Abstract
The frequency of freshwater cyanobacterial blooms is at risk of increasing as a consequence of climate change and eutrophication of waterways. It is increasingly apparent that abiotic data are insufficient to explain variability within the cyanobacterial community, with biotic factors such as heterotrophic bacterioplankton, viruses and protists emerging as critical drivers. During the Australian summer of 2012–2013, a bloom that occurred in a shallow ephemeral lake over a 6-month period was comprised of 22 distinct cyanobacteria, including Microcystis, Dolichospermum, Oscillatoria and Sphaerospermopsis. Cyanobacterial cell densities, bacterial community composition and abiotic parameters were assessed over this period. Alpha-diversity indices and multivariate analysis were successful at differentiating three distinct bloom phases and the contribution of abiotic parameters to each. Network analysis, assessing correlations between biotic and abiotic variables, reproduced these phases and assessed the relative importance of both abiotic and biotic factors. Variables possessing elevated betweeness centrality included temperature, sodium and operational taxonomic units belonging to the phyla Verrucomicrobia, Planctomyces, Bacteroidetes and Actinobacteria. Species-specific associations between cyanobacteria and bacterioplankton, including the free-living Actinobacteria acI, Bacteroidetes, Betaproteobacteria and Verrucomicrobia, were also identified. We concluded that changes in the abundance and nature of freshwater cyanobacteria are associated with changes in the diversity and composition of lake bacterioplankton. Given this, an increase in the frequency of cyanobacteria blooms has the potential to alter nutrient cycling and contribute to long-term functional perturbation of freshwater systems.
Introduction
Eutrophication and increased surface and water temperatures, as a consequence of intensive land management strategies and man-made climate change, are having profound impacts on the quality of our inland aquatic ecosystems (Barnett et al., 2004; Füreder, 2012; Winder, 2012; Grafton et al., 2013). The increasing frequency and severity of cyanobacterial blooms is of critical concern both with regards to maintaining ecosystem health as well as ensuring safe and equitable provision of water (Paerl and Huisman, 2009; Paerl and Paul, 2012; Sinha et al., 2012; Michalak et al., 2013). The occurrence of a cyanobacterial bloom is both an indicator of and a contributor to the declining quality of these aquatic ecosystems. The formation of cyanobacterial blooms has largely been linked to excessive eutrophication in the form of high levels of phosphorus (Schindler et al., 2008; Conley et al., 2009 (r11089); Posch et al., 2012; Ho and Michalak, 2015). However, the continuing proliferation of diazotrophic and non-diazotrophic cyanobacterial species in P-limited systems has reinforced the importance of nitrogen species to bloom formation (Paerl et al., 2014). Across the bloom period, the rapid accumulation of high levels of organic matter contributes to numerous changes to chemical properties of the water column. Periods of elevated cyanobacterial biovolume can result in oxygenation, nitrification, phosphorus loading of sediments and toxin production, followed by hypoxia owing to mass cell death (Li et al., 2012; Wilhelm et al., 2014). In addition, the natural or induced dispersion of cyanobacterial blooms poses a secondary detriment to the quality of water resources through the release of toxic secondary metabolites, collectively termed cyanotoxins (Carmichael, 2001; Ibelings and Chorus, 2007; Funari and Testai, 2008). The extent to which certain cyanobacterial species exhibit dominance over other phytoplankton, during bloom events or otherwise, has been shown to be dictated by differences in the physiological response of these organisms to prevailing abiotic factors (Paerl, 1996; Davis et al., 2009; Rouco et al., 2011; Neilan et al., 2012).
The Murray–Darling basin, which spans all five of Australia's eastern mainland states, contributes upwards of AUD18.6 billion (40%) annually to gross agricultural production (ABS 2008). Intensive water use places large demands on the basin ecosystem, and although water efficiency measures are being implemented, it is expected that changing climatic conditions will further contribute to reductions in flows within the river system (Kingsford, 2011; Grafton et al., 2013). As an indicator of future conditions, reduced river flows and increased water temperatures in the Murray River during 2009 saw the development of a cyanobacterial bloom spanning 1100 km of the river (Al-Tebrineh et al., 2011; Bowling et al., 2013). During the bloom, two potent cyanotoxins, cylindrospermopsin and saxitoxin, were observed, along with species with the genetic capacity to produce microcystins (Al-Tebrineh et al., 2011). There is an evident difficulty in correlating many environmental parameters with cyanobacterial community composition, toxicity or toxigenicity across such studies (Rinta-Kanto et al., 2009; Al-Tebrineh et al., 2011; Otten et al., 2012; Lee et al., 2014; Ngwa et al., 2014). In part, this lack of correlation can be attributed to genomic variability among closely related strains (Humbert et al., 2013; Sinha et al., 2014), giving rise to differing growth optima and potential to produce a myriad of toxic and non-toxic metabolites (Humbert et al., 2013), changes in the genome copy number throughout the growth phase (Griese et al., 2011) and uncertainty regarding the control of regulatory systems that direct the expression of toxic metabolites (Kaebernick et al., 2000; Alexova et al., 2011; Carneiro et al., 2013; Neilan et al., 2013; Rzymski and Poniedziałek, 2014; Makower et al., 2015).
Freshwater microbial communities exhibit high compositional and functional variability across spatial and temporal scales (Newton et al., 2011), reflecting changes in water chemistry and nutrient concentrations (Allgaier et al., 2007; Newton et al., 2007; Dennis et al., 2013), hydrodynamic stability of the water column (Salcher et al., 2011) and climatological impacts (Wilhelm et al., 2014). Periods of high cyanobacterial biovolume are associated with elevated rates of denitrification (McCarthy et al., 2007) and carbon sequestration (Becker et al., 2011; Sandrini et al., 2014), applying additional perturbations to the water column that in turn contribute to already highly variable systems (Li et al., 2012). Numerous studies have highlighted the diversity of heterotrophic groups that occur within microbial communities associated with freshwater bloom-forming cyanobacteria species (Eiler and Bertilsson, 2004; Wu et al., 2007; Berg et al., 2008; Cheng et al., 2011; Dziallas and Grossart, 2011; Grossart et al., 2011; Wilhelm et al., 2011; Li et al., 2012; Li et al., 2012; Steffen et al., 2012; Woodhouse et al., 2012; Cai et al., 2013; Xing et al., 2013; Bagatini et al., 2014). Further, such studies have demonstrated that, for cyanobacterial-associated microbial communities, despite the identity of these communities being dependent on the cyanobacterial species present (Bagatini et al., 2014), water chemistry, temperature (Dziallas and Grossart, 2011; Xing et al., 2013) and ultimately the freshwater system within which the experiment is based, the function of these communities is largely conserved across local and continental spatial scales (Steffen et al., 2012; Penn et al., 2014; Steffen et al., 2015). Fewer studies have assessed how the nature of the cyanobacterial-associated microbial community changes over temporal scales (Wu et al., 2007; Tang et al., 2010; Li et al., 2012; Xing et al., 2013) with changes in cyanobacterial species dominance and biovolume likely to impact on both the composition and function of these groups.
The Murray–Darling basin represents a series of interconnected reservoirs, facilitated by construction of dams, and small-to-medium-sized ephemeral water bodies that occur within numerous floodplains (Kingsford, 2000). Yanga Lake represents a shallow ephemeral eutrophic freshwater lake largely representative of many of the water bodies present throughout the floodplains of the Murray–Darling Basin that are utilised for recreation, agriculture and rural drinking water (Kingsford and Thomas, 2004, Kobayashi et al., 2013). Yanga Lake was selected for this study as it is has been the subject of routine monitoring, has a prior history of cyanobacterial blooms (Kobayashi et al., 2013) and, as a consequence of being fed by a single tributary (Yanga Creek), can be readily isolated from the river system, limiting the impact of inflows. In this study, we aimed to utilise a systems framework (Bissett et al., 2013) that encompasses measurement of water quality information, cell enumeration and microbial community data sets to address how abiotic and biotic factors combine and interact during cyanobacterial blooms. We performed routine monitoring over a 6-month period and performed in situ measurements, elemental analyses, cyanobacterial species identification and enumeration and 16S rRNA amplicon sequencing at five sites across the lake. This study provides an understanding of how abiotic and biotic factors interact at various stages throughout a cyanobacterial bloom.
Materials and methods
Field data and phytoplankton sample collection
Yanga Lake (34°17′S, 143°36′E) is a shallow ephemeral lake covering 12.5 km2 with a maximum mean depth of 2–3 m. Yanga Lake receives occasional water inputs as a consequence of overflows from the Lowbidgee floodplains. The lake derives most of its water from the Murrumbidgee River, via Yanga Creek, through a weir located at the northern end of the lake. Water samples were collected at a depth of 0.5 m from Sites 1, 2, 3, 5 and 6 within Yanga Lake (Supplementary Figure S1) on 11 occasions across a 7-month period.
Water temperature, dissolved oxygen, pH, chlorophyll a and electrical conductivity were measured in situ using a Hydrolab DS5 water quality sonde. No in situ data were collected on the 23 April 2013. Samples were collected to provide an approximation of the cyanobacterial community composition and the bacterial community composition at these five sites during and following periods of high cyanobacterial biovolume. Cell enumeration and estimation of cyanobacterial biovolume were estimated using standard procedures (see Supplementary Materials). For the purposes of this study, a bloom was defined in accordance with the Water Quality Research Australia framework, when the biovolume of all cyanobacterial species exceeded 10 mm3 l−1. Given samples were taken from a 0.5-m depth, this value is only with respect to surface waters, with care taken to ensure surface scums were not disturbed during sampling. Nutrient analyses (see Supplementary Materials) was limited to the measurement of major the cations (NO3− and NO2−) owing to constraints around the availability of infrastructure, storage conditions and time frames necessary to take receipt of samples from the remote Yanga Lake. Elemental analysis (see Supplementary Materials) was performed to provide specific insight into the nature of ions contributing to variation in electrical conductivity (Na, Mg, Al, Si, P, S, K, Ca, Cr, Mn, Fe, Ni, Cu, Zn, Ar, Cd and Pb).
Lugol's persevered samples (0.25 litres) for cell enumerations and molecular analysis and a 0.5-litre sample for elemental analysis and measurement of major cations were collected on each but the first two occasions where only Lugol's preserved samples were obtained. Non-preserved samples were frozen on site at a NSW Office of Water field office (Hay, NSW, Australia) and shipped along with Lugol's preserved samples directly to UNSW Australia. Frozen samples were thawed immediately prior to analysis to limit the dissolution of particulate nutrients. Elemental analysis and measurement of major cations was performed at the Water Research Centre, UNSW Australia (see Supplementary Materials).
Molecular analysis
DNA was extracted from Lugol's preserved samples immediately on receipt at UNSW to prevent any additional introduction of bias that may have arisen owing to the variability in different prokaryotic groups to prolonged storage (Bowers et al., 2000). A 100-ml volume of Lugol's preserved sample was filtered through a 0.22-μM MF-Millipore Membrane Filter (Merck Millipore, Darmstadt, Germany). DNA was extracted from filters using the Power Water DNA Extraction Kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) in accordance with the manufacturer's specifications. Amplicon sequencing (2 × 300 bp) of the 16S rRNA gene V1–V3 region was performed using primers 28f (5′-GAGTTTGATCNTGGCTCAG-3′) and 519r (5′-GTNTTACNGCGGCKGCTG-3′) utilising the MiSeq platform at the Ramaciotti Centre for Gene Function Analysis, UNSW Australia. A complete description of the PCR conditions used for this primer set is available elsewhere (see Supplementary Materials). De-multiplexed sequences are available for download via the NCBI short read archive under BioProject PRJNA296748. Quality co-processing was performed using Mothur v 1.34.1 (see Supplementary Methods). Sequences were clustered at a distance threshold of 0.03 using the average neighbour method (Schloss and Westcott, 2011). The taxonomy of each operational taxonomic unit (OUT) was assigned using a combination of the Freshwater Microbial Field Guide (Newton et al., 2011) July 2012 release (https://github.com/mcmahon-uw/FWMFG) and the GreenGenes May 2013 release (DeSantis et al., 2006) (see Supplementary Materials). Following sequence processing, each sample retained at least 40 000 sequences. Subsampling was performed at this level to ensure consistency across the data set.
Statistical analyses
Permutation multivariate analysis of variance (PERMANOVA), as implemented in Primer v6, on the full set of 11 time points (54 samples), as well as the reduced set of 7 time points (35 samples), was implemented to examine differences in the composition of samples with respect to both cyanobacterial cell counts and 16S rRNA gene OTUs across both spatial and temporal scales. Step-wise distance-based redundancy analysis (dbRDA) was implemented in Primer v6 on a reduced set of 7 time points (34 samples in total) to determine the contribution of each measured environmental parameter to the variation observed within the 16S rRNA gene and cyanobacteria cell count matrices.
The degree of association of measured environmental variables, cyanobacterial cell counts and 16S rRNA gene OTUs with respect to one another across the entire bloom period was measured using the Pearson's correlation coefficient (r). The number of bacterial OTUs, defined at 0.03-distance threshold, was reduced by retaining only those that occurred within at least three samples and retaining only those that contributed at least 1% to any given sample. Of these OTUs that were retained, no alteration was made to the observed abundance value within any sample. A Pearson correlation coefficient r score and P-value were calculated pairwise for each bacterial OTU using the rcor.test algorithm, available from the ltm package (available from http://rwiki.sciviews.org/doku.php?id=packages:cran:ltm) as implemented in R version 3.0.2. P-values for each correlation were generated and the false discovery rate was kept below 5% using the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995). Visualisation of these associations was made with the Cytoscape package version 2.8.3 (available at: www.cytoscape.org). The r value was selected to support the generation of an edge-weighted spring-embedded network. Topological and node/edge metrics, including connectivity, density and betweeness centrality, were calculated using the Network Analysis plug-in (Assenov et al., 2008).
Results
Cyanobacterial community composition
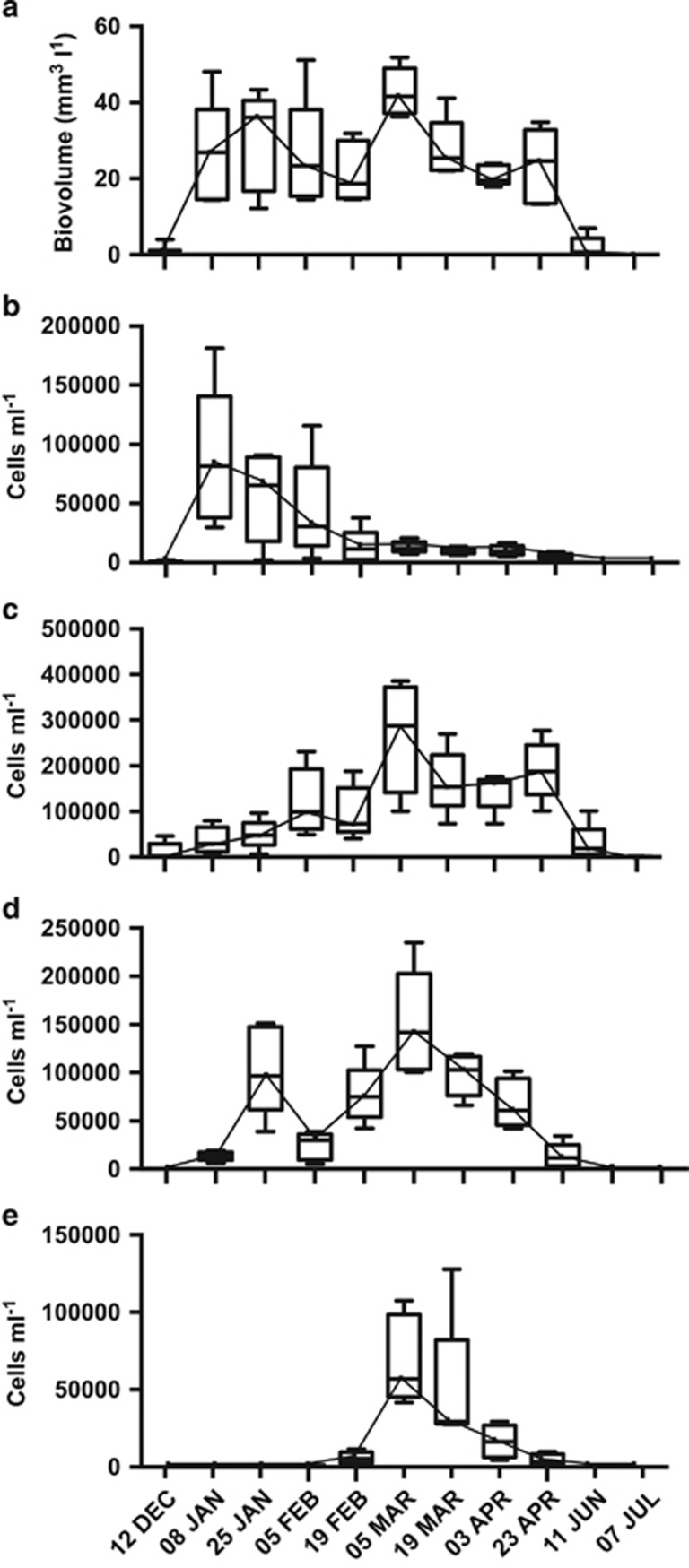
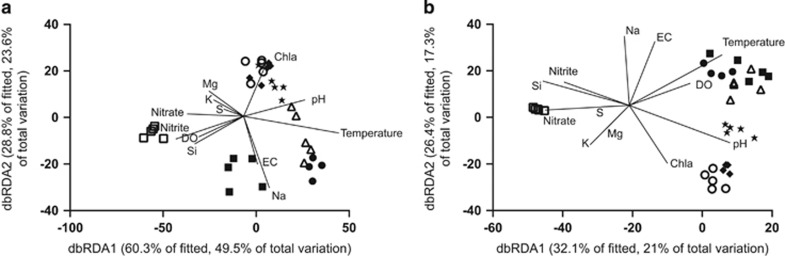
During the sampling period, the cyanobacterial bloom biovolume remained at levels >10 mm3 l−1 for a continuous period of 4 months, from 25 January to 23 April 2013 (Figure 1a). Over this period, 22 morphologically distinguishable cyanobacterial taxa were observed (see Supplementary Table S1). The dominant cyanobacteria during this period were Dolichospermum circinale (ex Anabaena circinalis), Microcystis, Aphanocapsa, Geitlerinema, Oscillatoria and Sphaerospermopsis (Figures 1b–e). Bray–Curtis similarities, for cyanobacterial cell counts, revealed a pattern indicative of temporal succession across the samples (Figure 2a). Step-wise distance-based linear modelling (distLM), utilising a reduced sampling set of only seven time points, identified temperature, electrical conductivity (EC), dissolved oxygen (DO) and pH, Si, Mg and Ca as predictor variables for the cyanobacterial community (Table 1), together accounting for 78.23% of the observed variation. Marginal tests further identified nitrate, nitrite, chlorophyll a, Na, S, and K as being able to account for the variation in the cyanobacterial community, although their contribution is readily described by environmental variables (pH, Si, Mg, Ca) already identified within the step-wise distLM (Figure 2a).
Figure 1.
Averaged (a) estimated cyanobacterial cell biovolume and (b) Dolichospermum/Anabaena spp., (c) Microcystis, (d) Oscillatoria and (e) Sphaerospermopsis cell counts across the bloom period as measured at five sites.
Figure 2.
Distance-based linear redundancy visualising the relative contribution of measured environmental parameters on (a) cyanobacterial community composition determined by cell enumeration and (b) total bacterial community composition determined by 16S rRNA gene amplicon sequencing. Symbols indicate sampling date: 25 January (•), 5 February (▪), 19 February (Δ), 5 March (▯), 19 March (♦), 3 April (○), and 9 July (□).
Table 1. Marginal tests of abiotic variables to both cyanobacterial and microbial community composition as determined by PERMANOVA and Distance-based linear modelling (DistLM).
|
Cyanobacteria |
Bacteria |
|||
|---|---|---|---|---|
| Pseudo-F | P | Pseudo-F | P | |
| Spatial | 31.5 | <0.001 | 6.84 | <0.001 |
| Temporal | 1.87 | 0.074 | 1.31 | <0.001 |
| Temperature | 22.4 | <0.001 | 7.44 | <0.001 |
| Electrical conductivity | 8.74 | <0.001 | 5.01 | <0.001 |
| Dissolved oxygen | 1.84 | 0.133 | 2.43 | 0.005 |
| pH | 16.3 | <0.001 | 8.13 | <0.001 |
| Nitrate | 17.5 | <0.001 | 7.19 | <0.001 |
| Nitrite | 18.6 | <0.001 | 6.40 | <0.001 |
| Chlorophyll a | 6.67 | <0.001 | 4.88 | <0.001 |
| Na | 7.85 | <0.001 | 4.56 | <0.001 |
| Mg | 6.24 | 0.004 | 3.52 | <0.001 |
| Al | 0.76 | 0.501 | 0.89 | 0.54 |
| S | 4.71 | <0.001 | 2.77 | <0.001 |
| Si | 18.6 | <0.001 | 7.57 | <0.001 |
| K | 8.20 | <0.001 | 4.19 | <0.001 |
| Ca | 0.86 | 0.466 | 2.30 | 0.01 |
| Fe | 2.33 | 0.124 | 1.86 | 0.018 |
| P | 2.39 | 0.063 | 2.47 | 0.006 |
Abbreviation: PERMANOVA, permutation multivariate analysis of variance.
Microbial community structure
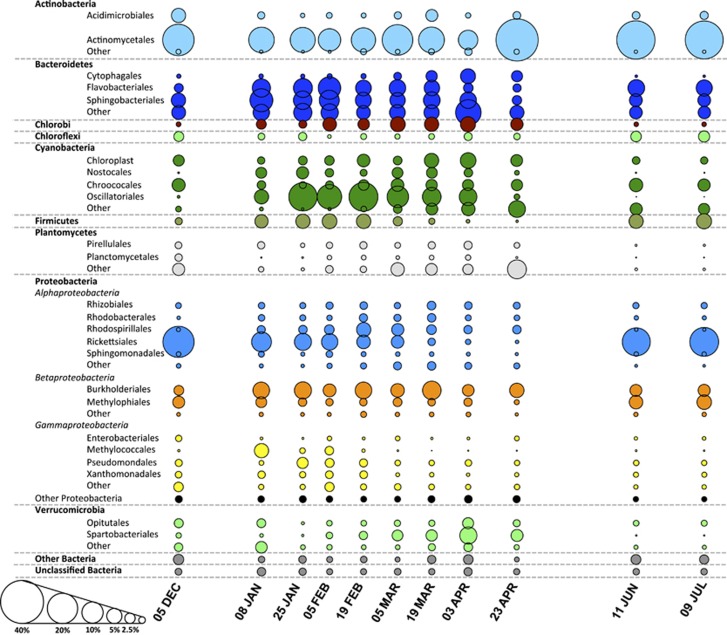
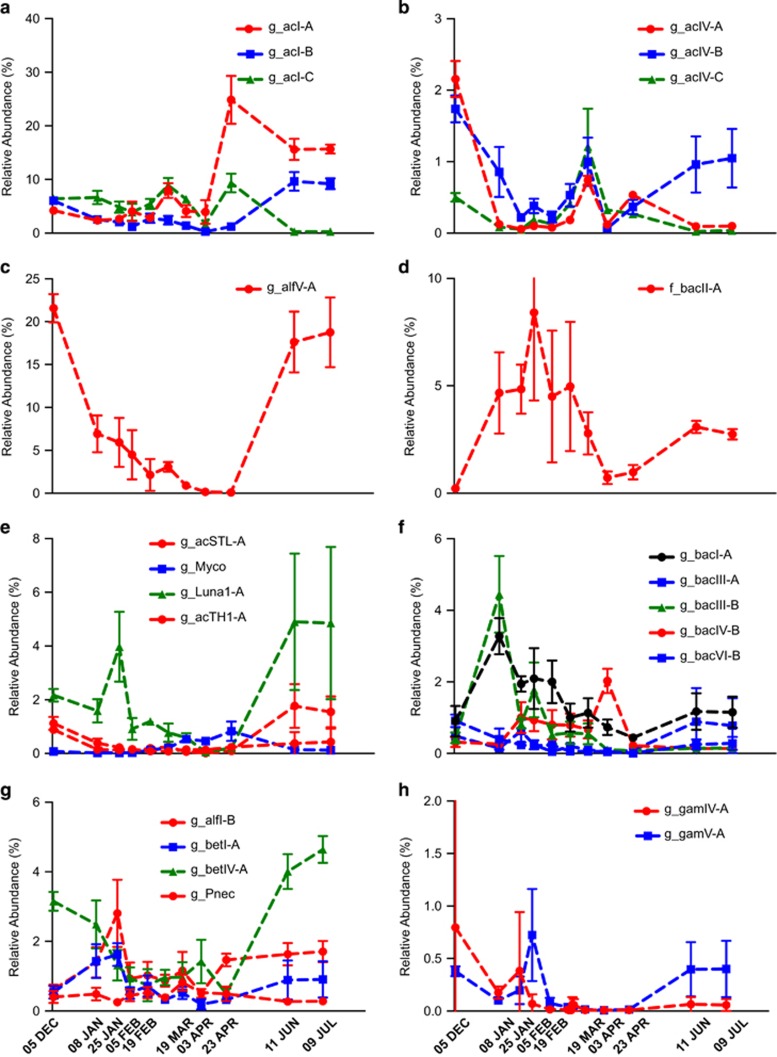
Bacterial community analysis, as assessed by sequencing the V1–V3 region of the 16 rRNA gene, identified 39 606 OTUs defined at 0.03-distance threshold across the bloom period (Supplementary Table S3). Phylotyping of these OTUs revealed a lake microbial community dominated by Actinobacteria and Alphaproteobacteria, when cyanobacterial biovolume were low, and by Actinobacteria, Bacteroidetes and Cyanobacteria when cyanobacterial biovolume were elevated (Figure 3). During periods of high cyanobacterial abundance, Betaproteobacteria, Gammaproteobacteria, Planctomycetes and Verrucomicrobia were also present at substantially greater abundance than at other periods. Cosmopolitan freshwater clades, including acI (Figure 4a), acIV (Figure 4b), alfV (Figure 4c) and bacII (Figure 4d), were the most abundant non-cyanobacterial taxa within Yanga Lake. AcI (Figure 4a) and alfV (Figure 4c) were distinct in that they were enriched during periods of low cyanobacterial biovolume, whereas acIV (Figure 4b) and bacII (Figure 4d) were most abundant during periods of high cyanobacterial biovolume. Several additional cosmopolitan clades were present at lower abundance, yet still exhibited distinct profiles in that they were enriched in accordance with periods of varying cyanobacterial biovolume (Figures 4d–h).
Figure 3.
Relative abundance of 16S rRNA gene phylotypes across the sampling period. The area of each circle reflects the relative abundance of that phylotype averaged across the five samples collected at each time point. The colour of each bubble is indicative of the phylum to which each phylotype belongs. A full colour version of this figure is available at the ISME Journal journal online.
Figure 4.
Relative abundance of cosmopolitan freshwater (a) acI, (b) acIV, (c) alfV, (d) bacII, (e) actinobacterial, (f) bacteroidetes, (g) alphaproteobacterial and betaproteobacterial and (h) gammaproteobacterial clades identified at the genus level.
Bray–Curtis similarities, calculated across the 39 606 OTUs (Figure 2b), were significantly correlated with the ecophysiological parameters (Rho statistic=0.697) and cyanobacterial cell counts (Rho statistic=0.809). PERMANOVA, across all 11 time points, supported both a significant temporal (Pseudo-F=8.3551, P(perm)=0.001) and, albeit less substantial, spatial (Pseudo-F=1.4565, P(perm)=0.001) succession (Supplementary Figure S2). Similarly, PERMANOVA supported a temporal and spatial separation of samples using the reduced data set of seven time points (Table 1). Step-wise distLM, utilising a reduced sampling set of only seven time points, identified pH, temperature, DO and EC as predictor variables for the bacterial community, accounting for 46.17% of the observed variation (Figure 2b). Marginal tests further identified, nitrate, nitrite (Pseudo-F=6.3983), chlorophyll a, Na, Mg, Si, P, S, and K as being able to account for variation within the total bacterial community (Table 1,Figure 2b).
The Chao1 richness and InvSimpson diversity indices for the microbial community varied across the sampling period (Supplementary Figure S3). Both the diversity and richness indices tended upwards with total cyanobacterial biovolume (Figure 1a), peaking around mid-March, coinciding with a decline in cyanobacterial biovolume.
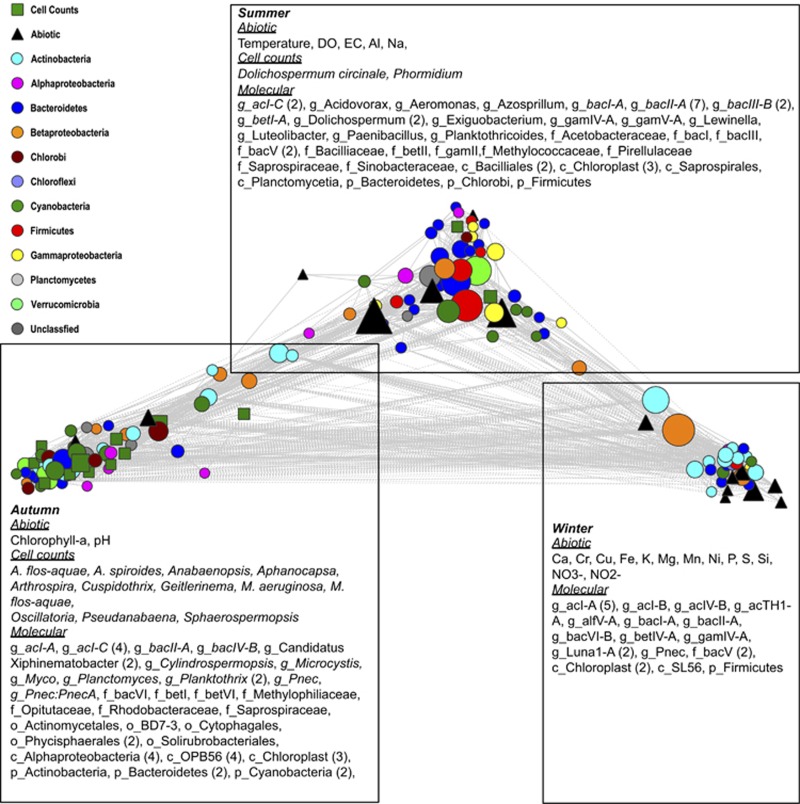
Covariance analysis of biotic and abiotic lake components
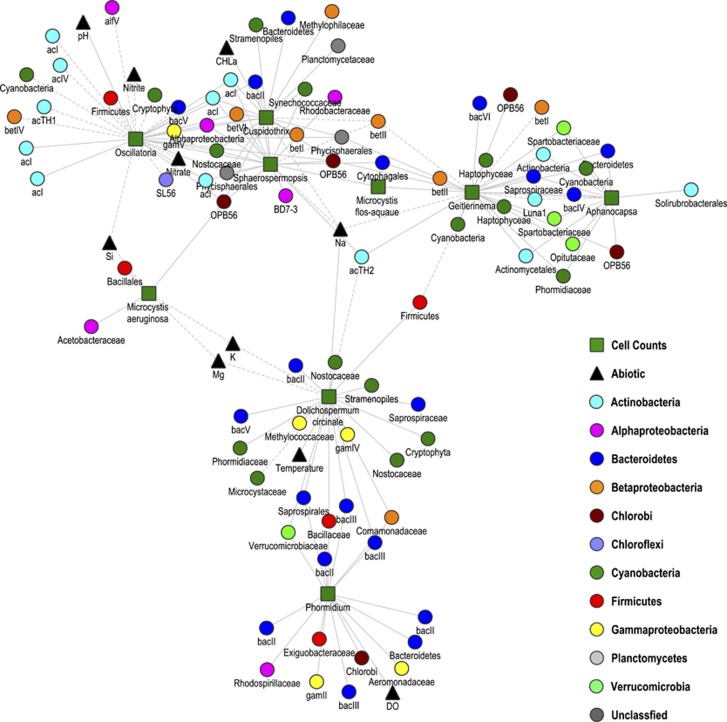
Of the 147 measured variables, 144, including 104 16S rRNA gene OTUs that contributed >1% to any sample, were shown to form a single interconnected network. Of the 10 731 tested correlations, only 1835 were considered significant (Supplementary Table S4). Edge-weighted spring-embedded visualisation of the network, utilising the r score as the edge-weight, revealed the arrangement of the variables along three axes (Figure 5). By scrutinising the distribution of measured environmental parameters and cyanobacterial cell counts, it was apparent that these three axes mirrored that observed within the dbRDA plots (Figure 2). The summer sampling period (January–February) was associated with high temperature, DO, EC, Al, Na, the cyanobacteria Dolichospermum circinale and Phormidium and 47 16S rRNA gene OTUs. The autumn period (March–April) was associated with high pH and chlorophyll a content, 12 cyanobacterial taxa and 45 16S rRNA gene OTUs. The winter period (July) was associated with high Ca, Fe, K, Mg, P, Si, NO3−, NO2− and 23 other bacterial 16S rRNA gene OTUs. The weak association of variables, such as temperature, within a single module suggests variables may be associated with more than one stage, with temperature-associated community shifts in summer and autumn stages when contrasted with the winter period. A sub-network, comprising only correlations between Microcystis spp., Dolichospermum circinale, Sphaerospermopsis, Oscillatoria, Aphanocapsa, Geitlerinema, Phormidium and Cuspidothrix cyanobacterial cell counts and either 16S rRNA gene OTUs or abiotic environmental parameters, revealed variability in the number and nature of unique and shared correlations between each of the cyanobacteria and other variables, including Na, and the taxa bacII, bacIII, bacIV, acIV and betII, within the network (Figure 6).
Figure 5.
Pearson correlation coefficient edge-weighted Spring-embedded network visualising significant correlations between measured biotic and abiotic variables. Node size is reflective of the betweeness centrality of the variable. Line types (solid=positive and dashed=negative) are indicative of the Pearson correlation coefficient.
Figure 6.
Organic correlation network visualising pairwise correlations between cyanobacteria and 16S rRNA gene OTUs and abiotic parameters. Line types (solid=positive and dashed=negative) are indicative of the Pearson correlation coefficient.
Network analysis supported the conclusions drawn from distLM that temperature (centrality=0.050) was strongly correlated with changes in bloom microbial composition. EC (centrality=0.027) and Na (centrality=0.037) were highlighted owing to an average decline of 30 μS cm−1 and 10 p.p.m., respectively, between the early and mid bloom periods associated with 29.4 mm of rainfall in surrounding areas. Ten 16S rRNA gene OTUs exhibited betweeness centrality >0.02. Firmicutes (centrality=0.041), Saprosipirales (centrality=0.036), Luteolibacter (centrality=0.036), Planktothricoides (centrality=0.024) Bacilliaceae (centrality=0.022) and Pirellulaceae (centrality=0.021) OTUs were associated with the summer period. The taxon Myco (centrality=0.039) and bacVI (centrality=0.021) were highlighted during the autumn period, whereas betII (centrality=0.042) and Luna1-A (centrality=0.0314) were only associated with the winter period.
Discussion
Cyanobacterial species composition
A bloom, defined by a combined biovolume of >10 mm3 l−1 of all cyanobacteria, was first observed in Yanga Lake on 8 January and persisted till 23 April 2013. During this period, it was evident that this single event was highly dynamic, with 22 morphologically distinguishable cyanobacteria observed across the period. A dbRDA model incorporating temperature, pH, EC and DO (Figure 2a) was able to describe much of the variability in the cyanobacterial community.
Measured abiotic factors, however, were less able to explain variability among individual taxon. It is worth mentioning that, owing to the remote location of Yanga Lake, samples were frozen on site, shipped and stored for several days. This process limited the analyses that could be performed on these samples and transformed an unquantifiable proportion of nutrients between soluble and particulate phases, which may account for these observations. Dolichospermum circinale, which was initially dominant, could only be directly correlated to Mg, K and Na and was the only cyanobacterial taxon correlated with temperature (Figure 6). D. circinale dominance of the surface waters during hotter months is consistent with an ability to persist in the euphotic zone, through regulating buoyancy, during periods of persistent stratification (Mitrovic et al., 2001), providing a competitive advantage over other taxa that, unlike Dolichospermum, exhibit even rather than distinct distribution through the water column. Decline of this species likely arose owing to a combination of multiple factors, either measured or otherwise, within this study, including decreasing temperature, changes in light availability (Brookes, 1999; McCausland et al., 2001; McCausland et al., 2005), P-limitation and dissolved N concentrations (Supplementary Table S2). The sudden collapse in the D. circinale population strongly implicates an external biological effector, such as viral lysis or bacterial predation, neither of which was addressed at length within the scope this study. In this regard, numerous studies have demonstrated biotic mechanisms for the rapid dispersal of cyanobacteria from surface waters that may explain this observation, including phage-induced lysis (Proctor and Fuhrman, 1990; Williamson et al., 2002; Matteson et al., 2011; Steffen et al., 2015), bacteria-induced lysis (Rashidan and Bird, 2001), grazing by protists (Ger et al., 2014), algicidal compounds (Luo et al., 2013; Shao et al., 2013) and cyanobacterial programmed cell death (Franklin, 2014).
Eubacterial diversity and composition
To the best of our knowledge, this study represents one of only a few studies to examine the total bacterial population of a freshwater lake on the Australian continent (Dennis et al., 2013) and the first Australian study to do so in the context of a multi-site time series comparable to those conducted elsewhere (Allgaier and Grossart, 2006; Wu et al., 2007; Tang et al., 2010; Dziallas and Grossart, 2011; Eckert et al., 2012; Paver et al., 2013; Wilhelm et al., 2014). During periods of low cyanobacterial biovolume, the microbial community present within the surface water was dominated by the taxa acI (-A and B) (Figure 4a) and alfV-A (Figure 4c). In the absence of cyanobacteria, these three lineages alone accounted for approximately 50% of the total sequences obtained from the five sites within Yanga Lake. During periods of elevated cyanobacterial biovolume, it was clear that acI (Figure 4a), alfV-A (Figure 4c), acTH1-A, Luna1-A (Figure 4e), betIV-A (Figure 4g), gamIV-A and gamV-A (Figure 4h) decreased as a proportion of the total sequence reads observed (Figure 4). Overall, the bacterial community composition could only partially be explained by those factors that defined cyanobacterial species composition. Increased heterogeneity between samples (Figure 2b) during periods of elevated biomass is consistent with high levels of variability reported among freshwater microbial communities during periods of increasing cyanobacterial biovolume (Wu et al., 2007; Li et al., 2012).
AlfV-A, sister group to the marine Pelagibacter (SAR11), represents a free-living ultramicrobacterium that was largely thought to be oligotrophic with its small cell size enabling it to persist at low nutrient concentrations. Consistent with the presence of this taxon in the eutrophic Yanga Lake (Kobayashi et al., 2013), an analysis of the literature, demonstrating that the abundance of alfV-A can be linked to increased surface water temperatures and high nutrient concentrations, concluded that there was clear evidence that functional heterogeneity occurs within this lineage (Newton et al., 2011). A caveat to this is that the absolute abundance of alfV is overwhelmingly dependent on water turbidity, with phytoplankton blooms drastically decreasing its abundance (Salcher et al., 2011) in a manner akin to that observed in this study. The emergence of acI, in Yanga Lake, subsequent to the bloom (Figure 4e) is consistent with the genomic potential of these organisms to take advantage of cyanobacterial exudates, specifically cyanophycin, following the lysis of cyanobacteria (Ghylin et al., 2014). Albeit contributing a smaller proportion of the total community, methylotrophs betIV and gamI (Figures 4g and h) exhibited similar distributions consistent with observations that the heterotrophic decomposition of cyanobacterial biomass leads to an accumulation of acetate, selecting for taxa capable of acetoclastic methanogenesis under either oxic or anoxic conditions (Bogard et al., 2014). The late emergence of methylotrophs following cyanobacterial blooms is also implicated as contributing to the accumulation of methane derived from archaeal methanogenesis in surface waters (Grossart et al., 2011).
During the summer and autumn periods, the total bacterial community was dominated by a number of OTUs assigned to the Nostocales, Chroococales and Oscillatoriales cyanobacterial orders (Figure 3), consistent with the presence of these cyanobacteria taxa. Actinobacteria remained a major part of the bacterial community during periods of high cyanobacterial biovolume, with clear increases in the relative abundance of the acIV taxon (Figure 4b). Studies have pointed to a number of actinobacteria as associated with particles, with several demonstrating the acI-C and acIV lineages form species-specific associations with cyanobacteria (Allgaier et al., 2007). Similar increases were observed for the particle-associated betI and betIV (Figure 4g), which have been noted for their attachment to prokaryotic (Bagatini et al., 2014) and eukaryotic phytoplankton (Paver et al., 2013), as well as the metabolism of the cyanobacterial toxin microcystin (Mou et al., 2013). Among the Bacteroidetes, bacI-A, bacIII-B, bacIV-B and bacVI-B (Figure 4f) were more dominant during periods of high biovolume. BacI-A and bacIII-B were most abundant during the summer period, suggesting that their presence is linked to the same factors that influenced the initial proliferation of D. circinale cell numbers or to the cyanobacterium itself. BacII-A (Figure 4g) contributed upwards of 10% of the total microbial community during the first 2 months of the bloom period. It is unclear whether the sharp decrease in the relative abundance of bacII-A or other taxa during the summer period arose simply owing to displacement by other taxa or on account of some external factor, coinciding with the proliferation of additional cyanobacterial taxa during the autumn period.
Covariance of biotic and abiotic factors
A covariance approach was applied to visualise how biotic and abiotic factors interact to define patterns within the cyanobacterial and bacterial communities using an edge-weighted spring-embedded network (Figure 6). In contrast with other studies, examining soil and sediment-bound microbial communities (Bissett et al., 2013; Sun et al., 2013), which have observed a lack of correlations between absolute abiotic measurements and 16S rRNA OTU relative abundances, numerous correlations were observed between biotic and abiotic factors. Although consideration should be made that such approaches, wherein relative abundances are used to derive correlations between species, may overestimate the numbers of true correlations (Friedman, 2012; r14933), this analysis was effective at classifying factors characteristic of the three observed periods, namely summer, autumn and winter periods, also observed in the dbRDA analysis (Figure 2). In this context, with cyanobacterial cell counts as the driver, 16S rRNA gene OTUs, cell counts and abiotic data were considered to be associated with each of the distinct periods when the observed variables exhibited strong positive correlations to either the cell counts or to one another (Figure 5).
The summer period was defined by high cell numbers of the cyanobacteria Dolichospermum circinale and Phormidium, elevated temperature, DO and EC, a value influenced by elevated Na and Al levels. Recruitment of bloom-forming cyanobacteria from littoral zones (Kononen et al., 1996) has been demonstrated to be significantly enhanced by high water temperatures and disturbance of these sediments (Rengefors et al., 2004). Although littoral recruitment cannot be ruled out, the shallow ephemeral nature of the lake suggests that mixing is not critical for the recruitment of cyanobacteria from the sediment. Similarly, it is plausible that major inflows arrived from upstream and that the rapidly moving water was sufficient for recruitment of cyanobacteria and suspension of nutrients from river and creek sediments. The closing of the northern weir, following the initial identification of the bloom on 8 January suggests that a similar mechanism was not responsible for the succession of the bloom from one dominated by Dolichospermum to the one dominated by Microcystis and Oscilltoria.
A difference in the number and nature of variables correlated with Dolichospermum and Phormidium and the low proportion of heterotrophic taxa that are correlated to either genus was evident (Figure 6). Dolichospermum was the only cyanobacterial taxon positively correlated with temperature and was negatively correlated with Na, Mg and K, while Phormidium was positively correlated with DO. The bacII and bacIII OTUs, as well as several γ-proteobacterial OTUs, were overrepresented in both species-specific and shared correlations (Figure 6). The presence of particle-associated Bacteroidetes lineages following bloom events have led to the assertion that postbloom conditions provide favourable conditions for proliferation of heterotrophs (Li et al., 2012; Shao et al., 2013). Consistent with this, bacII reached its relative abundance maxima in the period following peak Dolichospermum cell counts. Peak levels of bacIII corresponded with peak Dolichospermum cell counts, indicating that their levels are more explicitly linked to viable cyanobacterial numbers, rather than the concentration of cyanobacterial exudates.
The autumn period was defined by multiple cyanobacterial genera, with Microcystis, Oscillatoria, Sphaerospermopsis, Cuspidothrix, Aphanocapsa and Geitlerinema present at levels exceeding 105 cells ml−1. High levels of these cyanobacteria were associated with high chlorophyll a and pH levels during the mid bloom period. Decreased dissolved oxygen in the mid bloom compared with the early bloom period indicated that heterotrophic metabolism, established following the dispersal of Dolichospermum, continues despite the proliferation of multiple cyanobacterial species. Covariance analysis showed that the Na concentration was an important associating factor in the cyanobacterial community composition, with Dolichospermum positively correlated to Na, whereas Cuspidothrix, Geitlerinema and Sphaerospermopsis were negatively correlated. Na levels and EC were only slightly higher in the summer bloom period (average [Na]=28.2 p.p.m., average EC=399 μS cm−1) than in the autumn period (average [Na]=18.9 p.p.m., average EC=361 μS cm−1). Although it is unlikely that Na concentration was sufficient to suppress the growth of any cyanobacterial species, several studies have demonstrated a link between ionic flux and toxin production (Pomati et al., 2004; Wilhelm et al., 2011; Carneiro et al., 2013), presenting a credible route through which subtle changes in Na concentrations may influence, via secondary and tertiary mechanisms, the cyanobacterial and bacterial community composition (Wilhelm et al., 2011; Mou et al., 2013).
In the autumn period, patterns of covariance between cyanobacterial cell counts and bacterial OTUs were not distinct for each taxa. The observed bacterial community associated with Aphanocapsa and Geitlerinema was similar to that associated with Phormidium and Dolichospermum, being overrepresented by OTUs identified as unclassified bacteria and Bacteroidetes. However, the bacterial community associated with Aphanocapsa and Geitlerinema differed to that of the autumn period with a greater number of OTUs identified as belonging to the Verrucomicrobial orders Opitutales and Spartobacteriales. The Verrucomicrobia have previously been reported in cyanobacterial blooms and metagenomic analysis, suggesting that they possess numerous pathways for the assimilation of cyanobacterial extracellular polymeric substances, as well as exudates (Mou et al., 2013; Bagatini et al., 2014). The bacterial groups associated with the cyanobacteria Microcystis, Oscillatoria, Sphaerospermopsis and Cuspidothrix were overrepresented by particle-associated groups, including betI, acI-C, acIV-C (Allgaier et al., 2007; Paver et al., 2013; Bagatini et al., 2014) and the methylotrophic taxon betIV, and Methylophiliaceae (Grossart et al., 2011).
The winter period was defined by the absence of cyanobacteria, high levels of nitrate/nitrite, likely as a consequence of a lack of cyanobacterial nitrate reduction and an increased relative abundance of numerous acI(-A and B), alfV and betIV OTUs. Two OTUs annotated as Luna1-A and Pnec were positively correlated with variables from both the early bloom and postbloom period (Figure 5), suggesting that their abundance is more closely linked to the decomposition of cyanobacterial organic matter rather than the other postbloom-associated OTUs. Endemic lake members, including acI(A-B), betIV and alfV, were very strongly (r<−0.94) correlated with pH, which mirrors the findings of similar studies (Newton et al., 2007). Cyanobacterial carbon fixation, which increases pH, may contribute to the observed seasonality of acI, particularly its decreased abundance between the late spring and early autumn maxima. AcI is incapable of metabolising carboxylic acids, the primary product of cyanobacterial carbon fixation (Ghylin et al., 2014). However, their capacity to utilise the cyanobacterial pigment cyanophycin (Ghylin et al., 2014) and the action of particle-associated heterotrophic groups during the dispersal of the cyanobacterial biomass may ensure sufficient dissolved organic carbon for the rapid proliferation of both acI and alfV in the periods following a decline in cyanobacterial biovolume.
Conclusion
This study highlights the dynamic nature of freshwater microbial communities, during periods of elevated cyanobacteria cell numbers, with regard to both the cyanobacterial species composition and the composition of particle-associated and free-living lake bacteria. The proliferation of cyanobacteria in Yanga Lake was shown to enrich in a strain-specific manner for particle-associated, opportunistic heterotrophs. Despite the identification of microbial taxa, whose relative abundance within surface waters was substantially altered during periods of high cyanobacterial abundance and the evident strain-specific nature of this, additional research is required to identify the nature of the effects that specific particle-associated or free-living microbial have on the cyanobacteria and nutrient cycling in freshwaters. A continued effort to establish the dynamics of microbial organisms in freshwater systems, particularly with an emphasis of cyanobacteria blooms, over the mid and long term is necessary to determine the impact of rising surface water temperatures and increased eutrophication on nutrient cycling and the security of these critical resources.
Acknowledgments
This research was supported under Australian Research Council's Discovery Projects funding scheme (project number DP130102254).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Al-Tebrineh J, Merrick C, Ryan D, Humpage A, Bowling L, Neilan BA. (2011). Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1,100 Kilometers of the Murray River. Appl Environ Microbiol 78: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexova R, Haynes PA, Ferrari BC, Neilan BA. (2011). Comparative protein expression in different strains of the bloom-forming cyanobacterium Microcystis aeruginosa. Mol Cell Proteomics 10: M110.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier M, Brückner S, Jaspers E, Grossart H-P. (2007). Intra- and inter-lake variability of free-living and particle-associated Actinobacteria communities. Environ Microbiol 9: 2728–2741. [DOI] [PubMed] [Google Scholar]
- Allgaier M, Grossart H-P. (2006). Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl Environ Microbiol 72: 3489–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenov Y, Ramírez F, Schelhorn SE, Lengauer T, Albrecht M. (2008). Computing topological parameters of biological networks. Bioinformatics 24: 282–284. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics. (2008). Water and the Murray-Darling Basin - A Statistical Profile, 2000-01 to 2005-06 (4610.0.55.007). Retrieved from http://www.abs.gov.au/ausstats/abs@.nsf/mf/4610.0.55.007.
- Bagatini IL, Eiler A, Bertilsson S, Klaveness D, Tessarolli LP, Vieira AA. (2014). Host-specificity and dynamics in bacterial communities associated with bloom-forming freshwater phytoplankton. PLoS One 9: e85950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T, Malone R, Pennell W, Stammer D, Semtner B, Washington W. (2004). The effects of climate change on water resources in the west: introduction and overview. Clim Change 62: 1–11. [Google Scholar]
- Becker S, Van Donk E, Visser PM, Huisman J. (2011). Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J 5: 1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57: 289–300. [Google Scholar]
- Berg KA, Lyra C, Sivonen K, Paulin L, Suomalainen S, Tuomi P et al. (2008). High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J 3: 314–325. [DOI] [PubMed] [Google Scholar]
- Bissett A, Brown MV, Siciliano SD, Thrall PH. (2013). Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecol Lett 16: 128–139. [DOI] [PubMed] [Google Scholar]
- Bogard MJ, del Giorgio PA, Boutet L, Chaves MCG, Prairie YT, Merante A et al. (2014). Oxic water column methanogenesis as a major component of aquatic CH4 fluxes. Nat Commun 5: 5350. [DOI] [PubMed] [Google Scholar]
- Bowers HA, Tengs T, Glasgow HB, Burkholder JM, Rublee PA, Oldach DW. (2000). Development of real-Time PCR assays for rapid detection of Pfiesteria piscicida and related Dinoflagellates. Appl Environ Microbiol 66: 4641–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling LC, Merrick C, Swann J, Green D, Smith G, Neilan BA. (2013). Effects of hydrology and river management on the distribution, abundance and persistence of cyanobacterial blooms in the Murray River, Australia. Harmful Algae 30: 27–36. [Google Scholar]
- Brookes J. (1999). The influence of light and nutrients on buoyancy, filament aggregation and flotation of Anabaena circinalis. J Plankton Res 21: 327–341. [Google Scholar]
- Cai H-Y, Yan Z-S, Wang A-J, Krumholz LR, Jiang H-L. (2013). Analysis of the attached microbial community on mucilaginous cyanobacterial aggregates in the eutrophic Lake Taihu reveals the importance of Planctomycetes. Microb Ecol 66: 73–83. [DOI] [PubMed] [Google Scholar]
- Carmichael WW. (2001). Health effects of toxin-producing cyanobacteria: 'The CyanoHABs'. Hum Ecol Risk Assess 7: 1393–1407. [Google Scholar]
- Carneiro R, Pacheco A, de Oliveira e Azevedo S. (2013). Growth and saxitoxin production by Cylindrospermopsis raciborskii (Cyanobacteria) correlate with water hardness. Marine Drugs 11: 2949–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Zaichao Z, Aizhong D, Jiayan W, Jingfa X. (2011). Bar-coded pyrosequencing reveals the bacterial community during Microcystis water Bloom in Guanting Reservoir, Beijing. Procedia Eng 18: 341–346. [Google Scholar]
- Conley DJ, Paerl HW, Howarth RW, Boesch DF. (2009). Controlling eutrophication: nitrogen and phosphorus. Science 322: 1014–1015. [DOI] [PubMed] [Google Scholar]
- Davis TW, Berry DL, Boyer GL, Gobler CJ. (2009). The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8: 715–725. [Google Scholar]
- Dennis PG, Seymour J, Kumbun K, Tyson GW. (2013). Diverse populations of lake water bacteria exhibit chemotaxis towards inorganic nutrients. ISME J 7: 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K et al. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziallas C, Grossart H-P. (2011). Temperature and biotic factors influence bacterial communities associated with the cyanobacterium Microcystis sp. Environ Microbiol 13: 1632–1641. [DOI] [PubMed] [Google Scholar]
- Eckert EM, Salcher MM, Posch T, Eugster B, Pernthaler J. (2012). Rapid successions affect microbial N-acetyl-glucosamine uptake patterns during a lacustrine spring phytoplankton bloom. Environ Microbiol 14: 794–806. [DOI] [PubMed] [Google Scholar]
- Eiler A, Bertilsson S. (2004). Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ Microbiol 6: 1228–1243. [DOI] [PubMed] [Google Scholar]
- Franklin DJ. (2014). Explaining the causes of cell death in cyanobacteria: what role for asymmetric division? J Plankton Res 36: 11–17. [Google Scholar]
- Friedman J, Alm EJ. (2012). Inferring correlation networks from genomic survey data. PLoS Comput Biol 8: e1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funari E, Testai E. (2008) Human health risk assessment related to cyanotoxins exposure. CRC Crit Rev Toxicol 38: 97–125. [DOI] [PubMed] [Google Scholar]
- Füreder L. (2012). Freshwater ecology: melting biodiversity. Nat Clim Change 2: 318–319. [Google Scholar]
- Ger KA, Hansson L-A, Lürling M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw Biol 59: 1783–1798. [Google Scholar]
- Ghylin TW, Garcia SL, Moya F, Oyserman BO, Schwientek P, Forest KT et al. (2014). Comparative single-cell genomics reveals potential ecological niches for the freshwater acI Actinobacteria lineage. ISME J 8: 2503–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton RQ, Pittock J, Davis R, Williams J, Fu G, Warburton M et al. (2013). Global insights into water resources, climate change and governance. Nat Clim Change 3: 315–321. [Google Scholar]
- Griese M, Lange C, Soppa J. (2011). Ploidy in cyanobacteria. FEMS Microbiol Lett 323: 124–131. [DOI] [PubMed] [Google Scholar]
- Grossart H-P, Frindte K, Dziallas C, Eckert W, Tang KW. (2011). Microbial methane production in oxygenated water column of an oligotrophic lake. Proc Natl Acad Sci USA 108: 19657–19661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JC, Michalak AM. (2015). Challenges in tracking harmful algal blooms: a synthesis of evidence from Lake Erie. J Gt Lakes Res 41: 317–325. [Google Scholar]
- Humbert J-F, Barbe V, Latifi A, Gugger M, Calteau A, Coursin T et al. (2013). A tribute to disorder in the genome of the bloom-forming freshwater cyanobacterium Microcystis aeruginosa. PLoS One 8: e70747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelings BW, Chorus I. (2007). Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: a review. Environ Pollut 150: 177–192. [DOI] [PubMed] [Google Scholar]
- Kaebernick M, Neilan BA, Borner T, Dittmann E. (2000). Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl Environ Microbiol 66: 3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsford RT. (2000). Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol 25: 109–127. [Google Scholar]
- Kingsford RT. (2011). Conservation management of rivers and wetlands under climate change–a synthesis. Mar Freshw Res 62: 217–222. [Google Scholar]
- Kingsford RT, Thomas RF. (2004). Destruction of wetlands and waterbird populations by dams and irrigation on the Murrumbidgee River in arid Australia. Environ Manag 34: 383–396. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ralph TJ, Ryder DS, Hunter SJ. (2013). Gross primary productivity of phytoplankton and planktonic respiration in inland floodplain wetlands of southeast Australia: habitat-dependent patterns and regulating processes. Ecol Res 28: 833–843. [Google Scholar]
- Kononen K, Kuparinen J, Mäkelä K, Laanemets J, Pavelson J, Nommann S. (1996). Initiation of cyanobacterial blooms in a frontal region at the entrance to the Gulf of Finland, Baltic Sea. Limnol Oceanogr 41: 98–112. [Google Scholar]
- Lee TA, Rollwagen-Bollens G, Bollens SM, Faber-Hammond JJ. (2014). Environmental influence on cyanobacteria abundance and microcystin toxin production in a shallow temperate lake. Ecotoxicol Environ Saf 114: 318–325. [DOI] [PubMed] [Google Scholar]
- Li H, Xing P, Wu QL. (2012). Characterization of the bacterial community composition in a hypoxic zone induced by Microcystis blooms in Lake Taihu, China. FEMS Microbiol Ecol 79: 773–784. [DOI] [PubMed] [Google Scholar]
- Li H, Xing P, Wu QL. (2012). The high resilience of the bacterioplankton community in the face of a catastrophic disturbance by a heavy Microcystis bloom. FEMS Microbiol Ecol 82: 192–201. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Tang S, Liang J, Lin W, Luo L. (2013). Isolation and identification of algicidal compound from Streptomyces and algicidal mechanism to Microcystis aeruginosa. PLoS One 8: e76444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makower AK, Schuurmans JM, Groth D. (2015). Transcriptomics-aided dissection of the intracellular and extracellular roles of microcystin in Microcystis aeruginosa PCC 7806. Appl Environ Microbiol 81: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson AR, Loar SN, Bourbonniere RA, Wilhelm SW. (2011). Molecular enumeration of an ecologically important cyanophage in a Laurentian Great Lake. Appl Environ Microbiol 77: 6772–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Lavrentyev PJ, Yang L, Zhang L, Chen Y, Qin B et al. (2007) Nitrogen Dynamics and Microbial Food Web Structure During a Summer Cyanobacterial Bloom in a Subtropical, Shallow, Well-Mixed, Eutrophic Lake (Lake Taihu, China). Eutrophication of Shallow Lakes With Special Reference to Lake Taihu, China 194 Springer: Dordrecht, Netherlands, 195–207. [Google Scholar]
- McCausland MA, Thompson PA, Blackburn SI. (2001). The effect of changes in light availability caused by mixing on the growth of Anabaena circinalis (Nostocales, Cyanobacteria) and Aulacoseira sp.(Centrales, Bacillariophyceae). Phycologia 40: 530–541. [Google Scholar]
- McCausland MA, Thompson PA, Blackburn SI. (2005). Ecophysiological influence of light and mixing on Anabaena circinalis (Nostocales, Cyanobacteria). Eur J Phycol 40: 9–20. [Google Scholar]
- Michalak AM, Anderson EJ, Beletsky D, Boland S, Bosch NS, Bridgeman TB et al. (2013). Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc Natl Acad Sci 110: 6448–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic SM, Bowling LC, Buckney RT. (2001). Vertical disentrainment of Anabaena circinalis in the turbid, freshwater Darling River, Australia: quantifying potential benefits from buoyancy. J Plankton Res 23: 47–55. [Google Scholar]
- Mou X, Lu X, Jacob J, Sun S, Heath R. (2013). Metagenomic identification of bacterioplankton taxa and pathways involved in microcystin degradation in Lake Erie. PLoS One 8: e61890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilan BA, Pearson LA, Muenchhoff J, Moffitt MC, Dittmann E. (2012). Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ Microbiol 15: 1239–1253. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Pearson LA, Muenchhoff J, Moffitt MC, Dittmann E. (2013). Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ Microbiol 15: 1239–1253. [DOI] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. (2011). A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75: 14–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Helmus MR, McMahon KD. (2007). Phylogenetic ecology of the freshwater actinobacteria acI lineage. Appl Environ Microbiol 73: 7169–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa FF, Madramootoo CA, Jabaji S. (2014). Comparison of cyanobacterial microcystin synthetase (mcy) E gene transcript levels, mcy E gene copies, and biomass as indicators of microcystin risk under laboratory and field conditions. Microbiol Open 3: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten TG, Xu H, Qin B, Zhu G, Paerl HW. (2012). Spatiotemporal patterns and ecophysiology of toxigenic microcystis blooms in Lake Taihu, China: implications for water quality management. Environ Sci Technol 46: 3480–3488. [DOI] [PubMed] [Google Scholar]
- Paerl HW. (1996). A comparison of cyanobacterial bloom dynamics in freshwater, estuarine and marine environments. Phycologia 35: 25–35. [Google Scholar]
- Paerl HW, Gardner WS, McCarthy MJ, Peierls BL, Wilhelm SW. (2014). Algal blooms: noteworthy nitrogen. Science 346: 175. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Huisman J. (2009). Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep 1: 27–37. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Paul VJ. (2012). Climate change: links to global expansion of harmful cyanobacteria. Water Res 46: 1349–1363. [DOI] [PubMed] [Google Scholar]
- Paver SF, Hayek KR, Gano KA, Fagen JR, Brown CT, Davis-Richardson AG et al. (2013). Interactions between specific phytoplankton and bacteria affect lake bacterial community succession. Environ Microbiol 15: 2489–2504. [DOI] [PubMed] [Google Scholar]
- Penn K, Wang J, Fernando SC, Thompson JR. (2014). Secondary metabolite gene expression and interplay of bacterial functions in a tropical freshwater cyanobacterial bloom. ISME J 8: 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomati F, Rossetti C, Manarolla G, Burns BP. (2004). Interactions between intracellular Na+ levels and saxitoxin production in Cylindrospermopsis raciborskii T3. Microbiology 150: 455–461. [DOI] [PubMed] [Google Scholar]
- Posch T, Köster O, Salcher MM, Pernthaler J. (2012). Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat Clim Change 2: 809–813. [Google Scholar]
- Proctor LM, Fuhrman JA. (1990). Viral mortality of marine bacteria and cyanobacteria. Nature 343: 60–62. [Google Scholar]
- Rashidan KK, Bird DF. (2001). Role of predatory bacteria in the termination of a cyanobacterial bloom. Microb Ecol 41: 97–105. [DOI] [PubMed] [Google Scholar]
- Rengefors K, Gustafsson S, Stahl-Delbanco A. (2004). Factors regulating the recruitment of cyanobacterial and eukaryotic phytoplankton from littoral and profundal sediments. Aquat Microb Ecol 36: 213–226. [Google Scholar]
- Rinta-Kanto JM, Konopko EA, DeBruyn JM, Bourbonniere RA, Boyer GL, Wilhelm SW. (2009). Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 8: 665–673. [Google Scholar]
- Rouco M, López-Rodas V, Flores-Moya A, Costas E. (2011). Evolutionary changes in growth rate and toxin production in the cyanobacterium Microcystis aeruginosa under a scenario of eutrophication and temperature increase. Microb Ecol 62: 265–273. [DOI] [PubMed] [Google Scholar]
- Rzymski P, Poniedziałek B. (2014). In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Res 66C: 320–337. [DOI] [PubMed] [Google Scholar]
- Salcher MM, Pernthaler J, Posch T. (2011). Seasonal bloom dynamics and ecophysiology of the freshwater sister clade of SAR11 bacteria ‘that rule the waves' (LD12). ISME J 5: 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, Matthijs HCP, Verspagen J, Muyzer G, Huisman J. (2014). Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. ISME J 8: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ et al. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci USA 105: 11254–11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL. (2011). Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl Environ Microbiol 77: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Li R, Lepo JE, Gu J-D. (2013). Potential for control of harmful cyanobacterial blooms using biologically derived substances: problems and prospects. J Environ Manag 125: 149–155. [DOI] [PubMed] [Google Scholar]
- Shao K, Gao G, Chi K, Qin B, Tang X, Yao X et al. (2013). Decomposition of Microcystis blooms: Implications for the structure of the sediment bacterial community, as assessed by a mesocosm experiment in Lake Taihu, China. J Basic Microbiol 53: 549–554. [DOI] [PubMed] [Google Scholar]
- Sinha R, Pearson LA, Davis TW, Burford MA, Orr PT, Neilan BA. (2012). Increased incidence of Cylindrospermopsis raciborskii in temperate zones - is climate change responsible? Water Res 46: 1408–1419. [DOI] [PubMed] [Google Scholar]
- Sinha R, Pearson LA, Davis TW, Muenchhoff J, Pratama R, Jex A et al. (2014). Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities. BMC Genomics 15: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen MM, Belisle BS, Watson SB, Boyer GL, Bourbonniere RA, Wilhelm SW. (2015). Metatranscriptomic evidence for co-occurring top-down and bottom-up controls on toxic cyanobacterial communities. Appl Environ Microbiol 81: 3268–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen MM, Li Z, Effler TC, Hauser LJ, Boyer GL, Wilhelm SW. (2012). Comparative metagenomics of toxic freshwater cyanobacteria bloom communities on two continents. PLoS One 7: e44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MY, Dafforn KA, Johnston EL, Brown MV. (2013). Core sediment bacteria drive community response to anthropogenic contamination over multiple environmental gradients. Environ Microbiol 15: 2517–2531. [DOI] [PubMed] [Google Scholar]
- Tang X, Gao G, Chao J, Wang X. (2010). Dynamics of organic-aggregate-associated bacterial communities and related environmental factors in Lake Taihu, a large eutrophic shallow lake in China. Limnol Oceanogr 55: 469–480. [Google Scholar]
- Wilhelm SW, Farnsley SE, LeCleir GR, Layton AC. (2011). The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai), China. Harmful Algae 10: 207–215. [Google Scholar]
- Wilhelm SW, LeCleir GR, Bullerjahn GS, McKay RM, Saxton MA, Twiss MR et al. (2014). Seasonal changes in microbial community structure and activity imply winter production is linked to summer hypoxia in a large lake. FEMS Microbiol Ecol 87: 475–485. [DOI] [PubMed] [Google Scholar]
- Williamson SJ, Houchin LA, McDaniel L, Paul JH. (2002). Seasonal variation in lysogeny as depicted by prophage induction in Tampa Bay, Florida. Appl Environ Microbiol 68: 4307–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder M. (2012). Limnology: lake warming mimics fertilization. Nat Clim Change 2: 771–772. [Google Scholar]
- Woodhouse JN, Ongley SE, Brown MV, Neilan BA. (2012). Microbial diversity and diazotrophy associated with the freshwater non-heterocyst forming cyanobacterium Lyngbya robusta. J Appl Phycol 25: 1039–1045. [Google Scholar]
- Wu X, Xi W, Ye W, Yang H. (2007). Bacterial community composition of a shallow hypertrophic freshwater lake in China, revealed by 16S rRNA gene sequences. FEMS Microbiol Ecol 61: 85–96. [DOI] [PubMed] [Google Scholar]
- Xing P, Zheng J, Li H, Liu Q. (2013). Methanogen genotypes involved in methane formation during anaerobic decomposition of Microcystis blooms at different temperatures. World J Microb Biot 29: 373–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.