Abstract
Skin pigmentation is one of the most strikingly variable phenotypes in humans, therefore making cutaneous pigmentation disorders frequent symptoms manifesting in a multitude of forms. The most common among them include lentigines, postinflammatory hyperpigmentation, dark eye circles, and melasma. Variability of skin tones throughout the world is well-documented, some skin tones being reported as more susceptible to pigmentation disorders than others, especially in Asia and India. Furthermore, exposure to ultraviolet radiation is known to trigger or exacerbate pigmentation disorders. Preventive strategies for photoprotection and treatment modalities including topical and other medical approaches have been adopted by dermatologists to mitigate these disorders. This review article outlines the current knowledge on pigmentation disorders including pathophysiology, molecular profiling, and therapeutic options with a special focus on the Indian population.
Keywords: Hyperpigmentation disorders, reconstructed skin models, sunscreen, ultraviolet radiation, ultraviolet protection
Introduction
What was known?
Skin hyperpigmentations represent one of the major dermatological concerns for populations with pigmented skin phototypes, with a high prevalence in the Indian population. The usual treatments are based on the use of topical depigmenting agents to decrease the amount of melanin content or laser technologies. However the role of solar exposure is often ignored.
Variability of constitutive pigmentation around the world is well-established, with some skin tones, especially in Asian and Indian subjects, reported to be more susceptible to pigmentation disorders than other human groups.[1,2] This review mostly focuses on the skin pigmentation and its variation, as well as associated pigmentary disorders among the Indian population. The influence of ultraviolet (UV) exposures on skin pigmentation and their role in pigmentary disorders will be discussed with insights in UV-induced biomarkers that were previously identified, using reconstructed skin models relevant for decoding the underlying mechanisms. Furthermore, treatment strategies as well as photoprotective measures for pigmentation disorders are included.
Melanogenesis and Skin Complexion
Melanin is the primary pigment that determines the color of the skin. Melanin is produced by epidermal melanocytes through enzymatic oxidation of tyrosine, a process known as melanogenesis which takes place within specific organelles, melanosomes.[3] After maturation, melanin is transferred into the surrounding keratinocytes. The epidermal melanin unit is defined as the association that involves one melanocyte and about forty keratinocytes. Constitutive pigmentation depends on the amount of melanins, their quality and relative composition between eumelanin (brown/black pigment) and pheomelanin (yellow-reddish pigment), the mode of transfer and processing of melanosomes inside the keratinocytes, and not on the number of melanocytes, which is relatively constant in a given skin site, irrespective of the color skin type. The type and amount of melanin are under the control of several genes with a great number of alleles, resulting in wide variations of skin colors. While pheomelanin is thought to be photoreactive, eumelanin has been found to dissipate >99.9% of absorbed UV and visible rays and therefore acts as primary photoprotectant.[4] Interestingly, a recent study shows that human epidermis comprises approximately 74% of eumelanin and 26% pheomelanin, regardless of the degree of pigmentation.[5] The same study showed a good correlation between constitutive pigmentation and total melanin content assessed by three different methods.
Variation in skin tone is one of the prominent distinguishable features of human beings. Skin pigmentation in humans is variable and has evolved predominantly to regulate the penetration of UV levels.[2] A direct correlation has been observed between the geographical distribution of UV radiation and skin pigmentation worldwide.[6] Dark-skinned populations are predominantly seen in geographical areas located closer to the equator as these areas receive high amounts of UVB radiation. Light-skinned populations are observed to be located far from the tropics and closer to the poles, which receive low-intensity UVB radiance.[7] In females, the lighter skin complexion is thought to have evolved to allow UVB-induced synthesis of Vitamin D3 required for pregnancy and lactation.[2]
As mentioned above, human skin pigmentation is a heritable trait controlled by a number of genes. In a study by Shriver et al.,[8] genotyping studies revealed differences in the allelic frequencies of several genes in three populations of different descent. Among these, TYR and OCA2 genes were found to play a role in skin pigmentation in African and European populations. In another genome-wide association study, polymorphisms in SLC24A5, TYR, and SLC45A2 genes were found to be significantly associated with skin pigmentation in a population of South Asian descent.[9]
The color of the Indian skin shows a wide variability. Hourblin et al. studied a range of skin parameters including color, among 1204 women volunteers across Indian four cities.[10] They reported that the complexion in Indian population ranged from whitish to brown and that skin complexion ranged from fairer in North India to darker in South India [Figure 1]. This wide diversity in pigmentation among the Indian population can occur due to the presence of several gene polymorphisms. In this context, Mukherjee et al. studied allelic frequencies of single nucleotide polymorphisms in four pigmentation-related genes including SLC45A2, SLC24A5, MC1R, and TYRP1 in 749 individuals from 11 sub-populations suggesting significant allelic variations across studied populations.[11]
Figure 1.
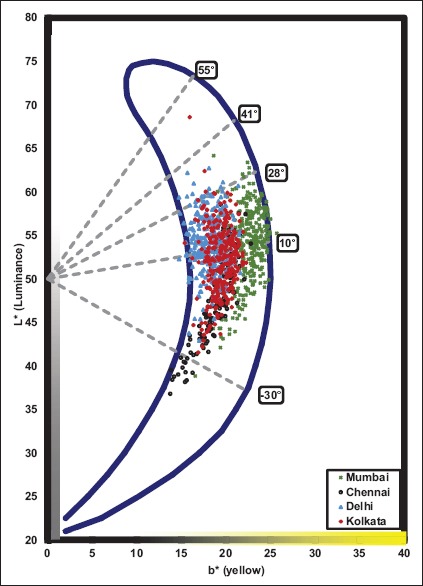
Indian skin colorimetric classification in the skin color volume projected on the L*/b* plane of the L*a*b* space (CIE 1976). The vertical axis L* is the luminance or lightness of the skin and the horizontal axis b* is the yellow component of the skin. Skin color categories from fair to intense dark and individual typological angles are indicated
Common Pigmentary Disorders - Focus on the Indian Scenario
This section includes studies carried out on the variation of Indian skin tones across various geographical locations and their susceptibility to common pigmentation disorders. A large sample study across four Indian cities revealed that more than 80% of the population present skin color heterogeneity on the face, irrespective of age and gender.[10] This heterogeneity mainly results from hyper-pigmented spots, melasma and ill-defined pigmented macules and dark circles.[10] In addition, these different lesions often coexist. In this study, hypopigmentary disorders are less frequent, such as vitiligo, pityriasis alba, pityriasis versicolor, hypopigmented leprosy, nevus achromicus, and albinism and some hyper-pigmentary disorders such as idiopathic guttate and confluent hypermelanosis, lichen amyloidosis, and nevus of Ota.[12,13] Pigmentary disorders cause psychological distress and negatively impact the quality of life of an individual.[14] Three important hyperpigmentary disorders in India, namely, melasma, postinflammatory hyperpigmentation (PIH) and actinic lentigines will be detailed below, and the role of UV exposure in their pathogenesis will be emphasized.
Melasma
Melasma, an acquired pigmentary disorder is characterized by hyperpigmented brown to grayish brown macules on the face. It occurs mainly in women (90% cases) and 10% of males of all ethnic and racial groups.[15] In India, 20–30% of 40–65 years old women present a facial melasma.[10] The current classification of melasma is based on the site of lesion and on the depth of pigmentation that is determined histologically or instrumentally within the epidermis, dermis or both.[16,17,18,19] While the exact cause of melasma is currently unknown, exposure to UV, increased estrogen levels (observed mainly during pregnancy or use of oral contraceptives), genetic predisposition and phototoxic drugs are known to play a major role in the development of this hypermelanosis disorder.[18] Other factors implicated in its etiology include ovarian dysfunction, thyroid and/or liver diseases.[16] The role of UV exposure has been shown to being crucial in development but mainly exacerbation of melasma.[20,21,22] Epidemiological studies revealed that in more than 25% of cases, an association with sun exposure has been declared.[23,24] At the molecular level, it is well-established that exposure to UV rays induce increased production of alpha-melanocyte-stimulating hormone and corticotrophin as well as interleukin (IL)-1 that, in turn, contribute to increased melanin production. In addition, overexpression of dermal stem cell factor and its receptor, c-kit, have been identified in melasma lesions and are believed to increase melanogenesis.[25] More generally in melasma, paracrine melanogenic factors have been identified from keratinocytes, mast cells or dermal fibroblasts.[20] However, it seems that melasma results from complex interactions of various causative factors, UV exposure(s) included.
Postinflammatory hyperpigmentation
PIH is an acquired pigmentary skin disorder.[26] It occurs as a result of an inflammatory reaction, induced by cutaneous diseases including acne vulgaris, atopic dermatitis, psoriasis, impetigo, lichen planus, pityriasis rosea, irritant and allergic contact, photocontact-dermatitis and insect bites as well as a complication of laser therapy. It has been shown that severity and frequency of PIH are both increased in individuals with skin of color of both genders.[27,28] In India, a majority of subjects with an acne history present pigmented postinflammatory marks: More than 70% before 35 years old, both in women and men. This prevalence rapidly decreases with age to involve <10% of people older than 50 years. This observation is directly related to the high prevalence of acne in progress observed in the study.[10] PIH can last from months to years and may significantly impair the quality of life of affected individuals. The severity of PIH has been observed to be higher in prolonged and/or recurrent inflammation when compared with short-term acute inflammation.[29] Inflammation of the epidermis results in the production and release of several cytokines, prostaglandins, and leukotrienes, that stimulate the epidermal melanocytes leading to an increased synthesis of total melanin.[27,30] Most of these factors are also produced under solar stimulation supporting the role of UV exposure in the initiation and exacerbation of PIH. In addition, cutaneous inflammation also causes damage to the basal layer resulting in a leakage of melanins from basal keratinocytes and the subsequent accumulation of melanophages in the dermis thereby exacerbating dermal hyperpigmentation. Esthetic procedures and light-based treatments can also induce PIH, especially in darker-skinned patients.[31]
Lentigines
Actinic lentigines also called solar lentigines or lentigo senilis, are light brown to dark brown, spots, even-colored or reticulated patches occurring mainly in sun-exposed areas. The dorsal aspects of the hands, extensor forearms, upper trunk and face are the most commonly affected sites. They might be sometimes solitary, but these lesions are more often multiple. Actinic lentigines are considered to be a clinical sign of photoaging and their frequency increases with age.[32,33,34,35] Their number is an indicator of the amount of sun exposure over the course of a life-time and therefore shows an increased risk for developing skin cancers.[36,37,38] They are more characteristic of fair to the medium photoaged skin, and prevalence in India is quite close to other Asian countries. It affects one-third of women about 50 years old in the large sample study carried out in India and concerns half of the population over 70 years old.[10] Actinic lentigines are sometimes difficult to distinguish from seborrheic keratosis or simplex lentigos. The presence of the three entities often causes distress to the individual due to their color, number, size, location, and their known link to aging for two of them. At the histological level, actinic lentigines are characterized by a hyperpigmented basal layer which is due to an increased total melanin content of the epidermis (hypermelaninosis).[39] The number of melanocytes was found increased in some studies (hypermelanocytosis),[40] whereas others did not reveal any differences.[39] The global architecture of the epidermis is often disorganized with broadened rete-ridges and includes long, short, crowded, and bulbous buds.[34,41,42,43] In addition, the rete-ridges of the dermal-epidermal junction are altered and elongated resulting in a protrusion of the epidermis into the dermis.[41,42,44] Although a strong association between actinic lentigines development and UV exposure has been observed, the underlying molecular mechanisms are still not fully understood. a potential role of keratinocyte growth factor has been recently suggested as well as the involvement of other dermal factors.[43,45]
Periorbital hyperpigmentation
Periorbital hyperpigmentation (POH) also known as dark circles or periorbital melanosis or periocular hyperpigmentation which surround the eyelids is a significant problem found especially in Asians. The overall prevalence of Indian women is 50% with moderate to severe dark circles on the upper eyelid and increases with age. In a study, the most common form of POH was constitutional/genetic type (n = 103, 51.5%) followed by postinflammatory type (n = 45, 22.5%) with very little knowledge about etiology.[46] Other studies describe POH as acquired idiopathic patterned facial pigmentation[47] or idiopathic cutaneous hyperchromia at the orbital region (ICHOR)[48] that manifests due to genetically determined increased pigmentary functional activity. Both studies have indicated sun exposure as a risk factor for aggravation. Ranu et al.[49] attributed vascular factors, constitutional factors and postinflammatory pigmentation and shadow effects as reasons for ICHOR. Verschoore et al.[48] also found vascular factors to be causative in periorbital pigmentation. Malakar et al.[50] concluded that periorbital melanosis was an extension of pigmentary demarcation lines of the face.
Ultraviolet-induced Damage: A Cause for Exacerbation of Pigmentation Disorders
Considering that UV exposure is the most important factor that influences skin pigmentation, and more so in fairer skin tones (photo-types I–IV) and the major environmental stress involved in the development of several hyperpigmentation disorders, researches on the precise biological effects of UV exposure are crucial. UVB (290–320 nm) are the more energetic of the UV rays and are capable of inducing direct DNA damage through the production of cyclobutane pyrimidine dimers and 6–4 photoproducts.[51] In contrast, UVA rays (320–400 nm) mainly cause the production of reactive oxygen species (ROS) that, in turn, can be responsible for indirect DNA damage and activation of several pathways. UVA rays can also penetrate deep into the skin, reaching the basal layer of the epidermis and dermis.[52] UVA rays are considered as major actors for the photoaging process. Several works studied the effect of UV radiation on the skin at the molecular level. Reconstructed skin models have been shown to mimic the biological properties of human skin in vivo, such as the three-dimensional architecture, the formation of a fully differentiated epidermis and a dermal equivalent that comprises matrix components and living dermal fibroblasts. The effects of both UVA and UVB on keratinocytes and fibroblasts were examined in a reconstructed skin model.[53] After UVB exposure, several well-known epidermal biomarkers of a sunburn reaction were found induced similarly to the in vivo situation, such as DNA lesion formation, p53 accumulation or sunburn cell formation.[54] Apart from the classical activation of melanogenesis by UV exposure, it has been shown that both DNA damage but also the subsequent accumulation of p53 protein act as stimulators of melanogenesis, resulting in an increased pigmentation.[55,56] UVA exposure led to ROS formation in both compartments, with direct alterations in the dermal equivalent, and an increase in inflammatory mediators such as IL-1 or IL-6.[57] Both UVA and UVB induced expression of matrix degrading enzymes such as matrix metalloproteinases 1 (MMP-1), either directly in dermal fibroblasts after UVA exposure or through secretion of soluble epidermal factors after UVB exposure.[58] Marionnet et al. recently studied the biological effects of UVA1 (340–400 nm) exposure. UVA1 is the most abundant part of the sun UV spectrum that reaches the earth surface, leading to high annual doses in Asia and India.[59] It was observed that although less energetic than UVB or UVA2, UVA1 induced ROS production in the whole skin depth, thymine dimer formation, lipid peroxidation and apoptosis in fibroblasts. Further, using a full genome transcriptomic study, a differential regulation of 461 genes in epidermal keratinocytes and 480 genes in dermal fibroblasts was found induced by exposure to UVA1, of which genes encoding heat shock and oxidative stress proteins were clearly up-regulated. Genes belonging to a variety of functional families were also found modulated by UVA1 exposure, especially genes related to immune function, including proinflammatory actors. Among them, several have been shown participating in UV-induced pigmentation. Altogether, this data support the fact that the whole UV spectrum is responsible for cellular responses and tissue damage that are in turn involved in the stimulation of pigmentation and development or exacerbation of pigmentary lesions.
Correction of Pigmentary Disorders
Treatments of pigmentary disorders
Topical treatment is an effective treatment modality for pigmentary disorders and hydroquinone (HQ), a hydroxyphenol, has been widely used across the world as the treatment of choice.[60]
HQ inhibits tyrosinase and 4% can be used twice daily for up to 6 months for PIH. Nonphenolic agents, such as kojic acid and tretinoin,[61,62] are also proven effective topical depigmenting agents. Monteiro et al. found that 0.75% kojic acid cream was less efficacious and had a slower rate of clinical improvement when compared to 4% HQ cream which is a better topical hypopigmenting agent.[63]
However, monotherapy is often associated with undesirable side effects, in contrast, a combined therapy, such as Kligman and Willis's triple combination,[64] is favored since offering an improved efficacy coupled with minimized side-effects.
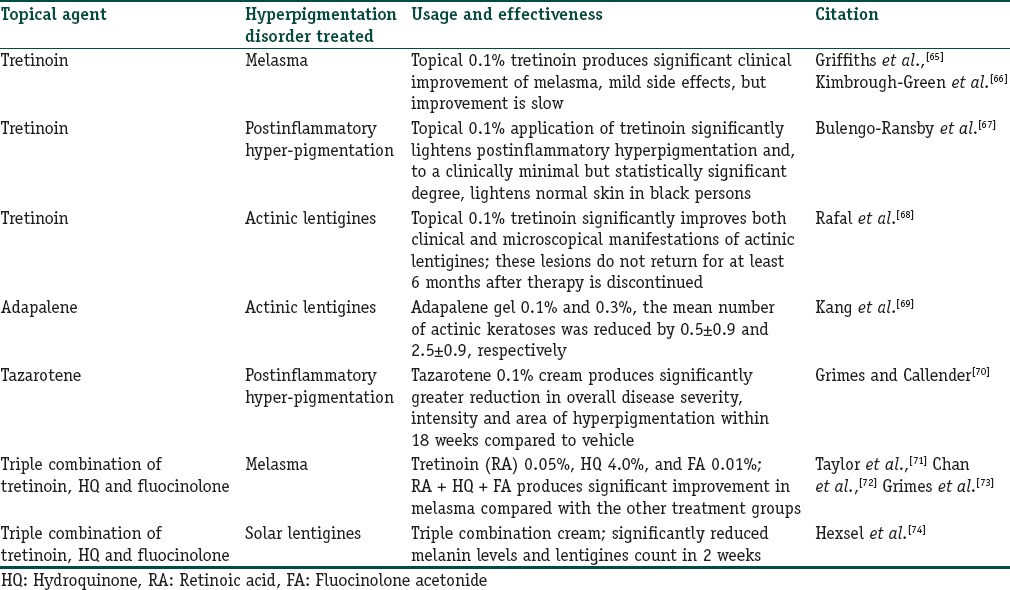
Several studies have been performed to explore the role of topical retinoids in the treatment of pigmentary disorders including melasma, actinic lentigines, and PIH.[28] Clinical studies have demonstrated the efficacy of retinoids such as tretinoin (all-trans retinoic acid [RA]), isotretinoin (13-cis-RA), tazarotene, and adapalene in the treatment of these disorders. A summary of retinoids in the treatment of hyperpigmentation disorders is provided in Table 1. Adapalene gel also improved actinic lentigines in another randomized trial.[69] HQ and triple combination formulations are currently the gold standard for treating melasma.[75] Other groups have studied triple combination consisting of tretinoin, HQ and fluocinolone acetonide, a corticosteroid in multi-center, randomized, investigator-blind studies, and observed reduction in melasma.[71,72,73] Triple combination creams were also found effective in lightening actinic lentigines in a randomized clinical trial.[74] Other topical agents including azelaic acid, kojic acid, ascorbic acid, glycolic acid, and salicylic peels have also been tried with variable degrees of success.[76] Newer topical agents including soy extracts, licorice extract, mulberroside F, N-acetyl glucosamine, niacinamide, resveratrol, rucinol, dioic acid, and ellagic acid have revealed considerable amount of success in the treatment of hyperpigmentation disorders.[77]
Table 1.
A summary of various retinoids in the treatment of hyperpigmentation disorders
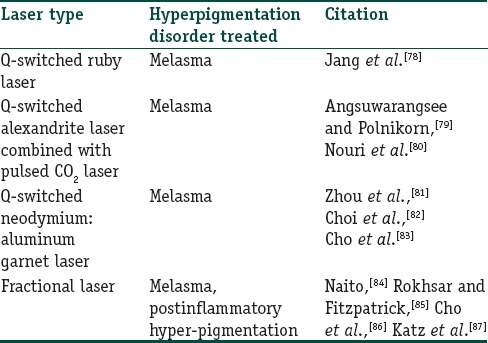
During the last decade, laser technologies have been increasingly used in the treatment of dermatological disorders. Currently, several laser options are available for the treatment of hyperpigmentation disorders including Q-switched (QS) ruby, QS alexandrite, QS neodymium:yttrium-aluminum-garnet (Nd:YAG), and fractional photothermolysis. A summary of different lasers used in the treatment of hyperpigmentation disorders is provided in Table 2. In a study by Taylor et al., QS ruby laser-based treatment of eight subjects with melasma and PIH was found ineffective.[88] However, multiple sessions of the low dose QS ruby laser was found beneficial in the treatment of melasma in fifteen Korean women, leading to decreased melasma area and severity index scores after the final treatment.[78] Treatment of melasma with QS alexandrite laser alone showed mixed results. However, improved outcome of treatment with QS alexandrite laser was observed when combined with pulsed CO2 laser.[79] The use of QS Nd:YAG laser was found effective in the treatment of melasma. In a study comprising 25 women with melasma, 44% of the women showed marked improvement with QS Nd:YAG treatment during a 2-month follow-up posttreatment.[83] Fractional laser photothermolysis is commonly used in the treatment of hyperpigmentation disorders. Rokhsar and Fitzpatrick examined the efficacy of fractional photothermolysis in the treatment of melasma and found that 60% of the patients showed 75–100% improvement.[85] Fractional photothermolysis was found to confer at least 20% improvement in six Chinese patients with resistant melasma.[84] Further, fractional photothermolysis was found effective in the treatment of PIH[87] and refractory arcuate hyperpigmentation.[86]
Table 2.
A summary of lasers in the treatment of hyperpigmentation disorders
Daily Photo-protection, a Way to Prevent Pigmentary Disorders
Since solar exposures have been clearly involved in several short- and long-term harmful effects on the skin including the development of pigmentary disorders, strategies to achieve an even tone complexion and treat skin hyperpigmentation include exogenous protection approaches such as photoprotection, for reducing the deleterious effects of UV exposure.[89] They comprise a battery of exogenous and complementary photoprotective cautions or items such as sun avoidance, clothing or use of broad-spectrum sunscreens. Clothing and hats have been proposed as an important photoprotective strategy[90] with some advantages such as the high compliance from patients. Studies by Gambichler et al. concluded that 67% of the 236 tested summer apparels offered a UV protection factor (UPF) value of 15 and above.[90] They also observed that 70% of the wool, polyester, and fabric blends brought UPF values of above thirty as compared to <30% of cotton, linen, and viscose fabrics. Other studies have shown that rather than the nature of the garment, their weaving (tight or not) is absolutely crucial.[91,92]
However, clothing does not offer protection against UV exposure to the uncovered anatomical sites such as a facial region for which the use of sunscreens remains the most efficient exogenous photoprotective strategy.
Two types of sunscreens have to be distinguished, i.e., organic or mineral-based sunscreens. The first ones act by strong absorption of wavelengths over a defined spectrum (in UVB or UVA or both), whereas those of mineral origin (titane dioxide and zinc oxide) reflect or diffuse UV rays along the all UV band.[93] This explains why these mineral-based sunscreens are most often combined with organic sunscreens to extend the broadness of protection. Currently used organic sunscreens comprise butyl methoxydibenzoylmethane (BMDM or avobenzone), terephthalylidene dicamphor sulfonic acid (TDSA or Mexoryl® SX), drometrizole trisiloxane (DTS or Mexoryl® XL), methylene bis-benzotriazolyl tetramethylbutylphenol and bis-ethylhexyloxyphenol methoxyphenyl triazine.[94] TDSA or Mexoryl® SX has been previously shown to prevent DNA damage,[95] photoaging,[96] UV induced-pigmentation,[97] and UV-induced immunosuppression.[98] Final sunscreen products are usually a combination of UVB and UVA absorbers (and mineral sunscreens) leading to an absorption profile against the majority of the UV spectrum. Broad-spectrum sunscreens (UVB-UVA) offer considerable protection against UV exposure, especially in daily UV exposure conditions, representative of realistic everyday life sun exposure conditions. This was illustrated by Marionnet et al., who assessed gene expression in reconstructed skin models following exposure to standard UV daylight spectrum (daily UV radiation [DUVR]) using quantitative polymerase chain reaction arrays.[99] DUVR exposure led to altered regulation of 35 genes in fibroblasts and 66 genes in keratinocytes, which were involved in a host of processes including oxidative stress response, cell growth, and inflammation among others. In contrast, DUVR-exposed models in the presence of a sunscreen product with sun protection factor of 13 and UVA protection factor of 10.5 showed a significant reduction in DUVR-induced alterations of gene expression. Regular use of broad spectrum sunscreen has been shown to be effective in reducing the development of squamous cell carcinoma as well as actinic keratoses[100] and clinical signs of photoaging.[101] Regarding pigmentary disorders, ICHOR has been shown being prevented using sunscreens.[48]
In India, high incidence of melasma in high-altitude, sun-exposed environments confirms that skin disorders often seen in darker skin phenotypes are related to UV exposure.[102] Further, the use of sunscreens has been shown to offer protection against hyperpigmentation disorders[103] The primary treatment of PIH aims at preventing and treating the underlying inflammatory condition. A study in Moroccan pregnant women demonstrated the effectiveness of photoprotection from melasma using sunscreen on the darker skin sites.[104] Only 3% of the 185 patients who used sunscreens developed melasma. As Indians present with several sun-induced damages including hyperpigmentation and photoaging,[10] the use of sunscreens is therefore of paramount importance in the Indian scenario. In a previous study with more than 300 Indian patients with melasma, only 10% of the patients were found to use sunscreens.[105] This emphasizes the need for greater awareness of UV-induced skin disorders and their prevention in the Indian society.
Future Outlooks
Hyperpigmentation disorders are common within the Indian population. Most of these disorders are attributed to or exacerbated by solar exposure. Photoprotection has been recommended as the best and primary strategy to achieve a flawless skin to inhibit the triggering events. One of the most efficient strategies to achieve an efficient photoprotection implies the regular use of a broad-spectrum sunscreen. In India, photoprotection is often ignored and hence regular sunscreen use is not followed. Indian population also presents a large variety of sun-induced damage including hyperpigmentation and photoaging. Campaigns to raise awareness of UV-induced skin hyperpigmentation are necessary to educate the general public and enhance the strict use of sunscreen for the management of these disorders.
Molecular characterization of the Indian skin needs to be carried out to develop better skin products targeted specifically to the Indian market. Testing of new ingredients that modulate pigmentation through controlled clinical trials would also be desirable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
The role of solar UV exposures in the development or exacerbation of pigmentary disorders is now indisputable. The contribution of the whole UV spectrum, including UVB, UVA2 and long UVA1 wavelengths is now evidenced up to the molecular level. A first line of prevention and treatment of hyperpigmented disorders is therefore a regular use of a broad spectrum photoprotection, together with education of the population.
References
- 1.Taylor SC, Cook-Bolden F, Rahman Z, Strachan D. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 Suppl):S98–106. doi: 10.1067/mjd.2002.120791. [DOI] [PubMed] [Google Scholar]
- 2.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 3.Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009;22:563–79. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Meredith P, Riesz J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol. 2004;79:211–6. doi: 10.1562/0031-8655(2004)079<0211:rcrqyf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Del Bino S, Ito S, Sok J, Nakanishi Y, Bastien P, Wakamatsu K, et al. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res. 2015;28:707–17. doi: 10.1111/pcmr.12410. [DOI] [PubMed] [Google Scholar]
- 6.Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169(Suppl 3):33–40. doi: 10.1111/bjd.12529. [DOI] [PubMed] [Google Scholar]
- 7.Jablonski NG, Chaplin G. Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962–8. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–99. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 9.Stokowski RP, Pant PV, Dadd T, Fereday A, Hinds DA, Jarman C, et al. A genomewide association study of skin pigmentation in a South Asian population. Am J Hum Genet. 2007;81:1119–32. doi: 10.1086/522235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hourblin V, Nouveau S, Roy N, de Lacharrière O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol. 2014;80:395–401. doi: 10.4103/0378-6323.140290. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee M, Mukerjee S, Sarkar-Roy N, Ghosh T, Kalpana D, Sharma AK. Polymorphisms of four pigmentation genes (SLC45A2, SLC24A5, MC1R and TYRP1) among eleven endogamous populations of India. J Genet. 2013;92:135–9. doi: 10.1007/s12041-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 12.Pasricha JS, Khaitan BK, Dash S. Pigmentary disorders in India. Dermatol Clin. 2007;25:343–52, viii. doi: 10.1016/j.det.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Dogra S, Sarangal R. Pigmentary disorders: An insight, Pigment International. 2014;1:5–7. [Google Scholar]
- 14.Ortonne JP, Passeron T, Srinivas C. In: Pigmentary Disorders, Prevention, Treatment and Cosmetics Contributions. Basic Science for Modern Cosmetic Dermatology. Srinivas C, Verschoore M, editors. New Delhi: Jaypee Brothers, Medical Publishers Pvt. Limited; 2015. pp. 75–90. [Google Scholar]
- 15.Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006;54(5 Suppl 2):S272–81. doi: 10.1016/j.jaad.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Katsambas A, Antoniou C. Melasma. Classification and treatment. J Eur Acad Dermatol Venereol. 1995;4:217–23. [Google Scholar]
- 17.Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: Histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228–37. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 18.Grimes PE. Melasma. Etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453–7. doi: 10.1001/archderm.131.12.1453. [DOI] [PubMed] [Google Scholar]
- 19.Gilchrest BA, Fitzpatrick TB, Anderson RR, Parrish JA. Localization of malanin pigmentation in the skin with Wood's lamp. Br J Dermatol. 1977;96:245–8. doi: 10.1111/j.1365-2133.1977.tb06132.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee AY. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28:648–60. doi: 10.1111/pcmr.12404. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC., Jr Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698–710. doi: 10.1016/s0190-9622(81)70071-9. [DOI] [PubMed] [Google Scholar]
- 22.Sivayathorn A. Melasma in orientals. Clin Drug Investig. 1995;10:34–40. [Google Scholar]
- 23.Achar A, Rathi SK. Melasma: A clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380–2. doi: 10.4103/0019-5154.84722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151–6. doi: 10.1111/j.1468-3083.2011.04430.x. [DOI] [PubMed] [Google Scholar]
- 25.Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, et al. The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol. 2006;154:1094–9. doi: 10.1111/j.1365-2133.2006.07179.x. [DOI] [PubMed] [Google Scholar]
- 26.Callender VD, St Surin-Lord S, Davis EC, Maclin M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87–99. doi: 10.2165/11536930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Lacz NL, Vafaie J, Kihiczak NI, Schwartz RA. Postinflammatory hyperpigmentation: A common but troubling condition. Int J Dermatol. 2004;43:362–5. doi: 10.1111/j.1365-4632.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 28.Davis EC, Callender VD. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Maldonado R, Orozco-Covarrubias ML. Postinflammatory hypopigmentation and hyperpigmentation. Semin Cutan Med Surg. 1997;16:36–43. doi: 10.1016/s1085-5629(97)80034-x. [DOI] [PubMed] [Google Scholar]
- 30.Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989;92:565–72. doi: 10.1111/1523-1747.ep12709595. [DOI] [PubMed] [Google Scholar]
- 31.Eimpunth S, Wanitphadeedecha R, Manuskiatti W. A focused review on acne-induced and aesthetic procedure-related postinflammatory hyperpigmentation in Asians. J Eur Acad Dermatol Venereol. 2013;27(Suppl 1):7–18. doi: 10.1111/jdv.12050. [DOI] [PubMed] [Google Scholar]
- 32.Ortonne JP. The effects of ultraviolet exposure on skin melanin pigmentation. J Int Med Res. 1990;18(Suppl 3):8C–17C. [PubMed] [Google Scholar]
- 33.Hölzle E. Pigmented lesions as a sign of photodamage. Br J Dermatol. 1992;127(Suppl 41):48–50. doi: 10.1111/j.1365-2133.1992.tb16989.x. [DOI] [PubMed] [Google Scholar]
- 34.Ber Rahman S, Bhawan J. Lentigo. Int J Dermatol. 1996;35:229–39. doi: 10.1111/j.1365-4362.1996.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 35.Bastiaens M, Hoefnagel J, Westendorp R, Vermeer BJ, Bouwes Bavinck JN. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17:225–9. doi: 10.1111/j.1600-0749.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 36.Dubin N, Pasternack BS, Moseson M. Simultaneous assessment of risk factors for malignant melanoma and non-melanoma skin lesions, with emphasis on sun exposure and related variables. Int J Epidemiol. 1990;19:811–9. doi: 10.1093/ije/19.4.811. [DOI] [PubMed] [Google Scholar]
- 37.Green A, Battistutta D. Incidence and determinants of skin cancer in a high-risk Australian population. Int J Cancer. 1990;46:356–61. doi: 10.1002/ijc.2910460303. [DOI] [PubMed] [Google Scholar]
- 38.Kricker A, Armstrong BK, English DR, Heenan PJ. Pigmentary and cutaneous risk factors for non-melanocytic skin cancer – A case-control study. Int J Cancer. 1991;48:650–62. doi: 10.1002/ijc.2910480504. [DOI] [PubMed] [Google Scholar]
- 39.Mehregan AH. Lentigo senilis and its evolutions. J Invest Dermatol. 1975;65:429–33. doi: 10.1111/1523-1747.ep12608175. [DOI] [PubMed] [Google Scholar]
- 40.Yamada T, Hasegawa S, Inoue Y, Date Y, Arima M, Yagami A, et al. Comprehensive analysis of melanogenesis and proliferation potential of melanocyte lineage in solar lentigines. J Dermatol Sci. 2014;73:251–7. doi: 10.1016/j.jdermsci.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Andersen WK, Labadie RR, Bhawan J. Histopathology of solar lentigines of the face: A quantitative study. J Am Acad Dermatol. 1997;36(3 Pt 1):444–7. doi: 10.1016/s0190-9622(97)80224-1. [DOI] [PubMed] [Google Scholar]
- 42.Cario-Andre M, Lepreux S, Pain C, Nizard C, Noblesse E, Taïeb A. Perilesional vs. lesional skin changes in senile lentigo. J Cutan Pathol. 2004;31:441–7. doi: 10.1111/j.0303-6987.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 43.Cardinali G, Kovacs D, Picardo M. Mechanisms underlying post-inflammatory hyperpigmentation: Lessons from solar lentigo. Ann Dermatol Venereol. 2012;139(Suppl 4):S148–52. doi: 10.1016/S0151-9638(12)70127-8. [DOI] [PubMed] [Google Scholar]
- 44.Montagna W, Hu F, Carlisle K. A reinvestigation of solar lentigines. Arch Dermatol. 1980;116:1151–4. [PubMed] [Google Scholar]
- 45.Lin CB, Hu Y, Rossetti D, Chen N, David C, Slominski A, et al. Immuno-histochemical evaluation of solar lentigines: The association of KGF/KGFR and other factors with lesion development. J Dermatol Sci. 2010;59:91–7. doi: 10.1016/j.jdermsci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Sheth PB, Shah HA, Dave JN. Periorbital hyperpigmentation: A study of its prevalence, common causative factors and its association with personal habits and other disorders. Indian J Dermatol. 2014;59:151–7. doi: 10.4103/0019-5154.127675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarma N, Chakraborty S, Bhattacharya SR. Acquired, idiopathic, patterned facial pigmentation (AIPFP) including periorbital pigmentation and pigmentary demarcation lines on face follows the lines of Blaschko on face. Indian J Dermatol. 2014;59:41–8. doi: 10.4103/0019-5154.123492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verschoore M, Gupta S, Sharma VK, Ortonne JP. Determination of melanin and haemoglobin in the skin of idiopathic cutaneous hyperchromia of the orbital region (ICHOR): A study of Indian patients. J Cutan Aesthet Surg. 2012;5:176–82. doi: 10.4103/0974-2077.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranu H, Thng S, Goh BK, Burger A, Goh CL. Periorbital hyperpigmentation in Asians: An epidemiologic study and a proposed classification. Dermatol Surg. 2011;37:1297–303. doi: 10.1111/j.1524-4725.2011.02065.x. [DOI] [PubMed] [Google Scholar]
- 50.Malakar S, Lahiri K, Banerjee U, Mondal S, Sarangi S. Periorbital melanosis is an extension of pigmentary demarcation line-F on face. Indian J Dermatol Venereol Leprol. 2007;73:323–5. doi: 10.4103/0378-6323.34009. [DOI] [PubMed] [Google Scholar]
- 51.Sinha RP, Häder DP. UV-induced DNA damage and repair: A review. Photochem Photobiol Sci. 2002;1:225–36. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 52.Tyrrell RM, Keyse SM. New trends in photobiology. The interaction of UVA radiation with cultured cells. J Photochem Photobiol B. 1990;4:349–61. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- 53.Vioux-Chagnoleau C, Lejeune F, Sok J, Pierrard C, Marionnet C, Bernerd F. Reconstructed human skin: From photodamage to sunscreen photoprotection and anti-aging molecules. J Dermatol Sci Suppl. 2006;2:S1–12. [Google Scholar]
- 54.Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol. 1997;183:123–38. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- 55.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–64. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 56.Atoyan RY, Sharov AA, Eller MS, Sargsyan A, Botchkarev VA, Gilchrest BA. Oligonucleotide treatment increases eumelanogenesis, hair pigmentation and melanocortin-1 receptor expression in the hair follicle. Exp Dermatol. 2007;16:671–7. doi: 10.1111/j.1600-0625.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 57.Bernerd F, Asselineau D. UVA exposure of human skin reconstructed in vitro induces apoptosis of dermal fibroblasts: Subsequent connective tissue repair and implications in photoaging. Cell Death Differ. 1998;5:792–802. doi: 10.1038/sj.cdd.4400413. [DOI] [PubMed] [Google Scholar]
- 58.Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293:576–83. doi: 10.1007/s00403-001-0271-1. [DOI] [PubMed] [Google Scholar]
- 59.Marionnet C, Pierrard C, Golebiewski C, Bernerd F. Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS One. 2014;9:e105263. doi: 10.1371/journal.pone.0105263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandra M, Levitt J, Pensabene CA. Hydroquinone therapy for post-inflammatory hyperpigmentation secondary to acne: Not just prescribable by dermatologists. Acta Derm Venereol. 2012;92:232–5. doi: 10.2340/00015555-1225. [DOI] [PubMed] [Google Scholar]
- 61.Spann CT. Ten tips for treating acne vulgaris in Fitzpatrick skin types IV-VI. J Drugs Dermatol. 2011;10:654–7. [PubMed] [Google Scholar]
- 62.Rossi AB, Leyden JJ, Pappert AS, Ramaswamy A, Nkengne A, Ramaswamy R, et al. A pilot methodology study for the photographic assessment of post-inflammatory hyperpigmentation in patients treated with tretinoin. J Eur Acad Dermatol Venereol. 2011;25:398–402. doi: 10.1111/j.1468-3083.2010.03798.x. [DOI] [PubMed] [Google Scholar]
- 63.Monteiro RC, Kishore BN, Bhat RM, Sukumar D, Martis J, Ganesh HK. A comparative study of the efficacy of 4% hydroquinone vs. 0.75% kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58:157. doi: 10.4103/0019-5154.108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40–8. [PubMed] [Google Scholar]
- 65.Griffiths CE, Finkel LJ, Ditre CM, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br J Dermatol. 1993;129:415–21. doi: 10.1111/j.1365-2133.1993.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 66.Kimbrough-Green CK, Griffiths CE, Finkel LJ, Hamilton TA, Bulengo-Ransby SM, Ellis CN, et al. Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch Dermatol. 1994;130:727–33. [PubMed] [Google Scholar]
- 67.Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, Finkel LJ, Hamilton TA, Ellis CN, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438–43. doi: 10.1056/NEJM199305203282002. [DOI] [PubMed] [Google Scholar]
- 68.Rafal ES, Griffiths CE, Ditre CM, Finkel LJ, Hamilton TA, Ellis CN, et al. Topical tretinoin (retinoic acid) treatment for liver spots associated with photodamage. N Engl J Med. 1992;326:368–74. doi: 10.1056/NEJM199202063260603. [DOI] [PubMed] [Google Scholar]
- 69.Kang S, Goldfarb MT, Weiss JS, Metz RD, Hamilton TA, Voorhees JJ, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: A randomized trial. J Am Acad Dermatol. 2003;49:83–90. doi: 10.1067/mjd.2003.451. [DOI] [PubMed] [Google Scholar]
- 70.Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: A double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45–50. [PubMed] [Google Scholar]
- 71.Taylor SC, Torok H, Jones T, Lowe N, Rich P, Tschen E, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67–72. [PubMed] [Google Scholar]
- 72.Chan R, Park KC, Lee MH, Lee ES, Chang SE, Leow YH, et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008;159:697–703. doi: 10.1111/j.1365-2133.2008.08717.x. [DOI] [PubMed] [Google Scholar]
- 73.Grimes P, Kelly AP, Torok H, Willis I. Community-based trial of a triple-combination agent for the treatment of facial melasma. Cutis. 2006;77:177–84. [PubMed] [Google Scholar]
- 74.Hexsel D, Hexsel C, Porto MD, Siega C. Triple combination as adjuvant to cryotherapy in the treatment of solar lentigines: Investigator-blinded, randomized clinical trial. J Eur Acad Dermatol Venereol. 2015;29:128–33. doi: 10.1111/jdv.12484. [DOI] [PubMed] [Google Scholar]
- 75.Ascenso A, Ribeiro H, Marques HC, Oliveira H, Santos C, Simões S. Is tretinoin still a key agent for photoaging management? Mini Rev Med Chem. 2014;14:629–41. doi: 10.2174/1389557514666140820102735. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar R, Chugh S, Garg VK. Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol. 2012;78:417–28. doi: 10.4103/0378-6323.98071. [DOI] [PubMed] [Google Scholar]
- 77.Konda S, Geria AN, Halder RM. New horizons in treating disorders of hyperpigmentation in skin of color. Semin Cutan Med Surg. 2012;31:133–9. doi: 10.1016/j.sder.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Jang WS, Lee CK, Kim BJ, Kim MN. Efficacy of 694-nm Q-switched ruby fractional laser treatment of melasma in female Korean patients. Dermatol Surg. 2011;37:1133–40. doi: 10.1111/j.1524-4725.2011.02018.x. [DOI] [PubMed] [Google Scholar]
- 79.Angsuwarangsee S, Polnikorn N. Combined ultrapulse CO2 laser and Q-switched alexandrite laser compared with Q-switched alexandrite laser alone for refractory melasma: Split-face design. Dermatol Surg. 2003;29:59–64. doi: 10.1046/j.1524-4725.2003.29009.x. [DOI] [PubMed] [Google Scholar]
- 80.Nouri K, Bowes L, Chartier T, Romagosa R, Spencer J. Combination treatment of melasma with pulsed CO2 laser followed by Q-switched alexandrite laser: A pilot study. Dermatol Surg. 1999;25:494–7. doi: 10.1046/j.1524-4725.1999.08248.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhou X, Gold MH, Lu Z, Li Y. Efficacy and safety of Q-switched 1,064-nm neodymium-doped yttrium aluminum garnet laser treatment of melasma. Dermatol Surg. 2011;37:962–70. doi: 10.1111/j.1524-4725.2011.02001.x. [DOI] [PubMed] [Google Scholar]
- 82.Choi M, Choi JW, Lee SY, Choi SY, Park HJ, Park KC, et al. Low-dose 1064-nm Q-switched Nd:YAG laser for the treatment of melasma. J Dermatolog Treat. 2010;21:224–8. doi: 10.3109/09546630903401462. [DOI] [PubMed] [Google Scholar]
- 83.Cho SB, Kim JS, Kim MJ. Melasma treatment in Korean women using a 1064-nm Q-switched Nd:YAG laser with low pulse energy. Clin Exp Dermatol. 2009;34:e847–50. doi: 10.1111/j.1365-2230.2009.03599.x. [DOI] [PubMed] [Google Scholar]
- 84.Naito SK. Fractional photothermolysis treatment for resistant melasma in Chinese females. J Cosmet Laser Ther. 2007;9:161–3. doi: 10.1080/14764170701418814. [DOI] [PubMed] [Google Scholar]
- 85.Rokhsar CK, Fitzpatrick RE. The treatment of melasma with fractional photothermolysis: A pilot study. Dermatol Surg. 2005;31:1645–50. doi: 10.2310/6350.2005.31302. [DOI] [PubMed] [Google Scholar]
- 86.Cho SB, Lee SJ, Kang JM, Kim YK, Oh SH. Treatment of refractory arcuate hyperpigmentation using a fractional photothermolysis system. J Dermatolog Treat. 2010;21:107–8. doi: 10.3109/09546630902936794. [DOI] [PubMed] [Google Scholar]
- 87.Katz TM, Goldberg LH, Firoz BF, Friedman PM. Fractional photothermolysis for the treatment of postinflammatory hyperpigmentation. Dermatol Surg. 2009;35:1844–8. doi: 10.1111/j.1524-4725.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 88.Taylor CR, Anderson RR. Ineffective treatment of refractory melasma and postinflammatory hyperpigmentation by Q-switched ruby laser. J Dermatol Surg Oncol. 1994;20:592–7. doi: 10.1111/j.1524-4725.1994.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang SQ, Balagula Y, Osterwalder U. Photoprotection: A review of the current and future technologies. Dermatol Ther. 2010;23:31–47. doi: 10.1111/j.1529-8019.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 90.Gambichler T, Rotterdam S, Altmeyer P, Hoffmann K. Protection against ultraviolet radiation by commercial summer clothing: Need for standardized testing and labelling. BMC Dermatol. 2001;1:6. doi: 10.1186/1471-5945-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sinclair SA, Diffey BL. Sun protection provided by ladies stockings. Br J Dermatol. 1997;136:239–41. [PubMed] [Google Scholar]
- 92.Menter JM, Hatch KL. Clothing as solar radiation protection. Curr Probl Dermatol. 2003;31:50–63. doi: 10.1159/000072237. [DOI] [PubMed] [Google Scholar]
- 93.Forestier S. Rationale for sunscreen development. J Am Acad Dermatol. 2008;58(5 Suppl 2):S133–8. doi: 10.1016/j.jaad.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 94.Bernerd F, Moyal D, Pai SB, Srinivas C. In: xPigmentary Disorders, Prevention, Treatment and Cosmetics Contributions. Basic Science for Modern Cosmetic Dermatology. Srinivas C, Verschoore M, editors. New Delhi: Jaypee Brothers, Medical Publishers Pvt. Limited; 2015. pp. 91–113. [Google Scholar]
- 95.Young AR, Sheehan JM, Chadwick CA, Potten CS. Protection by ultraviolet A and B sunscreens against in situ dipyrimidine photolesions in human epidermis is comparable to protection against sunburn. J Invest Dermatol. 2000;115:37–41. doi: 10.1046/j.1523-1747.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 96.Séite S, Moyal D, Richard S, de Rigal J, Lévêque JL, Hourseau C, et al. Mexoryl SX: A broad absorption UVA filter protects human skin from the effects of repeated suberythemal doses of UVA. J Photochem Photobiol B. 1998;44:69–76. doi: 10.1016/s1011-1344(98)00122-5. [DOI] [PubMed] [Google Scholar]
- 97.Moyal D. Prevention of ultraviolet-induced skin pigmentation. Photodermatol Photoimmunol Photomed. 2004;20:243–7. doi: 10.1111/j.1600-0781.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 98.Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from the suppression of the elicitation phase of delayed-type hypersensitivity response in humans. J Invest Dermatol. 2001;117:1186–92. doi: 10.1046/j.0022-202x.2001.01545.x. [DOI] [PubMed] [Google Scholar]
- 99.Marionnet C, Pierrard C, Lejeune F, Bernerd F. Modulations of gene expression induced by daily ultraviolet light can be prevented by a broad spectrum sunscreen. J Photochem Photobiol B. 2012;116:37–47. doi: 10.1016/j.jphotobiol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Burnett ME, Wang SQ. Current sunscreen controversies: A critical review. Photodermatol Photoimmunol Photomed. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 101.Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed. 2014;30:55–61. doi: 10.1111/phpp.12109. [DOI] [PubMed] [Google Scholar]
- 102.Singh G, Chatterjee M, Grewal R, Verma R. Incidence and care of environmental dermatoses in the high-altitude region of Ladakh, India. Indian J Dermatol. 2013;58:107–12. doi: 10.4103/0019-5154.108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pandya AG. Guevara IL Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91–8, ix. doi: 10.1016/s0733-8635(05)70150-9. [DOI] [PubMed] [Google Scholar]
- 104.Lakhdar H, Zouhair K, Khadir K, Essari A, Richard A, Seité S, et al. Evaluation of the effectiveness of a broad-spectrum sunscreen in the prevention of chloasma in pregnant women. J Eur Acad Dermatol Venereol. 2007;21:738–42. doi: 10.1111/j.1468-3083.2007.02185.x. [DOI] [PubMed] [Google Scholar]
- 105.KrupaShankar DS, Somani VK, Kohli M, Sharad J, Ganjoo A, Kandhari S, et al. A cross-sectional, multicentric clinico-epidemiological study of melasma in India. Dermatol Ther (Heidelb) 2014;4:71–81. doi: 10.1007/s13555-014-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


