Abstract
Background
Obtaining high-quality images of cellular structures via immunofluorescence staining is critical for cellular localization studies. Often, these studies cannot be performed in parallel with certain oncology, virology, pharmacokinetic, and drug absorption studies due to model system technicalities requiring the cells to be cultured on porous membranes rather than glass or plastic.
Material/Methods
Here, we report a method of immunofluorescent staining of cells cultured on permeable membranes.
Results
As proof of principle, HeLa cells grown on Transwell® membrane supports were stained with fluorescently labeled antibodies using this modified immunofluorescence staining method and visualized by fluorescent microscopy.
Conclusions
This protocol is a convenient alternative to staining cells on glass coverslips, thereby expanding the scope and applications of this important research tool.
MeSH Keywords: Diffusion Chambers, Culture; Fluorescent Antibody Technique, Direct; Staining and Labeling
Backgruond
Cellular localization studies, which are commonly used for many basic science applications, often employ indirect immunofluorescence to monitor the distribution of proteins and organelles within cultured cells [1]. Traditionally, cells are cultured, fixed, and permeabilized on glass coverslips in preparation for immunofluorescent staining [2]. This requirement can potentially create limitations for various oncology, virology, pharmacokinetic, and drug absorption studies, since cells must often be cultured on a porous membrane rather than on glass or plastic. Currently, a standard protocol that describes applying immunofluorescent stains directly to cells cultured on permeable membranes is not available. This article describes the application of standard immunofluorescent staining techniques to various types of Transwell permeable membranes, thereby expanding the scope of this valuable research method.
Membrane filters have been used as cell growth supports for more than sixty years, dating as early as 1953. They were first used in transfilter metanephric induction studies of epithelio-mesenchymal interaction in cultured mouse embryo rudiments [3]. They have been adapted over the years to be compatible with different cell types and applications, such as the study of cellular trafficking and other functions. Culturing cells on permeable supports provides significant advantages over solid, impermeable cell growth surfaces. For example, apical-basal polarization is an important characteristic of mature epithelial layers that can be obtained by culturing epithelial cells on permeable supports, thereby simulating in vivo conditions [4]. Additionally, this configuration allows for the study of the transport of compounds across a polarized monolayer [5]. The use of permeable membranes is also crucial for studies of the blood-brain barrier where the membrane pore size can be manipulated to allow for close contact between two cell types without mixing of the cells [6]. This enables cellular differentiation-type events, such as tight junction and pinocytic vesicle formation, to proceed to higher levels, resulting in cells that more closely represent their in vivo counterparts [7]. Modifying standard immunofluorescent methods to allow cell staining directly on Transwell membranes greatly improves the study of cell structure and physiology.
The purpose of this study is to report a modification of standard immunofluorescent staining protocols to facilitate the direct staining and visualization of cells cultured on permeable membrane supports. This protocol allows scientists to study the cellular changes and effects on the monolayer of these polarized cells following co-culture, drug efflux, or other Transwell studies, thereby expanding the scope and application of this important research tool.
Material and Methods
Cell Culture
Cell culture reagents were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA), unless otherwise indicated; fetal bovine serum (FBS) was purchased from GE Healthcare HyClone (Logan, Utah, USA). HeLa human epithelial cell lines were purchased from ATCC #CCL-2 (Manassas, Virginia, USA) and cultured using the standard ATCC protocol. HeLa cells were cultured overnight on the apical compartment of a 12-well Transwell® apparatus (Corning Inc.; Corning, New York, USA). For our proof-of-principle experiments, three different Transwell permeable membrane materials were tested: polycarbonate (PC), polyester (PET), and collagen-coated polytetrafluoroethylene (PTFE) (Corning Inc.).
Immunofluorescent Staining
A modification of the standard immunofluorescent staining protocol was used [2]. Following overnight growth of HeLa cells, each Transwell membrane apparatus was transferred to a well of a 12-well plate containing PBS/1% FBS, and each membrane was released from the apparatus using a scalpel. The permeable membranes were then washed twice in PBS/1% FBS at room temperature. Cells on membranes were fixed and permeabilized with −20°C methanol (VWR, Radnor, Pennsylvania, USA) for 6 minutes following rehydration with PBS/1% FBS. Table 1 lists antibodies used in this study for staining of cells on membranes. Following antibody staining, membranes were mounted on glass slides using Prolong Gold antifade reagent with DAPI (Invitrogen™ Thermo Fisher Scientific, Waltham, Massachusetts, USA) and covered with coverslips. Control cells were grown on glass coverslips and stained using standard immunofluorescent staining protocols [2]. An Olympus IX81-DSU microscope was utilized to visualize the cells, and images were processed using Slidebook 5.0 software (Intelligent Imaging Innovations, Inc., Denver, Colorado, USA).
Table 1.
Primary and secondary antibodies utilized in this study.
| Antibody | Protein/cellular localization | Company & catalogue # | Dilution |
|---|---|---|---|
| Rabbit Polyclonal to pan Cadherin | Plasma membrane marker | Abcam #ab16505 | 1:500 |
| Alexa fluor® 488 Mouse anti-GM130 | Golgi marker | Invitrogen #560257 | 1:10 |
| Monoclonal anti-β-actin-FITC conjugate clone AC-15 | β-actin marker | Sigma-Aldrich #F3022 | 1:250 |
| Calnexin, mAb | Endoplasmic reticulum marker | Enzo Life Sciences #ALX-804-014 | 1:500 |
| Alexa fluor® 488 goat anti-mouse IgG | Secondary antibody | Invitrogen #A11029 | 1:1000 |
| Alexa fluor® 488 goat anti-rabit IgG | Secondary antibody | Invitrogen #A11034 | 1:1000 |
| Alexa fluor® 546 goat anti-mouse IgG | Secondary antibody | Invitrogen #A11030 | 1:1000 |
| Alexa fluor® 546 goat anti-rabbit IgG | Secondary antibody | Invitrogen #A11035 | 1:1000 |
Results
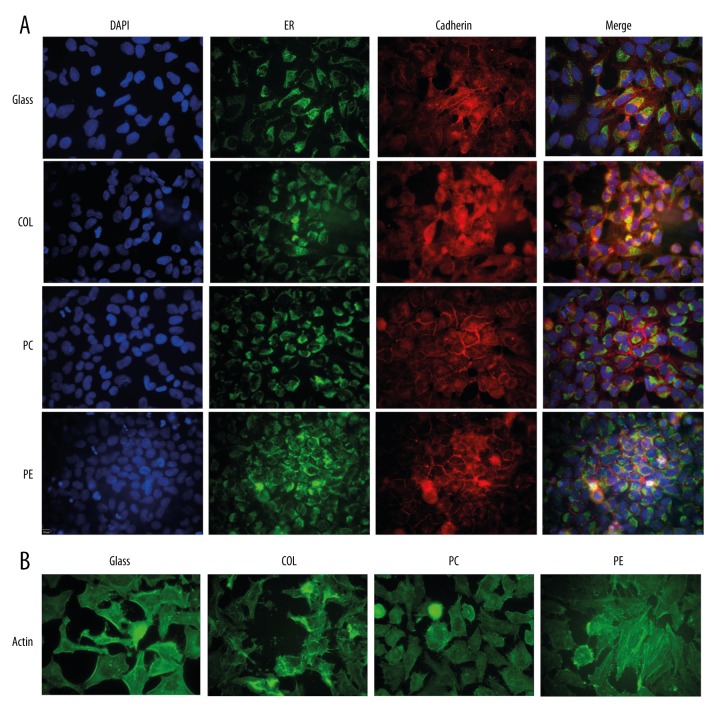
Cells stained with anti-ER, anti-Cadherin, or anti-actin antibodies showed no significant differences in localization patterns between cells cultured on any of the four surfaces tested (PC, PET, PTFE, or standard glass coverslips) (Figure 1). Furthermore, the intensities of the staining patterns were virtually identical. These results suggest that growth of cells on the three Transwell surfaces does not affect the ability of standard immunofluorescent staining protocols to successfully fix, permeabilize, and antibody-label cultured cells. Similarly, DAPI nuclear staining was also virtually identical, implying that nuclear access was similarly unaffected by the Transwell surface.
Figure 1.
Immunofluorescent staining of HeLa cells cultured and stained on four different materials. HeLa cells (ATCC, Manassas, Virginia, USA) were cultured overnight either on glass coverslips or on the apical compartment of 12 mm Transwell® inserts (Corning Inc.; Corning, New York, USA) composed of three membrane materials: collagen-coated polytetrafluoroethylene (COL), polycarbonate (PC), and polyester (PE). (A) Cells stained with Calnexin monoclonal antibody (endoplasmic reticulum marker) and rabbit polyclonal to pan Cadherin (cell membrane marker). (B) Cells stained with monoclonal anti-β-actin-FITC conjugate clone AC-15.
Discussion
The quality of images obtained from immunofluorescent staining of cells cultured on membranes as described here was virtually identical to the quality obtained when cells were cultured on glass coverslips (Figure 1). Here, we used methanol to fix and permeabilize the cells, but it should be noted that paraformaldehyde and Triton X-100 or saponin can also be used to fix and permeabilize cells prior to staining using this modified method. However in our hands, images were not as sharp as when methanol was used (data not shown).
Because permeable membranes allow for permeabilization of the basolateral side, often obscured by glass, the use of cells cultured on membranes may provide a superior image as compared to glass. This demonstrates that there is no need to limit immunofluorescent staining to cells cultured on glass or plastic supports.
Additionally, throughout the staining process, practical advantages of working with the cells cultured on Transwell membranes were identified. Because of their increased flexibility (compared to glass), membranes were easier to manipulate throughout the staining protocol, thereby significantly reducing the risk of breakage. It was observed, however, that collagen membranes were less pliant than the polycarbonate and polyester, yet still more flexible than glass. Furthermore, because cells adhere more strongly to membranes than to glass, there is a much lower rate of cell loss during staining, which is a common complication that causes a loss of both time and resources. It is also much easier to visualize “cell-side up” when using membranes, which is another vital component of the immunofluorescent staining method.
Possible future studies include exploring the permeability of the membranes by staining specific basolateral surface proteins through the Transwell support. Finally, studies requiring cells cultured on Transwell membranes, such as pharmacokinetic studies, can be performed in parallel with localization studies using this modified staining method.
Conclusions
The purpose of this study was to facilitate the staining and visualization of cells cultured on permeable membrane supports using a modification of protocols for standard immunofluorescent staining. The quality of images obtained using the modified method described here was virtually identical to the quality obtained when cells were cultured on glass coverslips. The ability to apply this valuable research tool to new practices gives researchers more flexibility and convenience in using immunofluorescence staining, hence increasing its possible applications and scientific impact.
Acknowledgments
The authors would like to acknowledge Corning® for Transwell® membranes to test this method. We would also like to thank Dr. Mary Peace McRae for critical review of the manuscript.
Footnotes
Source of support: Self financing
References
- 1.Kodiha M, Umar R, Stochaj U. Optimized immunofluorescence staining protocol to detect the nucleoporin Nup98 in different subcellular compartments. Protocol Exchange. 2009 doi: 10.1038/nprot.2009.16. [DOI] [Google Scholar]
- 2.Coling D, Kachar B. Principles and application of fluorescence microscopy. Curr Protoc Mol Biol. 2001;66:14.10.1–11. doi: 10.1002/0471142727.mb1410s44. [DOI] [PubMed] [Google Scholar]
- 3.Grobstein C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature. 1953;172:869–71. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- 4.Javaherian S, Paz AC, McGuigan AP. Micropatterning cells on permeable membrane filters. Methods Cell Biol. 2014;121:171–89. doi: 10.1016/B978-0-12-800281-0.00012-9. [DOI] [PubMed] [Google Scholar]
- 5.De Boer WI, van der Kwast TH, Chopin DK. technical bulletin #412. Corning Inc. Life Sciences; 2013. A Physiological and morphological in vitro model for normal human urothelium cultured on Falcon® cell culture permeable supports. [Google Scholar]
- 6.Demeuse P, Kerkhofs A, Struys-Ponsar C, et al. Compartmentalized co-culture of rat brain endothelial cells and astrocytes: A syngenic model to study the blood-brain barrier. J Neuroscience Methods. 2002;121:21–31. doi: 10.1016/s0165-0270(02)00225-x. [DOI] [PubMed] [Google Scholar]
- 7.Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Methods Mol Biol. 2014;1135:415–37. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]