ABSTRACT
Although current immunosuppression protocols improve the efficacy of clinical allogenic islet transplantation, T cell-mediated allorejection remains unresolved, and major histocompatibility complexes (MHCs) play a crucial role in this process. Papain, a cysteine protease, has the unique ability to cleave the extracellular domain of the MHC class I structure. We hypothesized that pretreatment of donor islets with papain would diminish the expression of MHC class I on islets, reducing allograft immunogenicity and contributing to prolongation of islet allograft survival. BALB/c islets pretreated with papain were transplanted into C57BL/6J mice as an acute allorejection model. Treatment with 1 mg/mL papain significantly prolonged islet allograft survival. In vitro, to determine the inhibitory effect on T cell-mediated alloreactions, we performed lymphocyte proliferation assays and mixed lymphocyte reactions. Host T cell activation against allogenic islet cells was remarkably suppressed by pretreatment of donor islet cells with 10 mg/mL papain. Flow cytometric analysis was also performed to investigate the effect of papain treatment on the expression of MHC class I on islets. One or 10 mg/mL papain treatment reduced MHC class I expression on the islet cell surface. Pretreatment of donor islets with papain suppresses MHC class I-mediated allograft rejection in mice and contributes to prolongation of islet allograft survival without administration of systemic immunosuppressants. These results suggest that pretreatment of human donor islets with papain may reduce the immunogenicity of the donor islets and minimize the dosage of systemic immunosuppressants required in a clinical setting.
KEYWORDS: allorejection, islet transplantation, MHC class I, papain, T cell
Introduction
Clinical allogenic islet transplantation has become an accepted procedure for treatment of type 1 diabetes mellitus.1-3 Furthermore, current combinatorial therapy by T cell depletion and anti-tumor necrosis factor-α antibody treatment has certainly enhanced graft survival.4 However, functional decline and graft loss remain barriers for successful islet transplantation. Furthermore, the required doses of systemic immunosuppressive drugs should be decreased as low as possible for reducing side effects such as infection and oncogenesis.5-7
Allorejection caused by T cell-mediated immune reactions is one of the major problems that lead to islet graft loss. There is no sufficient protocol to control allorejection to date.6 Alloreaction occurs through major histocompatibility complex (MHC) class I and II molecules that are both expressed on the cell surface. MHC class I molecules are expressed on whole nucleated cells of the body, whereas MHC class II molecules are only expressed on the surface of certain types of immune cells, including macrophages and dendritic cells, which are known as professional antigen-presenting cells. Host CD8+ T cells recognize allo-MHC class I-peptide complexes as alloantigens through T cell receptors, while host CD4+T cells recognize allo-MHC class II similarly.
The mechanism of allorecognition is divided into 3 pathways including direct, indirect and semidirect.8-11 Among the 3 pathways, the direct pathway, which involves both allo-MHC class I and II antigens, plays a crucial role in acute alloreactions.12 Host CD8+ T cells, the main effector cells of this phase, recognize allo-MHC class I molecules on grafts and elicit a destructive immune response.13 Makhlouf et al. showed that MHC class I-CD8+ T cell-mediated reactions are more involved in islet allograft rejection than MHC class II-CD4+ T cell reactions in C57BL/6 mice.14 Markmann et al. demonstrated a significant improvement of allograft survival in mice with MHC class I-deficient islets induced by gene disruption.15 Moreover, some donor MHC class I-removal strategies have shown successful outcomes in cellular transplantation such as insulin-secreting islets of Langerhans and fetal pig neuron transplants in animal models.16-23 In the present study, we explored a more applicable method to reduce MHC class I expression on islets.
Papain is a sulfhydryl protease isolated from the surface of the green fruit of papaya, which is known for its unique ability to cleave the extracellular domain of the MHC class I heavy chain.24-26 Galati et al. showed a papain dose-dependent removal effect on MHC class I from mouse lymphocytes in vitro.27 Moreover, Mera et al. reported that papain cleaves human leukocyte antigen class I from human lymphocytes in a dose-dependent manner and inhibits mixed lymphocyte reactions (MLRs) by reducing immunogenicity in vitro.28 From these back grounds, we hypothesized that pretreatment of donor islets with papain would diminish the expression of MHC class I on islets, reducing allograft immunogenicity and contributing to prolongation of islet allograft survival in mice.
Results
Papain pretreatment prolongs allograft survival of islet transplantation
We initially investigated the effects of papain pretreatment of islets on allograft survival in an acute rejection model by transplanting BALB/c islets into C57BL/6J mice. As shown in Fig. 1A and 1B, the 1 mg/mL papain treatment group showed the longest engraftment among the 4 groups (p < 0 .001). The median survival times (MSTs) of 0 (n = 10), 0.1 (n = 10), 1 (n = 11), and 10 mg/mL (n = 5) papain groups were 19, 19, 26, and 12 d, respectively (Table 1). A significant difference in MST was observed between 0 and 1 mg/mL papain treatment groups (p = 0.0088). Moreover, the mean graft survival of 0 and 1 mg/mL papain groups was 20.4 ± 2 .0 and 54.2 ± 13 .4 d (mean ± SE ), respectively (p < 0 .05). Two of 10 mice in the 0.1 mg/mL papain treatment group and 3 of 11 mice in the 1 mg/mL papain treatment group remained normoglycemic until 120 d after transplantation. The 0.1 mg/mL papain treatment showed a higher graft survival rate than the 0 mg/mL papain control, but the MST was not prolonged.
Figure 1.
Effect of papain pretreatment on islet allograft survival Isolated BALB/c islets were pretreated with various papain concentrations (0, 0.1, 1, and 10 mg/mL) prior to islet transplantation. Two hundred islets were transplanted under the left KC. (A) Individual blood glucose readings for a cohort of papain-pretreated islet-transplanted C57BL/6J mice. As indicated by the arrow, nephrectomies were performed on recipient mice that survived up to 120 d after receiving an islet allograft. (B) Survival of islet allografts (BALB/c to C57BL/6J) in recipient mice. The MSTs were calculated and compared using the Kaplan-Meier method. (C) Histological analysis of the grafts of 0 and 1 mg/mL papain groups (upper panels: 0 mg/mL papain (day 14); middle: 1 mg/mL papain (day 14); lower: 1 mg/mL papain (day 120)). Islet grafts were harvested on days 14 or 120 after transplantation. Paraffin-embedded sections were stained with HE, and anti-insulin and -Foxp3 antibodies. Arrowheads indicate Foxp3-positive cells. (HE and insulin staining: original magnification, ×100; Foxp3 staining: left, ×200 and right, ×400).
Table 1.
Graft survival of BALB/c islets pretreated with papain in C57BL/6J recipients.
| Group | n | Graft survival (days) | MST* (days) |
|---|---|---|---|
| Papain 0 mg/mL | 10 | 14, 14, 16, 17, 19, 19, 21, 24, 26, 34 | 19 |
| Papain 0.1 mg/mL | 10 | 14, 15, 17, 17, 19, 19, 19, 24, 120<, 120< | 19 |
| Papain 1 mg/mL | 11 | 17, 21, 21, 25, 25, 26, 31, 71, 120<, 120 <, 120< | 26 |
| Papain 10 mg/mL | 5 | 12, 12, 12, 14, 18 | 12 |
Median survival times
Nephrectomy was performed on the recipient mice at 120 d after transplantation. After nephrectomy, all mice became hyperglycemic, indicating that the normoglycemia was maintained by the islet grafts (Fig. 1A).
Histological examinations revealed intact islets in the 1 mg/mL papain treatment group at up to 120 d after transplantation. In contrast, severe infiltration of mononuclear cells and degenerated islets were observed in the 0 mg/mL papain group at 14 d after transplantation (Fig. 1C). Few Foxp3-positive regulatory T cells (Tregs) were observed in both 0 and 1 mg/mL papain groups (Fig. 1C).
Papain treatment of islet cells inhibits allogenic lymphocyte proliferation in vitro
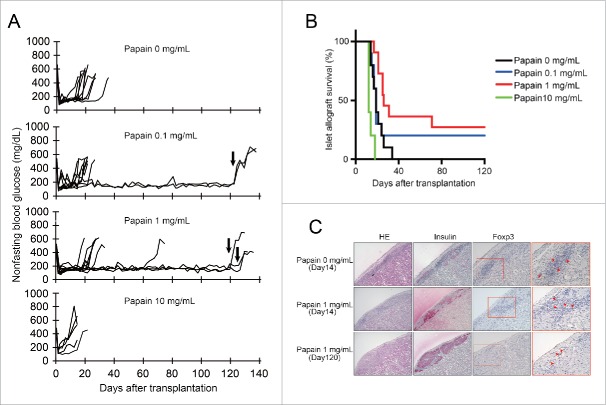
To investigate the effects of papain pretreatment of BALB/c islets on C57BL/6J lymphocyte proliferation in vitro, we assessed lymphocyte proliferation by MTT assays. Single islet cells derived from BALB/c mice as stimulators were pretreated with papain. As shown in Fig. 2, the proliferation of C57BL/6J lymphocytes against BALB/c islet cells pretreated with 10 mg/mL papain was significantly inhibited compared with 0 mg/mL papain at 24–120 h. However, there was no significant difference in lymphocyte proliferation between 0 and 1 mg/mL papain groups. These results indicated that treatment with 10 mg/mL papain inhibited lymphocyte activation against allogenic islet cells in vitro.
Figure 2.
Effects of papain pretreatment of isolated islets on prevention of lymphocyte proliferation against islet alloantigens Prior to the lymphocyte proliferation assay in vitro, BALB/c mouse islets were transplanted into C57BL/6J recipient mice to induce immunological sensitization. Ten days after transplantation, a lymphocyte proliferation assay was used to examine the proliferative response of the recipient spleen-derived lymphocytes to donor single islet cells (BALB/c). Proliferation of lymphocyte was measured by MTT assays after 24, 48, 72, 96, and 120 h of culture. The result of each well is presented as the mean ± SD (n = 7). The experiment was repeated 3 times. *p < 0 .001 compared with the corresponding 0 papain mg/mL group.
Papain treatment removes MHC class I molecules from islet cells
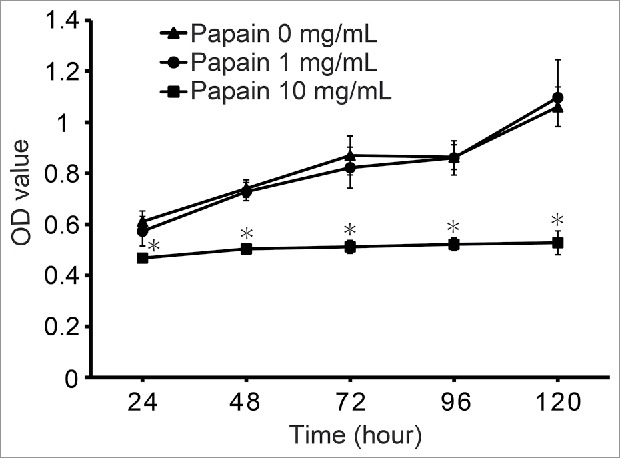
Previously, Mera et al. had reported that papain diminishes human leukocyte antigen class I molecules on the surface of human lymphocytes in a dose-dependent manner in vitro.28 To investigate whether papain performs cleavage of MHC class I on BALB/c mouse islet cells in vitro, we analyzed the expression of MHC class I by flow cytometric analysis. As shown in Fig. 3, the proportions of MHC class I-positive islet cells treated with 0, 0.1, 1, or 10 mg/mL papain were 53.0, 55.4 (data not shown), 43.5, and 39.9%, respectively. These results revealed a decrease in MHC class I expression on the surface of dispersed islets in a papain dose-dependent manner in vitro.
Figure 3.
MHC class I expression on papain-pretreated islet cells Flow cytometric analysis of MHC class I on papain-treated islet cells. Donor islets (BALB/c) were dissociated into single cells and treated with 0, 1, 10 mg/mL papain at 37°C for 15 min. Then, the dispersed islet cells were stained for MHC class I. The histograms are indicative of representative data of 3 separate experiments.
Papain treatment inhibits MLRs in vitro
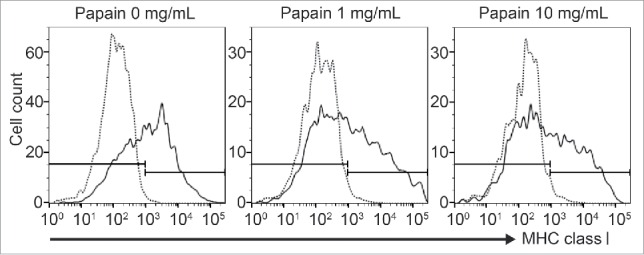
To confirm the results of lymphocyte proliferation against allo-islets (Fig. 2), we performed an MLR assay. BALB/c lymphocytes as stimulator cells were pretreated with papain to remove MHC class I before co-culture with responder T cells (C57BL/6J). The proliferation of responder CD3+ T cell was measured by flow cytometric analysis of CFSE with the decay of CFSE reflecting T cell proliferation. Briefly, the parent population is represented by a single peak (black). The cells proliferating from the parent population, i.e. the daughter population, are represented by 3 other linear histograms. Thus, the number of daughter generations as well as the number of cells in each peak represent the proliferative activity. As shown in Fig. 4A, the 0 mg/mL papain group showed a normal active proliferative response in the MLR. In contrast, the MLR response was remarkably inhibited by 10 mg/mL papain pretreatment of the stimulator cells between 24 and 96 h of culture. MTT assays were also performed at 72 h of culture to quantitatively evaluate the MLR. As a result, the proliferation of T cells was significantly inhibited by 10 mg/mL papain treatment compared with the 0 mg/mL papain group (Fig. 4B), which corresponds to the results of flow cytometry. These results indicated that 10 mg/mL papain treatment suppressed the T cell alloresponse.
Figure 4.
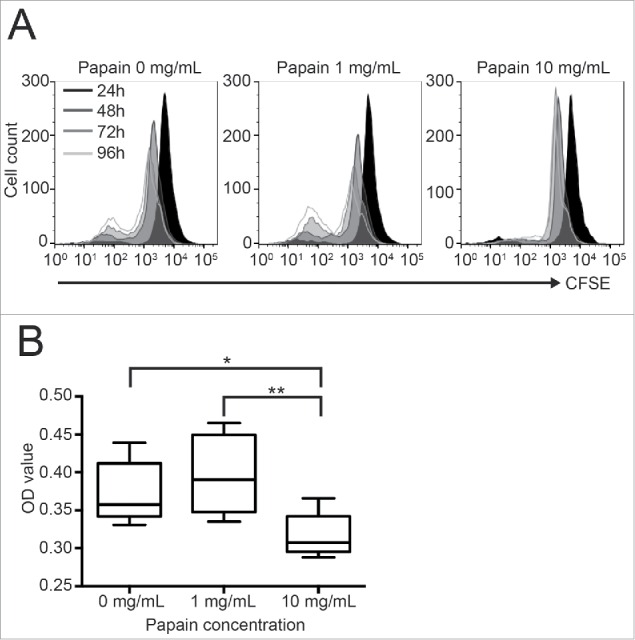
Effects of papain pretreatment on the reduction of an MLR Prior to T cell proliferation assays in vitro, BALB/c mouse islets were transplanted into C57BL/6J recipient mice to induce immunological sensitization. Ten days after transplantation, MLRs were performed to examine the proliferative response of the recipient spleen-derived T cells to donor-type spleen cells (BALB/c). (A) Time-dependent change of CD3+ T cell proliferation detected by flow cytometric analysis of CFSE-stained cells. The black shadowed area represents CD3+ T cell proliferation at 24 h, while the 3 other linear histograms represent the cell proliferation at 48, 72, and 96 h. A representative histogram of 3 samples is shown. (B) The MLR was also analyzed by an MTT assay after incubation for 72 h. The result of each well is presented as the mean ± SD (n = 8). The experiment was repeated 3 times. *p < 0 .05, **p < 0 .01.
Papain pretreatment has no negative effects on islet viability in vitro
Finally, we analyzed the effect of papain treatment on islet viability. As shown in Fig. 5A, there were few annexin V-positive islet cells treated with any papain concentration. Moreover, the PI-positive rate of islets treated with 0, 0.1, 1, or 10 mg/mL papain was 98 ± 1 .95, 99 ± 0 .47, 99 ± 0 .27, and 98 ± 0 .7%, respectively (Fig. 5B). These results demonstrated that papain pretreatment has no negative effects on islet viability.
Figure 5.

Effect of Papain on islet cell viability Fluorescence photomicrographs of isolated donor islets (BALB/c) treated with 0, 0.1, 1, and 10 mg/mL papain (37°C, 15 min). (A) Islets were stained with annexin V-FITC (green), PtdIns (red), and DAPI (blue) to reveal the number of viable, necrotic, and apoptotic cells. (B) Islets were stained with Hoechst 33342 and PI. The percentage of viable islet cells (viability) in each condition was calculated by the average of 10 islets. Viability (%) =100-PtdIns-positive ratio.
Discussion
Papain treatment has a long history as a useful analysis method of MHC class I structure and immunogenicity because of its well-known ability to cleave the MHC class I heavy chain.26,29-31 However, there has been no reports of allogenic cellular or organ transplantation using papain in animal models. Thus, it remains unclear how papain treatment to remove donor MHC class I on islet affects the outcome of allo-islet transplantation from the viewpoint of immunogenicity, especially alloreactions. In the present study, we demonstrated for the first time that papain treatment improves the outcomes of allo-islet transplantation, and revealed the inhibitory mechanism against the direct pathway involved in MHC class I-T cell-mediated alloreactions in mice.
The results of this study clearly showed that donor islets pretreated with 1 mg/mL papain had significantly prolonged allograft survival in mice. More than 35% of the 1 mg/mL papain-treated group showed graft survival for more 60 d. Furthermore, 3 of 11 mice in the 1 mg/mL papain-treated group showed remarkably longer allograft survival than 120 d after transplantation without immunosuppressive treatment. We assumed that these recipient mice might have completed immune tolerance at some point after transplantation. Generally, Tregs play an important role in controlling alloimmune tolerance, and some Treg-inducing strategies have achieved successful islet transplantation outcomes.32-35 Hence, we investigated the proportions of Tregs to mononuclear cells infiltrating into the graft site at day 120 (1 mg/mL papain group) in comparison with grafts at day 14 of 0 and 1 mg/mL papain groups by Foxp3 (Treg marker) staining. However, unexpectedly, there was no difference between the grafts at day 120 and 14. As a result, we concluded that papain treatment has little effect on Treg induction (Fig. 1). However, our in vivo results are quite similar to those of Markmann et al. who reported preparation of MHC class I-deficient islets by gene disruption, although the recipient mice were BALB/c.15 Thus, a strategy using papain may be comparable to a gene-targeting strategy.
In vitro, we revealed that both 1 and 10 mg/mL papain treatment reduced MHC class I on the islet cell surface by flow cytometric analysis (Fig. 3). Fifty-three percent of dispersed islet cells were positive for MHC class I, and 1 or 10 mg/mL papain treatment decreased MHC class I expression by 9.5 and 13.1%, respectively, compared with the control. These results suggested that cleavage of MHC class I on the islet cell surface by papain treatment is partial and temporary, because MHC class I may be re-expressed. Thus, papain pretreatment with systemic immunosuppression is thought to be an attractive therapy to reduce the use of systemic immunosuppressants.
The lymphocyte proliferation assay to determine the effect of papain on alloreactions showed that host lymphocyte activation against allogenic islet cells was significantly suppressed by 10 mg/mL papain treatment (Fig. 2). Furthermore, we confirmed that CD3+ T cell proliferation in MLRs was remarkably suppressed by 10 mg/mL papain treatment (Fig. 4). These results indicate that the decrease in MHC class I expression by papain treatment leads to suppression of T cell-mediated allorejection in mice.
Interestingly, only pretreatment of donor islet cells with 10 mg/mL papain inhibited host T cell activation against allogenic islet cells in vitro. However, the 10 mg/mL papain group showed the shortest graft survival among the 4 groups. There are several possible reasons to explain this phenomenon. First, we designed in vitro experiments using single islet cells instead of islet clusters, because single cells facilitate analysis of the papain efficacy compared with islet clusters. On the other hand, islet clusters were used for in vivo experiments. A form of the islet cells, such as single cells or clusters may affect the effective papain dosage between in vivo and in vitro conditions. Therefore, both results are acceptable. Second, although 10 mg/mL papain treatment had no negative effects on islet viability in vitro (Fig. 5), we found quite a few fragmented islets after 10 mg/mL papain treatment. Previously, Williams et al. reported that rat islets treated with papain became frangible and did not improve the outcome of islet transplantation in a syngenic model.36,37 Thus, their report supports our results. It is presumed that such a fragmented islet structure may accumulate damage over time and eventually affect islet viability and functions negatively in vivo, which would not support long-term engraftment. Third, papain is used to induce experimental pulmonary emphysema in animal models, because papain administration causes air space dimensions with destruction and thickening of lung tissue, which are involved in inflammation.38 Moreover, it has been previously reported that cytokines secreted from transplanted islets cause local inflammation that triggers the innate response and graft failure.39 Although we confirmed that the islets maintained their viability just after papain treatment in vitro (Fig. 5), it is possible that islets pretreated with a higher papain concentration cause stronger inflammation by releasing high levels of cytokines, leading to early graft loss. Taking these ideas into consideration, we presume that 1 mg/mL papain is the optimal concentration to balance positive and negative effects of papain in islet transplantation.
To date, some strategies to remove donor MHC class I have had success in cellular transplants including insulin-secreting islet transplants in murine models and even primates.17-20,22 Markmann et al. revealed that MHC class I-deficient islets improve allograft survival in mice.15 Moreover, CD8+ T cell-deficient recipients show prolonged survival because they lack MHC class I-CD8+ T cell-mediated alloreactions.20 Additionally, Coffman et al. concluded that genetic manipulations to reduce MHC class I expression may be effective to overcome some effects of MHC incompatibility in kidney transplants.40 These data strongly support our results and confirm that the MHC class I system serves as an essential component of the allorejection that is common to both cellular and organ transplantations. Because it is a well-known fact that mammals, including humans, have similar MHC systems, the ultimate goal of this study is practical application to a clinical setting. Thus far, the elimination of MHC antigen expression by gene targeting is not practical as a clinical procedure for islet transplantation in terms of both technical and ethical issues. However, although the effect of papain is somewhat limited, this procedure is much more convenient and worth trialing at the clinical stage. Mera at el. showed that it is possible to maintain the activity of papain at 4°C, suggesting that targeted enzymatic cleavage of donor MHC class I by papain has potential use in the whole organ transplant as well as tissue transplant setting.28 We also expect that papain pretreatment of allogenic grafts has the potential to decrease the required doses of systemic immunosuppressants. From this viewpoint, we propose combinatorial therapy with papain pretreatment of islets and low-dose systemic immunosuppressive drugs. Recently, Brooks et al. reported that antibody-mediated allo-rejection was important and caused by antibodies against mismatched donor HLA-A2 (MHC class I) in the recipients after transplantation.41 They revealed that MHC class I is a crucial antigen not only for cellular immunity but also humoral immunity. Thus, the MHC class I molecule as an immune antigen may have deeper implications going forward.
In conclusion, pretreatment of donor islets with papain suppresses MHC class I-mediated allograft rejection in mice and contributes to the prolongation of islet allograft survival without administrating systemic immunosuppressants. These results suggest that pretreatment of human donor islets with papain may reduce the immunogenicity of the donor islets and minimize the dosage of systemic immunosuppressants required in a clinical setting.
Materials and methods
Animals
Male BALB/c (H-2d) and C57BL/6J (H-2b) mice were purchased from Charles River Japan and CLEA Japan Inc., respectively. All mice were maintained under specific pathogen-free conditions. Recipient C57BL/6J mice were applied to experiments at 10–11 weeks of age. Diabetes was induced in the recipients by intravenous injection of streptozotocin (180 mg/kg body weight) (Sigma-Aldrich Co. S0130). Mice with persistent nonfasting blood glucose levels above 400 mg/dL at 2–3 d after streptozotocin injection were considered diabetic and used as transplant recipients. The experiments were approved by our Institutional Animal Care and Use Committee.
Islet isolation
Donor islets from BALB/c mice were isolated by the static digestion method using Type V collagenase (Sigma-Aldrich Co. C9263) and then separated by centrifugation on Ficoll-Conray gradients.42 Islets of 150–250 μm in diameter were hand selected using a Pasteur pipette with the aid of a dissecting microscope. Isolated islets were cultured in Dulbecco's modified Eagle's medium (DMEM) (Thermo Fisher Scientific 11885-084) supplemented with 10% fetal bovine serum (GE Healthcare 172012) and 1× Antibiotic-Antimycotic (Thermo Fisher Scientific 15240-062) overnight before transplantation.
Papain preparation
To display papain enzymatic activity from papaya latex (Sigma-Aldrich Co. P4762), 2 papain activating agents, L-cysteine (Sigma-Aldrich Co. 168149) and EDTA (Sigma-Aldrich Co. EDS) were required. Low glucose (1 g/L) DMEM supplemented with 0.05 M L-cysteine and 0.02 M EDTA, adjusted to pH 8.0 by 5 M NaOH was prepared freshly.28 Papain has excellent solubility to produce a high concentration solution. Papain at 0 (control), 0.1, 1, and 10 mg/mL was used for islet treatment.
Papain pretreatment and islet transplantation
Recipient mice were divided into 4 groups: 0, 0.1, 1, and 10 mg/mL papain groups. Prior to islet transplantation, 200 hand-picked islets were pretreated with each papain concentration at 37°C for 15 min and then transplanted under the left kidney capsule (KC) of recipient mice.
Nonfasting blood glucose levels and body weight were monitored 3 times a week in all recipients until 120 d after islet transplantation. Blood glucose was measured using a GlucoCard DIAmeter (Arkray).
Normoglycemia after transplantation was defined as 2 consecutive blood glucose level readings below 200 mg/dL. Graft rejection was defined as 2 consecutive hyperglycemias over 400 mg/dL. The first day confirmed as hyperglycemia was defined as the day of rejection. Nephrectomy was performed in recipients with functional grafts at more than 120 d after transplantation to ensure a normal glucose level had been maintained by the islet allograft.
Immunohistochemistry
Islet grafts with the kidney were fixed in 10% formaldehyde, processed, and embedded in paraffin. The sections were cut into 3 μm-thick sections and stained with hematoxylin-eosin (HE) or a Guinea pig anti-mouse insulin antibody (DAKO A056401) and rabbit anti-guinea pig secondary antibody (abcam ab97152) with a Warp Red™ Chromogen Kit (BIOCARE MEDICAL BRR806AH) in the presence of alkaline phosphatase for histochemical detection of insulin localization.43,44
Foxp3 staining was performed using an anti-Mouse/Rat Foxp3 antibody (Affymetrix eBioscience 14-5773-80) visualized by the chromogen diaminobenzidine and counterstaining with hematoxylin.
Lymphocyte proliferation assay
To analyze the activity of host lymphocytes against donor islet cells in vitro, BALB/c islet cells with or without papain pretreatment and C57BL/6J lymphocytes were co-cultured as a lymphocyte proliferation assay. For inducing immunological sensitization in recipient C57BL/6J mice, BALB/c islets were transplanted under the KC of the recipient C57BL/6J mice 10 d before harvesting splenocytes, and then the harvested splenocytes were used as responder cells. Donor islets were isolated from BALB/c mice, cultured at 24°C for 24 h, and then dissociated into single cells. After incubation at 37°C for 24 h, the dispersed islet cells were treated with 0, 1 or 10 mg/mL papain at 37°C for 15 min and used as stimulator cells. Then, stimulator cells (1×104 cells) and responder cells (5×105 cells) were co-cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 55 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin in 96-well V-bottomed plates (total volume: 200 μl at 37°C and a humidified atmosphere with 5% CO2). Proliferation was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays as a quantitative analysis using a Cell Counting Kit-8 (DOJINDO MOLECULAR TECHNOLOGIES, INC 347-07621) at 24, 48, 72, 96, and 120 h.
Flow cytometric analysis
Isolated islets were cultured at 24°C overnight and then dissociated into single cells by incubation with 0.04% EDTA and dispase I (GODO SHUSEI CO. 386-02271) at 37°C for 15 min.45 Dispersed islet cells were incubated at 37°C overnight and then stained with a phycoerythrin (PE)-anti mouse IgG2aκ antibody (isotype control; BioLegend 400211) or PE anti-mouse H-2Kd/H-2Dd antibody (BioLegend 114708). The cells were analyzed by a BD FACSVerse (BD Biosciences) and FlowJo software (Tree Star Inc.).
MLR
After inducing immunological sensitization, T cells were isolated from the spleens of recipient C57BL/6J mice using nylon wool fiber columns (Polysciences, Inc. 21759-1), incubated with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen C34554) at 37°C for 15 min, and then used as responder cells. Spleen cells obtained from naive BALB/c mice were pretreated with 25 μg/mL mitomycin C (Sigma-Aldrich Co. M4287) at 37°C for 1 h. Then, BALB/c spleen cells were treated with 0, 1, or 10 mg/mL papain at 37°C for 15 min and used as stimulator cells. The responder cells (C57BL/6J, 1×105 cells) were cultured in the presence of stimulator cells (BALB/c, 1×105 cells) in RPMI 1640 (Thermo Fisher Scientific 22400-089) supplemented with 10% fetal bovine serum, 55 μM 2-mercaptoethanol (Thermo Fisher Scientific 21985-023) and 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific 15140-122) in 96-well V-bottomed plates (total volume: 200 μl at 37°C in a humidified atmosphere with 5% CO2) and collected after 24, 48, 72 or 96 h of incubation. A PerCP/Cy5.5-conjugated anti-mouse CD3 antibody (BioLegend 100218) was used to determine CD3+ T cells, and then the CD3+-gated cell population was assessed by flow cytometric analysis at 24, 48, 72, and 96 h to detect changes in CFSE fluorescence. MTT assays were also performed as a quantitative analysis of proliferation using the Cell Counting Kit-8 after incubation for 72 h.
Islet viability assay
After culture for 24 h, isolated islets were treated with 0, 0.1, 1 or 10 mg/mL papain at 37°C for 15 min. The islets were then stained with annexin V-FITC and propidium iodide (PtdIns; TREVIGEN, Inc. 4830-01) to evaluate the number of apoptotic and necrotic cells, together with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Co. D9564) for nuclear staining. Isolated islets treated with 0, 0.1, 1 or 10 mg/mL papain were also stained with Hoechst 33342 and PI, and then the percentage of viable islet cells (viability) at each papain concentration was calculated using an average of 10 islets. Viability (%) was calculated by subtracting the PI-positive ratio from 100.
Statistical analysis
Survival data were analyzed using GraphPad Prism 6 statistic software and shown by Kaplan-Meier methods. Islet graft survival between experimental groups was compared using the Log-rank test. All data were statistically assessed by one-way analysis of variance, followed by the Tukey-Kramer test to compare 2 groups. P-values less than 0.05 was considered to be statistically significant.
Abbreviations
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- MST
median survival time
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- Treg
regulatory T cell
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Ms. Yuko Hata, Mr. Ryo Kawakami, and Ms. Yumie Tamura for their technical support.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society of the Promotion of Science (H.N., T.I., and S.K.) and the Intramural Foundation of Fukuoka University (S.K.).
References
- [1].Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343:230-8; PMID:10911004; http://dx.doi.org/ 10.1056/NEJM200007273430401 [DOI] [PubMed] [Google Scholar]
- [2].Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54:2060-9; PMID:15983207; http://dx.doi.org/ 10.2337/diabetes.54.7.2060 [DOI] [PubMed] [Google Scholar]
- [3].Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes 2014; 7:211-23; PMID:25018643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012; 12:1576-83; PMID:22494609; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03977.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sleater M, Diamond AS, Gill RG. Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. Am J Transplant 2007; 7:1927-33; PMID:17617855; http://dx.doi.org/ 10.1111/j.1600-6143.2007.01889.x [DOI] [PubMed] [Google Scholar]
- [6].Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation 2012; 93:1-10; PMID:22138818; http://dx.doi.org/ 10.1097/TP.0b013e31823cab44 [DOI] [PubMed] [Google Scholar]
- [7].Acuna SA, Fernandes KA, Daly C, Hicks LK, Sutradhar R, Kim SJ, Baxter NN. Cancer Mortality Among Recipients of Solid-Organ Transplantation in Ontario, Canada. JAMA Oncol 2016; 2:1-8; PMID:26768652 [DOI] [PubMed] [Google Scholar]
- [8].Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol 2004; 173:4828-37; PMID:15470023; http://dx.doi.org/ 10.4049/jimmunol.173.8.4828 [DOI] [PubMed] [Google Scholar]
- [9].Safinia N, Afzali B, Atalar K, Lombardi G, Lechler RI. T-cell alloimmunity and chronic allograft dysfunction. Kidney Int Suppl 2010; 119:S2-12; PMID:21116312; http://dx.doi.org/ 10.1038/ki.2010.416 [DOI] [PubMed] [Google Scholar]
- [10].Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev 2006; 58:194-243; PMID:16714486; http://dx.doi.org/ 10.1124/pr.58.2.6 [DOI] [PubMed] [Google Scholar]
- [11].Titus T, Badet L, Gray DW. Islet cell transplantation for insulin-dependant diabetes mellitus: perspectives from the present and prospects for the future. Expert Rev Mol Med 2000; 2:1-28; PMID:14585139 [DOI] [PubMed] [Google Scholar]
- [12].Mannon RB, Coffman TM. Gene targeting: applications in transplantation research. Kidney Int 1999; 56:18-27; PMID:10411675; http://dx.doi.org/ 10.1046/j.1523-1755.1999.00513.x [DOI] [PubMed] [Google Scholar]
- [13].Lenz P, Elbe A, Stingl G, Bergstresser PR. MHC class I expression on dendritic cells is sufficient to sensitize for transplantation immunity. J Invest Dermatol 1996; 107:844-8; PMID:8941672; http://dx.doi.org/ 10.1111/1523-1747.ep12331157 [DOI] [PubMed] [Google Scholar]
- [14].Makhlouf L, Yamada A, Ito T, Abdi R, Ansari MJ, Khuong CQ, Winn HJ, Auchincloss H Jr, Sayegh MH. Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice. J Am Soc Nephrol 2003; 14:2168-75; PMID:12874472; http://dx.doi.org/ 10.1097/01.ASN.0000079041.15707.A9 [DOI] [PubMed] [Google Scholar]
- [15].Markmann JF, Bassiri H, Desai NM, Odorico JS, Kim JI, Koller BH, Smithies O, Barker CF. Indefinite survival of MHC class I-deficient murine pancreatic islet allografts. Transplantation 1992; 54:1085-9; PMID:1465773; http://dx.doi.org/ 10.1097/00007890-199212000-00025 [DOI] [PubMed] [Google Scholar]
- [16].Pakzaban P, Deacon TW, Burns LH, Dinsmore J, Isacson O. A novel mode of immunoprotection of neural xenotransplants: masking of donor major histocompatibility complex class I enhances transplant survival in the central nervous system. Neuroscience 1995; 65:983-96; PMID:7617173; http://dx.doi.org/ 10.1016/0306-4522(94)00626-G [DOI] [PubMed] [Google Scholar]
- [17].Osorio RW, Ascher NL, Jaenisch R, Freise CE, Roberts JP, Stock PG. Major histocompatibility complex class I deficiency prolongs islet allograft survival. Diabetes 1993; 42:1520-7; PMID:8375593; http://dx.doi.org/ 10.2337/diab.42.10.1520 [DOI] [PubMed] [Google Scholar]
- [18].Osorio RW, Ascher NL, Melzer JS, Stock PG. Enhancement of islet allograft survival in mice treated with MHC class I specific F(ab')2 alloantibody. Transplant Proc 1994; 26:749; PMID:8171642 [PubMed] [Google Scholar]
- [19].Osorio RW, Ascher NL, Melzer JS, Stock PG. β-2 Microglobulin gene disruption prolongs murine islet allograft survival in NOD mice. Transplant Proc 1994; 26:752; PMID:8171644 [PubMed] [Google Scholar]
- [20].Osorio RW, Ascher NL, Stock PG. Prolongation of in vivo mouse islet allograft survival by modulation of MHC class I antigen. Transplantation 1994; 57:783-8; PMID:8154021; http://dx.doi.org/ 10.1097/00007890-199403270-00001 [DOI] [PubMed] [Google Scholar]
- [21].Mannon RB, Griffiths R, Ruiz P, Platt JL, Coffman TM. Absence of donor MHC antigen expression ameliorates chronic kidney allograft rejection. Kidney Int 2002; 62:290-300; PMID:12081591; http://dx.doi.org/ 10.1046/j.1523-1755.2002.00422.x [DOI] [PubMed] [Google Scholar]
- [22].Li X, Faustman D. Use of donor β 2-microglobulin-deficient transgenic mouse liver cells for isografts, allografts, and xenografts. Transplantation 1993; 55:940-6; PMID:8475570; http://dx.doi.org/ 10.1097/00007890-199304000-00046 [DOI] [PubMed] [Google Scholar]
- [23].Qian S, Fu F, Li Y, Lu L, Rao AS, Starzl TE, Thomson AW, Fung JJ. Impact of donor MHC class I or class II antigen deficiency on first- and second-set rejection of mouse heart or liver allografts. Immunology 1996; 88:124-9; PMID:8707337; http://dx.doi.org/ 10.1046/j.1365-2567.1996.d01-633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Balls AK, Lineweaver H, Thompson RR. Crystalline Papain. Science 1937; 86:379; PMID:17784205; http://dx.doi.org/ 10.1126/science.86.2234.379 [DOI] [PubMed] [Google Scholar]
- [25].Kimmel JR, Smith EL. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem 1954; 207:515-31; PMID:13163037 [PubMed] [Google Scholar]
- [26].Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987; 329:506-12; PMID:3309677; http://dx.doi.org/ 10.1038/329506a0 [DOI] [PubMed] [Google Scholar]
- [27].Galati G, Arcelloni C, Paroni R, Heltai S, Rovere P, Rugarli C, Manfredi AA. Quantitative cytometry of MHC class I digestion from living cells. Cytometry 1997; 27:77-83; PMID:9000588; http://dx.doi.org/ 10.1002/(SICI)1097-0320(19970101)27:1%3c77::AID-CYTO10%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- [28].Mera T, Faustman DL. Removal of donor human leukocyte antigen class I proteins with papain: translation for possible whole organ practices. Transplantation 2015; 99:724-30; PMID:25340603; http://dx.doi.org/ 10.1097/TP.0000000000000436 [DOI] [PubMed] [Google Scholar]
- [29].Bjorkman PJ, Strominger JL, Wiley DC. Crystallization and X-ray diffraction studies on the histocompatibility antigens HLA-A2 and HLA-A28 from human cell membranes. J Mol Biol 1985; 186:205-10; PMID:3878413; http://dx.doi.org/ 10.1016/0022-2836(85)90271-2 [DOI] [PubMed] [Google Scholar]
- [30].Zavazava N, Hausmann R, Kraatz E, Muller-Ruchholtz W. Clinical relevance of soluble HLA and interaction of papain derived class I molecules with alloreactive CTL. Transpl Int 1992; 5 Suppl 1:S606-8; PMID:14621888 [DOI] [PubMed] [Google Scholar]
- [31].Hausmann R, Zavazava N, Steinmann J, Muller-Ruchholtz W. Interaction of papain-digested HLA class I molecules with human alloreactive cytotoxic T lymphocytes (CTL). Clin Exp Immunol 1993; 91:183-8; PMID:8419081; http://dx.doi.org/ 10.1111/j.1365-2249.1993.tb03376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Krzystyniak A, Golab K, Witkowski P, Trzonkowski P. Islet cell transplant and the incorporation of Tregs. Curr Opin Organ Transplant 2014; 19:610-5; PMID:25304813; http://dx.doi.org/ 10.1097/MOT.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sugimoto K, Itoh T, Takita M, Shimoda M, Chujo D, SoRelle JA, Naziruddin B, Levy MF, Shimada M, Matsumoto S. Improving allogeneic islet transplantation by suppressing Th17 and enhancing Treg with histone deacetylase inhibitors. Transpl Int 2014; 27:408-15; PMID:24410777; http://dx.doi.org/ 10.1111/tri.12265 [DOI] [PubMed] [Google Scholar]
- [34].Muller YD, Mai G, Morel P, Serre-Beinier V, Gonelle-Gispert C, Yung GP, Ehirchiou D, Wyss JC, Bigenzahn S, Irla M, et al.. Anti-CD154 mAb and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS One 2010; 5:e10352; PMID:20436684; http://dx.doi.org/ 10.1371/journal.pone.0010352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ikemoto T, Tashiro S, Yasutomo K, Kishihara K, Kurita N, Miyake H. Donor-specific tolerance induced by simultaneous allogeneic islet transplantation with CD4+CD25+ T-cells into hepatic parenchyma in mice. J Med Invest 2004; 51:178-85; PMID:15460904; http://dx.doi.org/ 10.2152/jmi.51.178 [DOI] [PubMed] [Google Scholar]
- [36].Williams SJ, Huang HH, Kover K, Moore W, Berkland C, Singh M, Smirnova IV, MacGregor R, Stehno-Bittel L. Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis 2010; 6:115-24; PMID:20885858; http://dx.doi.org/ 10.4161/org.6.2.10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Williams SJ, Schwasinger-Schmidt T, Zamierowski D, Stehno-Bittel L. Diffusion into human islets is limited to molecules below 10 kDa. Tissue Cell 2012; 44:332-41; PMID:22717091; http://dx.doi.org/ 10.1016/j.tice.2012.05.001 [DOI] [PubMed] [Google Scholar]
- [38].Machado MN, Figueiroa SF, Mazzoli-Rocha F, Valenca Sdos S, Zin WA. Papain-induced experimental pulmonary emphysema in male and female mice. Respir Physiol Neurobiol 2014; 200:90-6; PMID:24931736; http://dx.doi.org/ 10.1016/j.resp.2014.06.005 [DOI] [PubMed] [Google Scholar]
- [39].Itoh T, Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, Kodama S, Yasunami Y. HMGB1-Mediated Early Loss of Transplanted Islets Is Prevented by Anti-IL-6R Antibody in Mice. Pancreas 2015; 44:166-71; PMID:25058889; http://dx.doi.org/ 10.1097/MPA.0000000000000188 [DOI] [PubMed] [Google Scholar]
- [40].Coffman T, Geier S, Ibrahim S, Griffiths R, Spurney R, Smithies O, Koller B, Sanfilippo F. Improved renal function in mouse kidney allografts lacking MHC class I antigens. J Immunol 1993; 151:425-35; PMID:8326135 [PubMed] [Google Scholar]
- [41].Brooks AM, Carter V, Liew A, Marshall H, Aldibbiat A, Sheerin NS, Manas DM, White SA, Shaw JA. De Novo Donor-Specific HLA Antibodies Are Associated With Rapid Loss of Graft Function Following Islet Transplantation in Type 1 Diabetes. Am J Transplant 2015; 15:3239-46; PMID:26227015; http://dx.doi.org/ 10.1111/ajt.13407 [DOI] [PubMed] [Google Scholar]
- [42].Okeda T, Ono J, Takaki R, Todo S. Simple method for the collection of pancreatic islets by the use of Ficoll-Conray gradient. Endocrinol Jpn 1979; 26:495-9; PMID:387387; http://dx.doi.org/ 10.1507/endocrj1954.26.495 [DOI] [PubMed] [Google Scholar]
- [43].Pelosi G, Fabbri A, Bianchi F, Maisonneuve P, Rossi G, Barbareschi M, Graziano P, Cavazza A, Rekhtman N, Pastorino U, et al.. DeltaNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: a novel two-hit, sparing-material approach. J Thorac Oncol 2012; 7:281-90; PMID:22071786; http://dx.doi.org/ 10.1097/JTO.0b013e31823815d3 [DOI] [PubMed] [Google Scholar]
- [44].Pelosi G, Gasparini P, Cavazza A, Rossi G, Graziano P, Barbareschi M, Perrone F, Barberis M, Takagi M, Kunimura T, et al.. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer 2012; 77:507-14; PMID:22705117; http://dx.doi.org/ 10.1016/j.lungcan.2012.05.093 [DOI] [PubMed] [Google Scholar]
- [45].Ono J, Takaki R, Fukuma M. Preparation of single cells from pancreatic islets of adult rat by the use of dispase. Endocrinol Jpn 1977; 24:265-70; PMID:410634; http://dx.doi.org/ 10.1507/endocrj1954.24.265 [DOI] [PubMed] [Google Scholar]