportion of terrestrial DOC consumed by bacteria:
where Consumed DOCTerr, BiomassTerr and Resp. CO2Terr correspond to the proportion of terrestrial DOC consumed, used for biomass production and respired by bacteria, respectively.
Results
Bacterial metabolic allocation of algal and terrestrial DOC
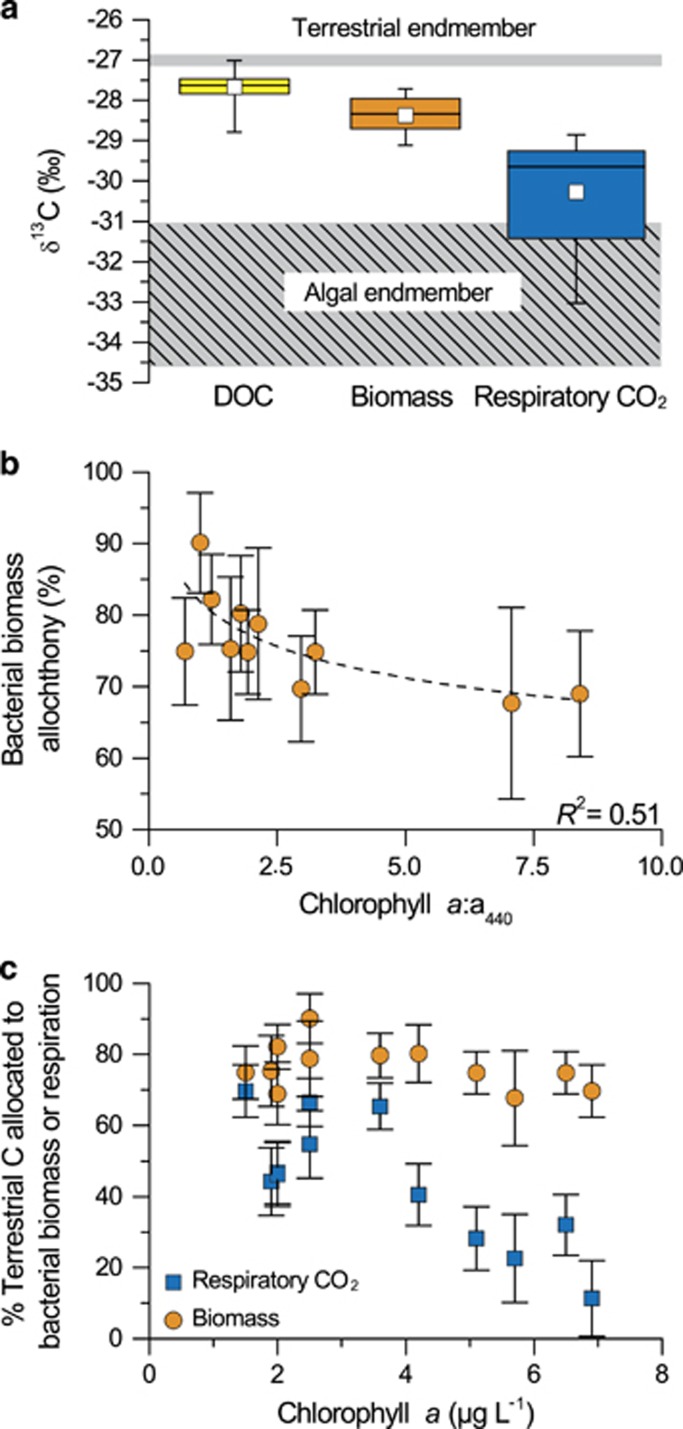
To assess the patterns in the bacterial metabolic allocation of algal and terrestrial DOC, we first estimated the proportion of both C sources in bacterial biomass, and compared this with the proportions in BR and in bulk DOC for the same incubations, the former having been reported in McCallister and del Giorgio (2008). For comparative purpose, the proportions of algal and terrestrial C estimated for respiratory CO2 from the study of McCallister and del Giorgio (2008) were recalculated here using the same procedure and assumptions as for bacterial biomass and bulk DOC (see Materials and methods). The isotopic signature of the bulk DOC pool minimally departed from the terrestrial endmember across lakes (−28.8 to −27.0‰ Table 1), and was negatively related to the ratio of total phosphorus to a440 (y=−27.16–0.69x, R2=0.67, P<0.01; Supplementary Figure S1) and positively to the ratio of a440 to lake DOC concentration (y=−29.02+4.38x, R2=0.65, P<0.01; Supplementary Figure S1), suggesting that the isotopic signature effectively captures shifts in the relative input of algal and terrestrial C on the bulk DOC pool composition. Similarly, the δ13C values of bacterial biomass were generally close to the terrestrial endmember across lakes (−29.1 to −27.7‰ Table 1), and did not differ significantly from that of bulk DOC on average (Figure 1a; one-way analysis of variance, post hoc Tukey test, P>0.05). As a result, the estimated allochthony of bacterial biomass was high across lakes (average of 76%±6; Table 1), with a lower limit approaching ~70% along a gradient of algal vs terrestrial influence (ratio of Chl a and a440; y=80.5–5.2 log (x), R2=0.48, P<0.05). In comparison, the isotopic signature of respiratory CO2 was systematically depleted compared with that of bulk DOC and bacterial biomass (Figure 1a), with δ13C values ranging from −33.0 to −28.9‰ (Table 1), and consequently the estimated proportion of terrestrial C in respiratory CO2 (44%±18) was significantly lower than in both bulk DOC (89%±10) and bacterial biomass (76%±6; one-way analysis of variance, post hoc Tukey test, P<0.001). The contribution of terrestrial C to respiration was previously found to decrease systematically with increasing lake trophy (McCallister and del Giorgio, 2008). The allochthony in bacterial biomass, however, did not follow this pattern, but rather remained relatively high and constant along the gradient of lake trophic status (Figure 1c).
Table 1. Estimated contribution of terrestrial C to bulk DOC, bacterial biomass and respiratory CO2.
| Lake | Algal endmember δ13C (‰) | Terrestrial endmember δ13C (‰) |
DOC |
Biomass |
Respiratory CO2 |
|||
|---|---|---|---|---|---|---|---|---|
| δ13C (‰) | % Terrestrial | δ13C (‰) | % Terrestrial | δ13C (‰) | % Terrestrial | |||
| Brome | −30.1a | −27.0b | — | — | −28.0c | 68±13 | −29.4 | 23±12 |
| Memphremagog | −34.6 | −27.0 | — | — | −28.5 | 80±6 | −29.6 | 65±6 |
| Des Monts 1 | −32.8 | −27.0 | −27.1 | 99±9d | −28.2 | 80±8 | −30.5 | 41±9 |
| Simoneau | −32.0 | −27.0 | −27.5 | 90±10 | −27.9 | 82±9 | −29.7 | 47±7 |
| Stukely 1 | −31.3 | −27.0 | −27.5 | 89±11 | −28.1 | 75±10 | −29.4 | 44±10 |
| Bran-de-scie 1 | −35.4 | −27.0 | −27.8 | 91±6 | −29.1 | 75±6 | −32.7 | 32±9 |
| Fraser 1 | −33.1 | −27.0 | −27.0 | 100±9 | −28.9 | 70±7 | −32.4 | 11±11 |
| Bran-de-scie 2 | −35.4 | −27.0 | −27.8 | 91±6 | −29.1 | 75±6 | −33.0 | 28±9 |
| Des Monts 2 | −34.2 | −27.0 | −27.8 | 89±7 | −27.7 | 90±7 | −29.4 | 67±7 |
| Stukely 2 | −31.3 | −27.0 | −27.5 | 89±11 | −27.9 | 79±11 | −29.0 | 55±10 |
| Bowker | −32.0 | −27.0 | −28.8 | 64±9 | −28.6 | 69±9 | −29.7 | 46±9 |
| Fraser 2 | −33.1 | −27.0 | −28.0 | 84±8 | −28.5 | 75±8 | −28.9 | 70±7 |
The algal endmember isotopic values are based on zooplankton δ13C signature (Karlsson et al., 2007; McCallister and del Giorgio, 2008) and assuming a 16% terrestrial C content (see Materials and methods for details).
The terrestrial endmember was set to −27.0‰ based on Lajtha and Marshall, 1994 and Boschker and Middelburg, 2002), and on the δ13C (−27.2‰±0.1, N=2) of a small forested stream sampled in this study.
Note that the reported δ13C values of bacterial biomass and respiratory CO2 were corrected for an isotopic fractionation factor of +0.6‰ and +0.5‰, respectively (see Materials and methods and Supplementary Table 2 for details). The isotopic signatures of respiratory CO2 were reported before (McCallister and del Giorgio, 2008), and are shown here for comparison purpose.
A two-source (algal and terrestrial) mixing model was resolved to calculate the contribution of terrestrial C to bulk DOC, bacterial respiratory CO2 and biomass using their respective δ13C signature, and the uncertainties in these estimates (expressed as±s.e.) were constrained using IsoError (Phillips and Gregg, 2001).
Figure 1.
Terrestrial and algal DOC metabolic allocation by lake bacterioplankton. (a) The isotopic signature (δ13C) of bulk DOC (yellow), bacterial biomass (orange) and respiratory CO2 (blue) measured in parallel in the same short-term lake water incubations. Open squares denote mean values. Also shown are the ranges in δ13C of the terrestrial and algal endmembers used in the mixing models (see Materials and methods for details). (b) The relationship between bacterial biomass allochthony and the ratio of chlorophyll a and the absorption coefficient measured at 440 nm (y=80.5–5.2 log (x), R2=0.48, P<0.05). (c) The allocation of terrestrial C to bacterial respiration (blue squares; y=72.2–7.6x, R2=0.64, P<0.01) and biomass (orange circles; non-significant relationship) as a function of lake chlorophyll a concentrations. The δ13C of respiratory CO2 data has been reported previously in McCallister and del Giorgio, 2008, and are shown here for comparison purpose. The error bars in (b) and (c) are ±s.e. calculated with IsoError (Phillips and Gregg, 2001) (see Materials and methods).
Incorporation efficiency of terrestrial DOC
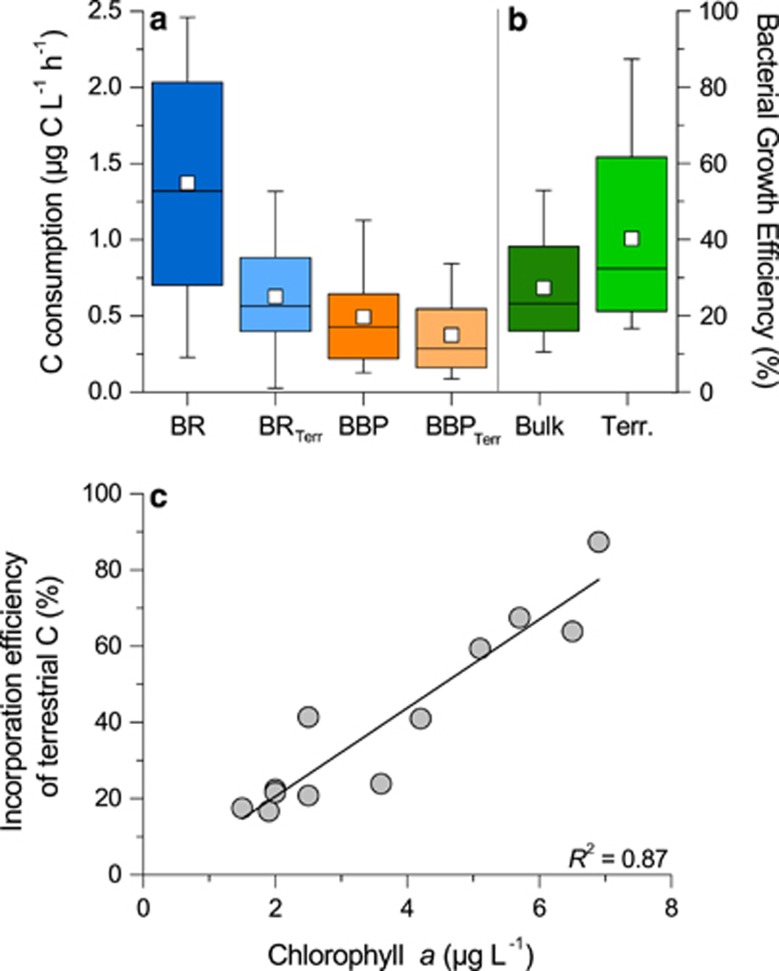
The consistently high levels of bacterial biomass allochthony across a lake productivity gradient (Figure 1c) implies that the incorporation efficiency of terrestrial C into biomass must increase along a similar gradient considering that: (1) the bulk, terrestrially dominated DOC pool of lakes becomes diluted by algal C, and that (2) algal C sustains a higher share of the overall bacterial metabolism in more productive lakes as suggested by the decreased allochthony in BR with increasing lake trophy (Figure 1c). To explore this possibility, we first calculated the actual rates of BR and biomass production based on terrestrial DOC (BRTerr and BBPTerr, respectively), by combining the measured BR and BBP rates with the estimated proportion of terrestrial C fueling both pathways described in the previous section. Across lakes, rates of BR based on terrestrial DOC varied from 0.03 to 1.20 μg C l−1 h−1, which were on average (0.63±0.38 μg C l−1 h−1) significantly lower than the overall rates of BR (1.34±0.73 μg C l−1 h−1; paired t-test, P<0.01; Figure 2a). On the other hand, BBPTerr ranged from 0.10 to 0.92 μg C l−1 h−1, but on average BBPTerr did not differ significantly from the average of overall BBP (0.38±0.27 μg C l−1 h−1 and 0.49±0.35 μg C l−1 h−1, for BBPTerr and BBP, respectively; paired t-test, P>0.05; Figure 2a). We then calculated the growth efficiency (or incorporation efficiency into biomass) associated to this terrestrial C (BGETerr), as BGETerr=BBPTerr/(BBPTerr+BRTerr). The resulting incorporation efficiency of terrestrial C was on average 40% higher than the bulk bacterial growth efficiency (bulk BGE), with values ranging from 17% up to 87% (Figure 2b). Further, we found that the incorporation efficiency of terrestrial C into bacterial biomass was strongly and positively related to Chl a concentrations (y=11.4 x, R2=0.87, P<0.001; Figure 2c), and less strongly to total phosphorus (y=3.72 x, R2=0.50, P=0.01), thus peaking in the most eutrophic lakes.
Figure 2.
Incorporation efficiency of terrestrial C. Ranges in total and terrestrial (a) bacterial respiration and biomass production, and (b) bacterial growth efficiency. Open squares denote mean values. (c) Incorporation efficiency of terrestrial C (BGE terrestrial) as a function of lake chlorophyll a concentrations (y=11.6x, R2=0.87, P<0.001).
Reconstructing the bacterial consumption of algal and terrestrial DOC
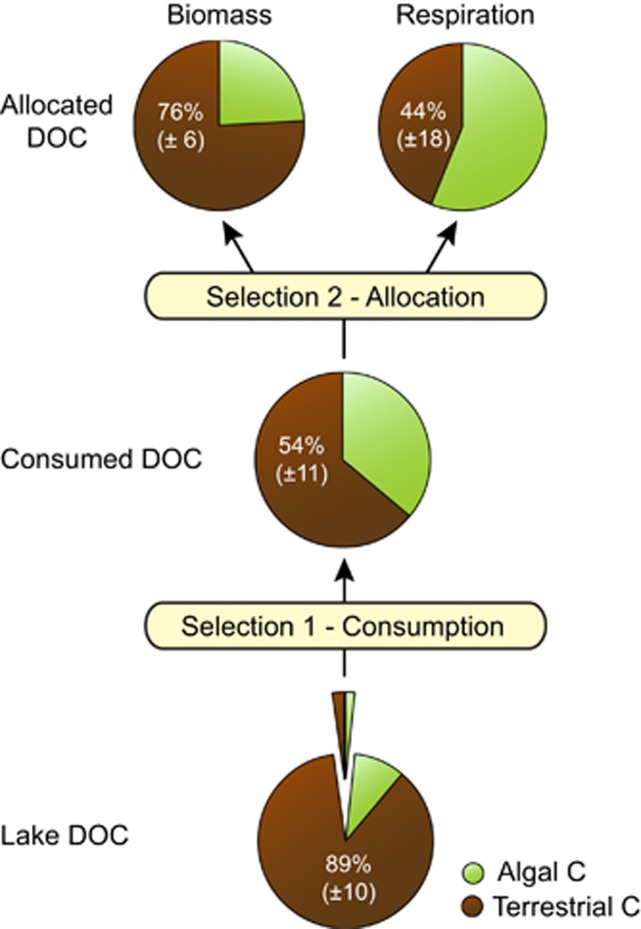
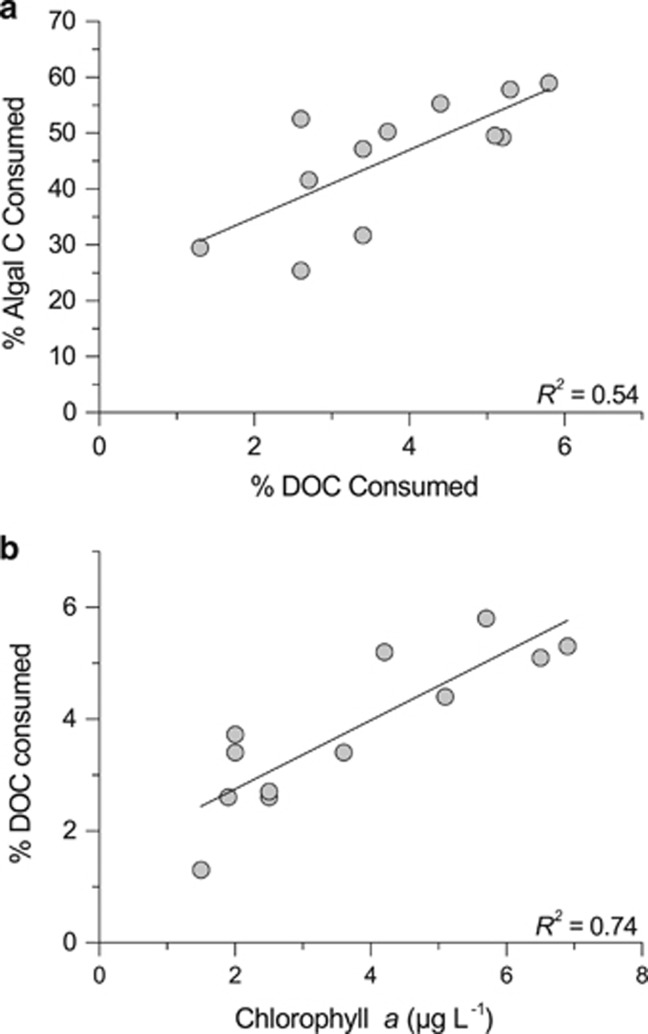
One of the aims of the study was to reconstruct the proportion of algal and terrestrial C consumed by bacterial communities to estimate their overall metabolic reliance on terrestrial C sources. We used a mass balance approach that combined the proportion of terrestrial C in bacterial biomass and in respiratory CO2, with the actual rates of bacterial production and respiration measured in parallel experiments (see Materials and methods). Our calculations reveal that, on average, bacteria consumed terrestrial C in much lower proportions than that present in bulk DOC (54%±11 versus 89%±10, for consumed and bulk DOC, respectively; Figure 3). It thus appears that bacteria selectively consumed and removed algal C from the bulk DOC pool of these lakes (Selection 1, Figure 3), but as mentioned above, do not allocate these two categories of substrate evenly to respiration and biomass. Rather, there was on average 22% more terrestrial C present in biomass than in the consumed DOC pool (one-way analysis of variance, post hoc Tukey test, P<0.001), and therefore less terrestrial C was channeled toward respiration (10% less on average, but not significant (one-way analysis of variance, post hoc Tukey test, P>0.05; Selection 2, Figure 3). Interestingly, the composition of consumed DOC could not be predicted from the composition of bulk DOC based on isotopic measurements (Supplementary Figure S2). Rather, we found that the fraction of DOC consumed that was of algal origin increased with the total amount of DOC consumed (that is, total bioavailable DOC; Figure 4a), which itself increased as a function of Chl a concentrations (Figure 4b).
Figure 3.
Proportion of algal (green) and terrestrial (brown) C in the consumed DOC pool, bacterial biomass and respiratory CO2. The diagram depicts the preferential consumption of algal vs terrestrial DOC (Selection 1) and the further allocation of the C sources to the respiration and biomass synthesis (Selection 2) in lake bacterioplankton. The terrestrial contribution to each component is derived from a δ13C, two-source (algal and terrestrial) mixing model (see Materials and methods). The average proportion was estimated in 10 of the 12 temperate lakes of southeastern Québec for which all comparative data were available, and is shown along with ±1 s.d. in brackets.
Figure 4.
Bioavailability of algal C. The relationships between (a) the proportion of algal C consumed and the fraction of bulk DOC consumed (y=22.9+6.0x, R2=0.54, P<0.01), and (b) the fraction of bulk DOC consumed over the incubation span and lake chlorophyll a concentrations (y=1.52+0.62x, R2=0.74, P<0.01).
Discussion
Our findings demonstrate that lake bacterial communities selectively consume pools of DOC of specific origin (Figure 3), and that the proportion of terrestrial C consumed by bacteria is not simply a reflection of the contribution of terrestrial C to the ambient DOC pool. Further, our results demonstrate that the terrestrial C allocated to growth and respiration is not simply a reflection of the proportion of terrestrial C originally consumed. Had we assumed that the level of terrestrial C observed in bacterial biomass was indicative of the proportion of terrestrial C that was originally consumed, we would have largely overestimated both the relative availability of this C in bulk DOC, and perhaps more importantly, the importance of terrestrial C to the overall metabolism of the bacterial compartment in our lakes (76% terrestrial C in biomass versus 54% of terrestrial C consumed as estimated by our mass balance calculation). In addition, our results suggest a strong interaction between the consumption and further respiration of algal C (Figure 4), and the incorporation of terrestrial C in bacterial biomass (Figure 2c), leading to high levels of terrestrial C in bacterial biomass even in highly productive lakes (Figure 1). These results are not only counterintuitive, but represent a major departure from the prevailing paradigm regarding bacterial cycling of algal versus terrestrial C in aquatic systems.
The results of our mass balance approach suggest that bacteria disproportionally removed algal DOC from a largely terrestrially dominated DOC pool, even in lakes of low primary productivity (Supplementary Figure S2). This preferential consumption of algal DOC by lake bacterial communities has been previously postulated, but past inferences were based on the isotopic signal of bacterial biomass (Kritzberg et al., 2004), of the respiratory CO2 (Guillemette et al., 2013) or on modeling (Berggren et al., 2010b), rather than on the combined amounts of respired and incorporated DOC as we present here. Our study thus provides novel and more robust support to the contention that algal DOC is preferentially consumed by bacteria (Figure 3). The high lability of algal DOC has also been traditionally equated to high nutritional quality (Thorp and Delong, 2002), and consequently to a greater potential to support growth. In contrast to this assumption, we found that the consumed algal pool was mainly diverted toward the respiratory pathway, and not to biomass production as previously suggested (Cole et al., 1988; Kamjunke et al., 2006). Although we can only speculate on the underlying mechanisms at this point, it is possible that the preferential respiration of algal compounds may in fact reflect a strategy related to growth under energy limitation in the pelagic environment of lakes (Smith and Prairie, 2004). The cellular energy flux is maximized by allocating high-energy substrates to respiration to maintain cellular functions such as membrane integrity, active transport systems, nutrient acquisition and enzyme production (Russell, 1991; del Giorgio and Cole, 1998). In this regard, past studies have shown that phytoplankton excrete compounds such as polysaccharides and carbohydrates that are not only easily accessible and quickly consumed by bacteria, but also energetically rich (Sundh, 1992; Amon and Benner, 1994; Weiss and Simon, 1999).
It is also possible that the apparent substrate selectivity does not occur at the cellular level, but rather at the community level. This community-level substrate selectivity could hypothetically result from shifts in the relative contribution of taxa that have intrinsically different strategies of C consumption and processing, such that the overall community C processing would reflect the relative proportion of these different functional types. Under this scenario, the apparent preferential respiration of algal C as suggested here would reflect the rapid turnover of specific populations within bacterial communities that are able to preferentially exploit labile algal C (Geller, 1986; De Nobili et al., 2001; Crump et al., 2003), but contribute very little to the overall biomass, which may be dominated by slower growing bacteria preferentially consuming terrestrially derived C. Unfortunately we did not collect DNA from these incubations, so we cannot test this hypothesis, and clearly, these alternative scenarios need to be further assessed. Regardless, our results are coherent with the strong correlation often observed between BR and Chl a or total phosphorus (both indicators of a higher algal C production), and the weaker correlation with DOC (that is, terrestrial C inputs) (Pace and Prairie, 2005).
In contrast to the pattern of preferential respiration of algal-derived C, much of the terrestrial DOC consumed was allocated to biosynthesis in our incubations, resulting in high levels of allochthony in bacterial biomass across lakes (76±6% Figure 3). Interestingly, our results suggest a lower limit in bacterial biomass allochthony in the order of ~70% even in more productive systems where terrestrial DOC becomes more diluted by algal C (Figure 1). These patterns are coherent with previous studies which noted similar high levels of allochthony of bacterial biomass (79%±16) across a wide range in lake productivity or color in Québec on the basis of the isotopic composition of bacterial fatty acids (Berggren et al., 2014), as well as in a lake in northern Sweden (>80%) (Karlsson et al., 2012) and in two Wisconsin lakes (Kritzberg et al., 2004), although lower estimates have also been reported in the latter (range of 35–70%). Our results are also consistent with a recent hypothesis, suggesting that the rapid transfer and efficient incorporation of compounds exported from soils and leaf litter, or produced via photochemical degradation, may account for a large share of total bacterial production in boreal streams and lakes (up to 80%) (Berggren et al., 2010a, b). These past studies, together with the observations presented here, are in stark contrast with the common assumption that terrestrially derived C is of poor nutritional quality or not particularly suited to support bacterial growth (Hobbie, 1988).
The relative constancy (76±6%, Figure 1) of the terrestrial signature in bacterial biomass across lakes is also remarkable given the range in lake productivity (Table 1), and in the proportion of terrestrial C consumed along this gradient (42–71%). It appears that as lakes become more productive, bacteria consume increasing proportions of algal C (Figure 4), yet the efficiency of incorporation of terrestrial C also increases along the same productivity gradient (Figure 2), such that the actual proportion of terrestrial C in bacterial biomass remains relatively constant across lakes. It has recently been hypothesized that an increase in the availability of labile compounds of algal origin may enhance the degradation of recalcitrant terrestrial DOC by aquatic bacterial communities (Danger et al., 2013; Guenet et al., 2014; Hotchkiss et al., 2014), although the actual occurrence and significance of this priming mechanism is still debated (Bengtsson et al., 2014; Catalán et al., 2015). Our experimental set up did not allow to directly test this hypothesis in terms of the enhancement of the consumption of terrestrial C, yet our results do suggest a significant interplay between the preferential allocation of algal-derived C to respiration and the incorporation of terrestrial DOC into bacteria biomass. The underlying mechanisms need to be further assessed, but this pattern nevertheless has important ecological consequences. The increase in terrestrial C incorporation efficiency into biomass with increasing system productivity implies that even in eutrophic systems, where bacteria overwhelmingly consume algal-derived C, the isotopic signature of bacterial biomass may still be largely terrestrial. There is now irrefutable evidence of widespread allochthony in freshwater zooplankton and fish (Cole et al., 2011; Karlsson et al., 2012; Berggren et al., 2014), but these metazoans have a low capacity to consume and grow on detrital terrestrial C (Brett et al., 2009), so this transfer must necessarily involve an efficient repackaging of terrestrial C in the form of aquatic bacterial biomass (Berggren et al., 2010b) that can then circulate within aquatic food webs. Given the large losses that occur during the transfer of C in food webs, the systematically high terrestrial signature of bacterial biomass may be one of the keys to sustaining the widespread presence of terrestrial C measured in zooplankton even in more productive or nutrient enriched systems (Cole et al., 2002). Our results thus provide support to the notion that bacteria are one of the key entry points of terrestrial C into aquatic food webs. Our results further elicit a re-evaluation of how we conceptualize and quantify the importance of terrestrial C in aquatic food webs, because the selective allocation of C sources also implies that the overall allochthony in bacteria, and potentially in other communities as well, cannot be inferred from the isotopic signature of either the respired or incorporated C. Thus, we postulate that both should be explicitly considered and assessed in future food-web allochthony studies.
The description of the patterns of resource utilization observed in this study was only possible because we simultaneously quantified the BR and incorporation of different sources of DOC in lakes. Our study relies, however, like other studies of its kind (Kritzberg et al., 2004; Karlsson et al., 2007; Attermeyer et al., 2014) on different assumptions concerning the isotopic values of the algal and terrestrial endmembers, and the level of bacterial fractionation during biosynthesis and respiration. Here we used previously published values for these parameters, and also a well-established isotopic model (IsoError; Phillips and Gregg, 2001) to account for their variability, but we cannot discard that some methodological biases, isotopic artifacts or secondary C incorporation pathways such as anaplerotic C fixation may have influenced our estimates of allochthony (See Supplementary Note 1 for a discussion of these issues). It should be emphasized that our estimates of bacterial biomass allochthony fall well within the range recently reported in other regions (Kritzberg et al., 2004; Karlsson et al., 2012; Berggren et al., 2014), and that the patterns in BR of terrestrial C also agree with previous reports, that is, that BR in unproductive lakes is dominated by terrestrial C (Karlsson et al., 2007; Karlsson et al., 2008). In addition, it is unlikely that the link that we found between the patterns of allocation of terrestrial versus algal C in the experimental incubations with ambient lake Chl a and phosphorous concentration (both of which being completely unconnected to the actual isotopic or metabolic mass balance), would emerge from chance or methodological bias, providing further evidence that these patterns in bacterial DOC utilization and allocation across lakes that we describe here are real.
In conclusion, the results presented here, while reinforcing the notion that lake bacterial communities may be strongly subsidized by terrestrial DOC inputs, show that once consumed, terrestrial and algal DOC have very different metabolic fates. Although algal DOC appears to be the preferred substrate, it is the terrestrial fraction that is primarily channeled to bacterial biosynthesis. Our results also suggest that increased availability of autochthonous DOC may facilitate the incorporation of terrestrial DOC into biomass, leading to the counterintuitive scenario wherein bacterial reliance on terrestrial DOC for growth may remain constant even if aquatic systems become more productive. This proposed pattern needs to be further tested, but provides a potential explanation to the widespread presence of a significant terrestrial contribution to bacterial communities and higher trophic levels in lakes. More generally, our findings suggest that allochthony in bacterial communities cannot be inferred from either the properties of the bulk DOC pool, or from the isotopic signature of the respired or incorporated C, because of the differential resource utilization strategies adopted by these communities. This may not just be a feature of freshwater bacterial communities, but rather a more generalized pattern within aquatic food webs, which should be considered in future studies assessing the influence of resource subsidies on organisms and ecosystem dynamics.
Acknowledgments
This work was founded by the National Science and Engineering Research Council of Canada (NSERC) #387312, and by NSF-DEB #0820725 and #1127962 to SLM. D Fréchette provided precious help in the laboratory and in the field, and C Beauchemin provided support for water chemistry analyses. We extend our thanks to P Middlestead of the G.G. Hatch Lab of the University of Ottawa for isotopic analysis. Finally, we thank Jay Lennon, Mario Muscarella, Martin Berggren, Jean-François Lapierre, Matthew Bogard and Josep Gasol for insightful comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Aitkenhead-Peterson JA, McDowell WH, Neff JC. (2003) Sources, production, and regulation of allochthonous dissolved organic matter inputs to surface waters. In: Findlay S, Sinsabaugh R (eds). Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press: New York, NY, USA, pp 26–70. [Google Scholar]
- Amon RMW, Benner R. (1994). Rapid cycling of high-molecular-weight dissolved organic matter in the ocean. Nature 369: 549–552. [Google Scholar]
- Attermeyer K, Hornick T, Kayler ZE, Bahr A, Zwirnmann E, Grossart HP et al. (2014). Enhanced bacterial decomposition with increasing addition of autochthonous to allochthonous carbon without any effect on bacterial community composition. Biogeosciences 11: 1479–1489. [Google Scholar]
- Bengtsson MM, Wagner K, Burns NR, Herberg ER, Wanek W, Kaplan LA et al. (2014). No evidence of aquatic priming effects in hyporheic zone microcosms. Sci Rep 4: 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren M, Lapierre J-F, del Giorgio PA. (2012). Magnitude and regulation of bacterioplankton respiratory quotient across freshwater environmental gradients. ISME J 6: 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren M, Laudon H, Haei M, Strom L, Jansson M. (2010. a). Efficient aquatic bacterial metabolism of dissolved low-molecular-weight compounds from terrestrial sources. ISME J 4: 408–416. [DOI] [PubMed] [Google Scholar]
- Berggren M, Ström L, Laudon H, Karlsson J, Jonsson A, Giesler R et al. (2010. b). Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecol Lett 13: 870–880. [DOI] [PubMed] [Google Scholar]
- Berggren M, Ziegler SE, St-Gelais NF, Beisner BE, del Giorgio PA. (2014). Contrasting patterns of allochthony among three major groups of crustacean zooplankton in boreal and temperate lakes. Ecology 95: 1947–1959. [DOI] [PubMed] [Google Scholar]
- Bertilsson S, Jones J. (2003). Supply of dissolved organic matter to aquatic ecosystems: autochthonous sources. In: Findlay S, Sinsabaugh R (eds). Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press: New York, NY, USA, pp 3–25. [Google Scholar]
- Bianchi TS. (2011). The role of terrestrially derived organic carbon in the coastal ocean: a changing paradigm and the priming effect. Proc Natl Acad Sci USA 108: 19473–19481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschker H, Middelburg J. (2002). Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol Ecol 40: 85–95. [DOI] [PubMed] [Google Scholar]
- Brett MT, Kainz MJ, Taipale SJ, Seshan H. (2009). Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proc Natl Acad Sci USA 106: 21197–21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán N, Kellerman AM, Peter H, Carmona F, Tranvik LJ. (2015). Absence of a priming effect on dissolved organic carbon degradation in lake water. Limnol Oceanogr 60: 159–168. [Google Scholar]
- Cattaneo A, Prairie YT. (1995). Temporal variability in the chemical characteristics along the Rivière de l'Achigan: how many samples are necessary to describe stream chemistry? Can J Fish Aquat Sci 52: 828–835. [Google Scholar]
- Cole JJ, Carpenter SR, Kitchell J, Pace ML, Solomon CT, Weidel B. (2011). Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proc Natl Acad Sci USA 108: 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JJ, Carpenter SR, Kitchell JF, Pace ML. (2002). Pathways of organic carbon utilization in small lakes: results from a whole-lake 13C addition and coupled model. Limnol Oceanogr 47: 1664–1675. [Google Scholar]
- Cole JJ, Carpenter SR, Pace ML, Van de Bogert MC, Kitchell JL, Hodgson JR. (2006). Differential support of lake food webs by three types of terrestrial organic carbon. Ecol Lett 9: 558–568. [DOI] [PubMed] [Google Scholar]
- Cole JJ, Findlay S, Pace ML. (1988). Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser 43: 1–10. [Google Scholar]
- Crump BC, Kling GW, Bahr M, Hobbie JE. (2003). Bacterioplankton community shifts in an Arctic lake correlate with seasonal changes in organic matter source. Appl Environ Microbiol 69: 2253–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danger M, Cornut J, Chauvet E, Chavez P, Elger A, Lecerf A. (2013). Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94: 1604–1613. [DOI] [PubMed] [Google Scholar]
- De Nobili M, Contin M, Mondini C, Brookes P. (2001). Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33: 1163–1170. [Google Scholar]
- del Giorgio PA, Cole JJ. (1998). Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Syst 29: 503–541. [Google Scholar]
- del Giorgio PA, France RL. (1996). Ecosystem-specific patterns in the relationship between zooplankton and POM or microplankton δ13C. Limnol Oceanogr 41: 359–365. [Google Scholar]
- del Giorgio PA, Pace ML, Fischer D. (2006). Relationship of bacterial growth efficiency to spatial variation in bacterial activity in the Hudson River. Aquat Microb Ecol 45: 55–67. [Google Scholar]
- Duarte C, Prairie Y. (2005). Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 8: 862–870. [Google Scholar]
- Geller A. (1986). Comparison of mechanisms enhancing biodegradability of refractory lakewater constituents. Limnol Oceanogr 31: 755–764. [Google Scholar]
- Guenet B, Danger M, Abbadie L, Lacroix G. (2010). Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91: 2850–2861. [DOI] [PubMed] [Google Scholar]
- Guenet B, Danger M, Harrault L, Allard B, Jauset-Alcala M, Bardoux G et al. (2014). Fast mineralization of land-born C in inland waters: first experimental evidences of aquatic priming effect. Hydrobiologia 721: 35–44. [Google Scholar]
- Guillemette F, McCallister SL, del Giorgio PA. (2013). Differentiating the degradation dynamics of algal and terrestrial carbon within complex natural dissolved organic carbon in temperate lakes. J Geophys Res Biogeosci 118: 963–973. [Google Scholar]
- Hobbie JE. (1988). A comparison of the ecology of planktonic bacteria in fresh and salt water. Limnol Oceanogr 33: 750–764. [Google Scholar]
- Hotchkiss ER, Hall RO, Baker MA, Rosi-Marshall EJ, Tank JL. (2014). Modeling priming effects on microbial consumption of dissolved organic carbon in rivers. J Geophys Res Biogeosci 119: 2013JG002599. [Google Scholar]
- Hullar M, Fry B, Peterson B, Wright R. (1996). Microbial utilization of estuarine dissolved organic carbon: a stable isotope tracer approach tested by mass balance. Appl Environ Microbiol 62: 2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamjunke N, Bohn C, Grey J. (2006). Utilisation of dissolved organic carbon from different sources by pelagic bacteria in an acidic mining lake. Arch Hydrobiol 165: 355–364. [Google Scholar]
- Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC. (1994). Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66: 4166–4170. [Google Scholar]
- Karlsson J, Ask J, Jansson M. (2008). Winter respiration of allochthonous and autochthonous organic carbon in a subarctic clear-water lake. Limnol Oceanogr 53: 948. [Google Scholar]
- Karlsson J, Berggren M, Ask J, Byström P, Jonsson A, Laudon H et al. (2012). Terrestrial organic matter support of lake food webs: Evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnol Oceanogr 57: 1042–1048. [Google Scholar]
- Karlsson J, Jansson M, Jonsson A. (2007). Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol Oceanogr 52: 603–608. [Google Scholar]
- Kirchman D. (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp P, Sherr BF, Sherr EB, Cole JJ (eds). Handbook of methods in aquatic microbial ecology. Lewis Publishers: FL, USA, pp 509–512. [Google Scholar]
- Kritzberg ES, Cole JJ, Pace ML, Granéli W, Bade DL. (2004). Autochthonous versus allochthonous carbon sources of bacteria: results from whole-lake 13C addition experiments. Limnol Oceanogr 49: 588–596. [Google Scholar]
- Lajtha K, Marshall JD. (1994). Sources of variation in the stable isotopic composition of plants. In: Michener RH, Lajtha K (eds). Stable isotopes in ecology and environmental science. Wiley-Blackwell: Oxford, UK, pp 1–21. [Google Scholar]
- Marty J, Planas D. (2008). Comparison of methods to determine algal δ13C in freshwater. Limnol Oceanogr Methods 6: 51–63. [Google Scholar]
- Mateles RI, Chian SK. (1969). Kinetics of substrate uptake in pure and mixed culture. Environ Sci Technol 3: 569–574. [Google Scholar]
- McCallister SL, del Giorgio PA. (2008). Direct measurement of the d13C signature of carbon respired by bacteria in lakes: Linkages to potential carbon sources, ecosystem baseline metabolism, and CO2 fluxes. Limnol Oceanogr 53: 1204–1216. [Google Scholar]
- McCallister SL, Guillemette F, del Giorgio PA. (2006). A system to quantitatively recover bacterioplankton respiratory CO2. Limnol Oceanogr Methods 4: 406–415. [Google Scholar]
- Mohamed MN, Taylor WD. (2009). Relative contribution of autochthonous and allochthonous carbon to limnetic zooplankton: a new cross-system approach. Fundam Appl Limnol 175: 113–124. [Google Scholar]
- Pace ML, Prairie YT. (2005) Respiration in lakes. In: del Giorgio PA, PJlB Williams (eds). Respiration in aquatic systems. Oxford University Press: Oxford, UK, pp 103–121. [Google Scholar]
- Phillips DL, Gregg JW. (2001). Uncertainty in source partitioning using stable isotopes. Oecologia 127: 171–179. [DOI] [PubMed] [Google Scholar]
- Russell JB. (1991). A re-assessment of bacterial growth efficiency: the heat production and membrane potential of Streptococcus bovis in batch and continuous culture. Arch Microbiol 155: 559–565. [DOI] [PubMed] [Google Scholar]
- Russell JB. (2007). The energy spilling reactions of bacteria and other organisms. J Mol Microbiol Biotechnol 13: 1–11. [DOI] [PubMed] [Google Scholar]
- Smith EM, Prairie YT. (2004). Bacterial metabolism and growth efficiency in lakes: the importance of phosphorus availability. Limnol Oceanogr 49: 137–147. [Google Scholar]
- St-Jean G. (2003). Automated quantitative and isotopic (13C) analysis of dissolved inorganic carbon and dissolved organic carbon in continuous-flow using a total organic carbon analyser. Rapid Commun Mass Spectrom 17: 419–428. [DOI] [PubMed] [Google Scholar]
- Sundh I. (1992). Biochemical composition of dissolved organic carbon derived from phytoplankton and used by heterotrophic bacteria. Appl Environ Microbiol 58: 2938–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp JH, Delong MD. (2002). Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 96: 543–550. [Google Scholar]
- Tranvik L. (1992). Allochthonous dissolved organic matter as an energy source for pelagic bacteria and the concept of the microbial loop. Hydrobiologia 229: 107–114. [Google Scholar]
- Vallino JJ, Hopkinson CS, Hobbie JE. (1996). Modeling bacterial utilization of dissolved organic matter: optimization replaces monod growth kinetics. Limnol Oceanogr 41: 1591–1609. [Google Scholar]
- Weiss M, Simon M. (1999). Consumption of labile dissolved organic matter by limnetic bacterioplankton: the relative significance of amino acids and carbohydrates. Aquat Microb Ecol 17: 1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.