Abstract
Background:
Cutaneous leishmaniasis (CL) usually occurs in areas with hot and dry climate. In India, the desert areas of Rajasthan, Gujarat, and the plains of Northwestern frontier are endemic for this disorder.
Aims and Objectives:
The present study was aimed to describe clinicoepidemiological profile of the cases of CL from South Rajasthan, which is a nonendemic area of Rajasthan.
Materials and Methods:
During a period of 4 years (2010–2014), a total of 23 patients with CL were diagnosed. All the suspected cases of CL were interrogated in detail regarding visit to areas where CL is known to occur. This was followed by clinical examination, relevant investigations, and treatment. All except one patient were treated with azole antifungals. In one patient, CO2 laser ablation was done.
Results:
There were 12 (52.17%) males and 11 (47.83%) females with age ranging from 3 to 72 years. Duration of disease ranged from 7 days to 10 months. Face (15; 65.22%) and extremities (12; 52.17%) were involved in majority of the patients. Common morphologies were noduloulcerative lesions and crusted plaques. Tissue smear for Leishmania donovani bodies was positive in all except one patient.
Conclusion:
The present report highlights occurrence of CL in nonendemic area. Further epidemiological studies are required for identification of vector and strain of Leishmania involved.
Keywords: Azole antifungals, cutaneous leishmaniasis, new focus, nonendemic area
Introduction
What was known?
Cutaneous leishmaniasis (CL) is common in certain endemic areas such as Western Thar Desert of Rajasthan
Antimonial agents are treatment of choice for CL.
Cutaneous leishmaniasis (CL) is a vector-borne protozoal infection of the skin caused by several species of Leishmania.[1] It is mainly transmitted by sandfly, Phlebotomus, of which more than 600 species have been identified. Phlebotomus papatasi is the most common vector for CL in India.[2] All over the world, there are about 1.5 million new cases of CL each year, of these over 90% are reported from seven countries, namely, Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.[3]
In India, indigenous cases of CL, anthroponotic, and zoonotic are mainly confined to hot and dry Northwestern region and are endemic in the Western Thar Desert of Rajasthan.[4] To the best of our knowledge, CL has not previously been reported from Udaipur. Herein, we report the clinical and epidemiological features of cases of CL from South Rajasthan with the aim of documenting the new focus.
Materials and Methods
The study was conducted in skin department at a teaching hospital of South Rajasthan. Spanning over a period of 4-year (2010–2014), a total of 23 patients with CL were enrolled. After obtaining demographic information, all the suspected cases of CL were interrogated in detail regarding visit to areas where CL is known to occur. Diagnosis of CL was made on the basis of clinical presentation, demonstration of amastigotes in Giemsa-stained smear of the lesions, or skin biopsy. In one patient, the diagnosis was confirmed by biopsy because amastigotes were not seen in tissue smear. All the patients were treated with azole antifungals except for one patient who underwent CO2 laser ablation. Liver enzymes were assessed at baseline, after 1 month, 3 months, and 6 months.
Results
Twenty-three patients, 12 males (52.17%) and 11 females (47.83%), were diagnosed as a case of CL. The details are summarized in Table 1. The age of the patients ranged from 3 to 72 years. The majority (21; 91.3%) of patients belonged to Udaipur district and adjacent villages. Two patients belonged to other districts. Eleven patients showed clustering and belonged to two adjoining areas as depicted in the map [Figure 1]. Duration of disease at the time of presentation ranged from 7 days to 10 months. Only two patients gave a definite history of visit to a known endemic region. One patient revealed history of similar lesions in a family member. The most common involved site was face (15; 65.22%) and extremities (12; 52.17%). The lesions were either noduloulcerative lesions or crusted plaques [Figures 2a and 3a]. Number of lesions ranged from 1 to 5. Twelve (52.17%) patients presented with single lesion while remaining 11 patients had 2–5 lesions. The size of lesions varied from 1 to 4 cm. A definite history of insect bite was revealed by two patients. Mucosal involvement over lip extended from cutaneous lesion was found in three patients. All the patients were subjected to smear examination, which was positive in 22 patients [Figure 4]. In one patient, the diagnosis was confirmed by biopsy because amastigotes were not seen in tissue smear. All except one patient were treated with azole antifungals. In one patient, CO2 laser ablation was done. The treatment was continued for 15 days to 1 month after clearance of lesions. Most patients showed regression of lesions over a period of 5–7 months [Figures 2b and 3b]. Scarring was seen in all but four patients. Three patients were lost to follow-up. On telephonic inquiry, they reported improvement in lesions without recurrence. None of the remaining patients reported relapse of the disease activity during a follow-up of 6 months.
Table 1.
Clinicoepidemiological profile of patients
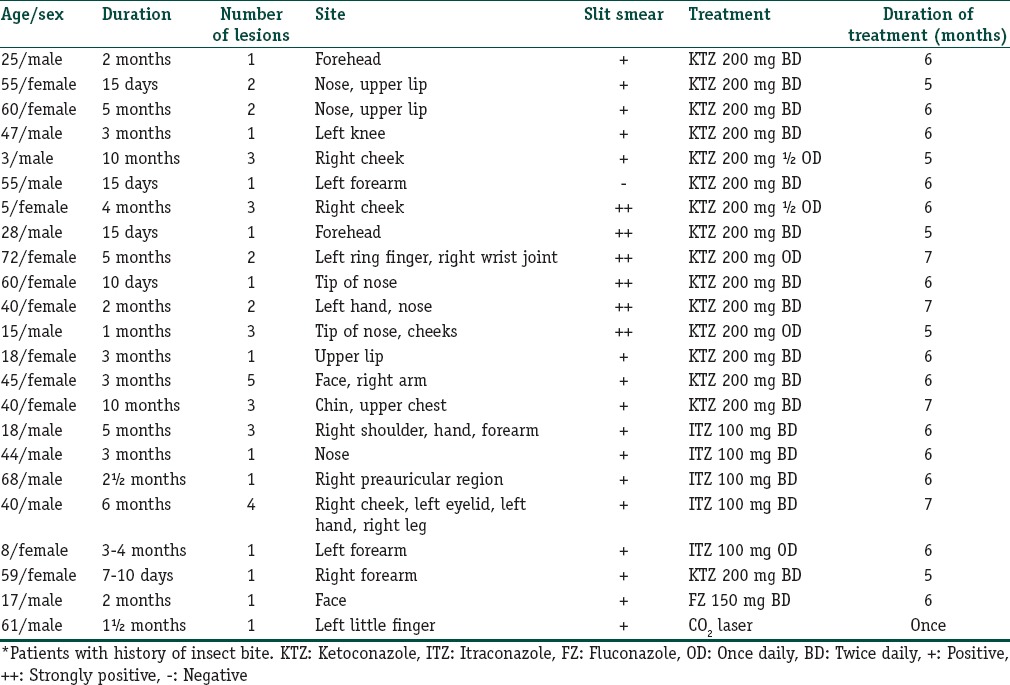
Figure 1.
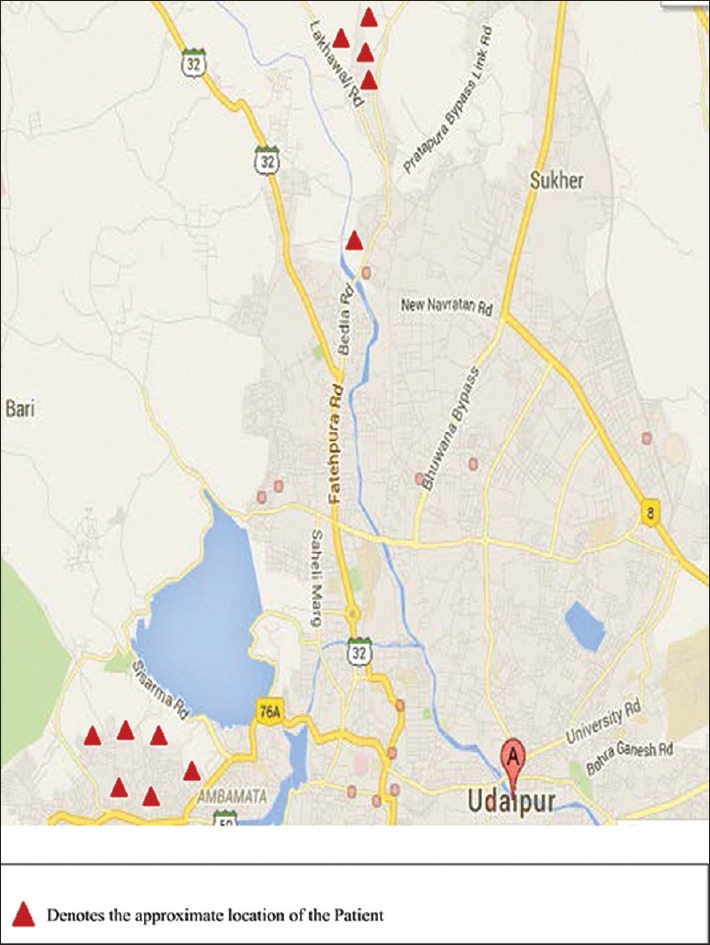
Map of Udaipur (Rajasthan) showing clustering of cases of cutaneous leishmaniasis patients in two adjoining areas
Figure 2.
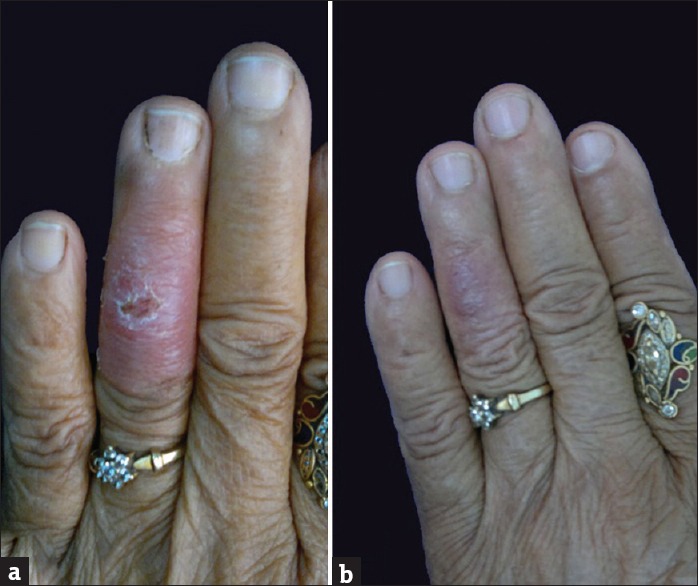
Crusted plaque over left ring finger (a) and 7 months posttreatment (b)
Figure 3.
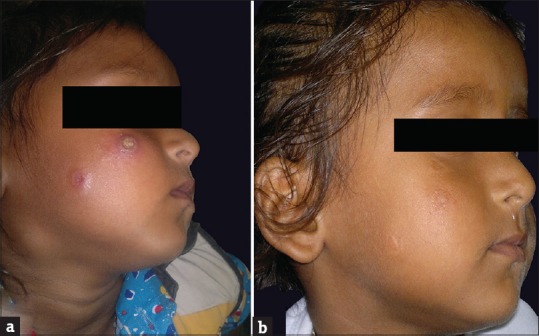
Noduloulcerative lesions over face, pretreatment (a) and 5 months posttreatment (b)
Figure 4.
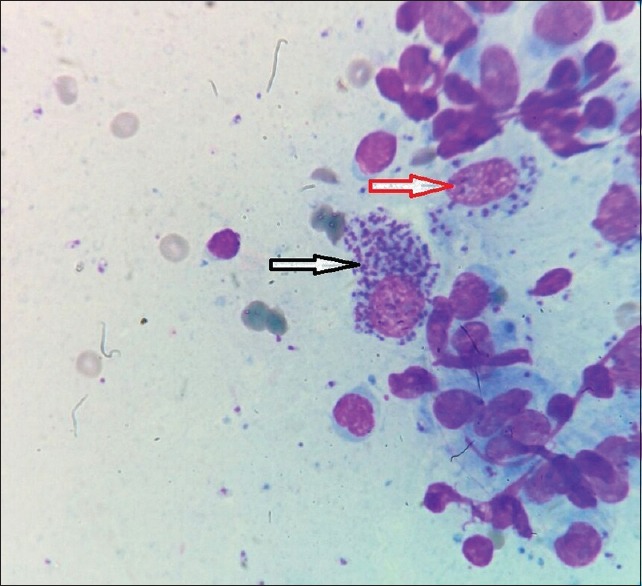
Slit skin smear showing multiple Leishmania donovani bodies within (red arrow) and outside (black arrow) macrophages (Giemsa, ×100)
Discussion
In India, surveys carried out during 1973 in whole of erstwhile endemic areas revealed the presence of sporadic cases in Fazilka (Punjab), Rajasthan canal zone at Hanumangarh (Sriganganagar district), Jodhpur city, and rural and urban areas of Bikaner district in Rajasthan.[5] Cases have also been reported from Assam,[6] Kerala,[7] Haryana,[8] and Himachal Pradesh.[9,10] CL has not been known to occur in South Rajasthan, and to the best of our knowledge, no reports are available so far from Udaipur district and adjoining areas. The patients evaluated in the current study were confined to Udaipur district and adjoining areas. Only two patients gave a definite history of visit to a known endemic region for the past 1 year. Epidemics of CL have been associated with deforestation, road construction, or any other activity, in which humans intrude in the habitat of the vector. Adaptation of the vector as well as the parasite to strange, often bizarre habitats and hosts is also well known.[2]
Various morphological forms of CL have been described,[11] of which noduloulcerative lesion and crusted plaque are most common. Similar observation was made in our cases also. Atypical clinical presentations of CL are increasingly seen nowadays. It is recommended that CL should be included in the differential diagnosis of common dermatological diseases such as erysipelas, chronic eczema, herpes zoster, and paronychia and uncommon disorders such as lupus vulgaris, squamous cell carcinoma, sporotrichosis, mycetoma, and other deep mycoses.[12] Demonstration of parasite in direct smears remains the easiest and the most specific method of diagnosis. A range of 30–96.23% smear positivity has been reported in the literature.[13] This depends on the age of lesions – younger lesions being more likely to yield the parasite.[14] In our study, a majority (69.6%) of the lesions were of 15 days to 3 months duration and tissue smear positivity was 95.6% which is in accordance with the study by Khatri et al.[15] However, in one patient with lesion of 15 days duration, tissue smear was negative and required confirmation of diagnosis by biopsy.
The various therapeutic modalities for the treatment of CL include physical agents, drugs such as antimonials,[9] azole antifungals,[16,17,18] amphotericin B,[19] and miltefosine,[20] and surgical procedures, but still there is no single uniformly effective treatment. Many of these drugs are not readily available and are also costly. Most of our patients were treated by azole antifungals either ketoconazole or itraconazole, and both of these were found to be efficacious without any side effects.
This study is of great epidemiological interest on account of focus of CL in nonendemic region of Rajasthan. Interestingly, there are no published data on exact incidence/prevalence of CL from Rajasthan. However, an article by Alvar et al.[21] on worldwide and global estimate of incidence of leishmaniasis in 98 countries has quoted a figure of 156 CL cases per year as shown in Table 1 from the Department of skin, STD, and leprosy, Bikaner, during a 5-year period (2005–2009).
Limitations of the study
The study population was small and the identification of the species of Leishmania and Phlebotomus involved could not be done.
Conclusion
The present report highlights a new focus of CL in a previously nonendemic area of South Rajasthan. Since no parasite isolation and characterization was carried out, further epidemiological studies are required for identification of vector and strain of Leishmania involved. Our 23 patients cannot be considered as true representation of magnitude of CL in our region because only suspected cases were recorded and we could have missed many nonsuspected Leishmania patients. Newer diagnostic modalities such as polymerase chain reaction may help in confirming the diagnosis of CL in other cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
We report a newer focus of cutaneous leishmaniasis (CL) that needs to be further investigated
Oral azole antifungals are still a promising agent in the treatment of CL.
References
- 1.Rastogi V, Nirwan PS. Cutaneous leishmaniasis: An emerging infection in a non-endemic area and a brief update. Indian J Med Microbiol. 2007;25:272–5. doi: 10.4103/0255-0857.34774. [DOI] [PubMed] [Google Scholar]
- 2.Kerdel-Vegas F, Harman R, Kerdel F, Dutta AK. In: Protozoan infections II. Clinical Tropical Dermatology. 2nd ed. Canizares O, Harman RC, editors. Massachusetts: Blackwell Science; 1992. pp. 293–312. [Google Scholar]
- 3.WHO. Cutaneous leishmaniasis: An overview: Symposium. J Postgrad Med. 2003;49:50. doi: 10.4103/0022-3859.928. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Control of the Leishmaniasis. WHO Technical Report Series 793. Geneva: WHO; 1990. pp. 66–94. [Google Scholar]
- 5.Sehgal S, Mittal V, Bhatia R. Manual on laboratory techniques in leishmaniasis. 2nd ed. Delhi: NICD; 1989. pp. 2–4. [Google Scholar]
- 6.Baishya BR, Hazarika NK. Cutaneous leishmaniasis in Assam. Indian J Dermatol Venereol Leprol. 1996;62:40. [PubMed] [Google Scholar]
- 7.Muhammed K, Narayani K, Aravindan KP. Indigenous cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 1990;56:228–9. [Google Scholar]
- 8.Verma KC, Bhargava NC, Joshi RK. Cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 1979;45:341–3. [PubMed] [Google Scholar]
- 9.Sharma RC, Mahajan VK, Sharma NL, Sharma A. A new focus of cutaneous leishmaniasis in Himachal Pradesh (India) Indian J Dermatol Venereol Leprol. 2003;69:170–2. [PubMed] [Google Scholar]
- 10.Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, et al. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in Himachal Pradesh, India. Am J Trop Med Hyg. 2005;72:819–24. [PubMed] [Google Scholar]
- 11.Momeni AZ, Aminjavaheri M. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int J Dermatol. 1994;33:260–5. doi: 10.1111/j.1365-4362.1994.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 12.Bari AU, Rahman SB. Many faces of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2008;74:23–7. doi: 10.4103/0378-6323.38402. [DOI] [PubMed] [Google Scholar]
- 13.Mashhood AA, Ahmad S, Malik MM. Is slit-skin smear an alternate to histopathology in the diagnosis of cutaneous leishmaniasis? J Pak Assoc Dermatologists. 2014;24:34–9. [Google Scholar]
- 14.Kubba R, Al-Gindan Y. Some recent observations in cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 1989;55:7–17. [PubMed] [Google Scholar]
- 15.Khatri ML, Di Muccio T, Gramiccia M. Cutaneous leishmaniasis in North-Western Yemen: A clinicoepidemiologic study and Leishmania species identification by polymerase chain reaction-restriction fragment length polymorphism analysis. J Am Acad Dermatol. 2009;61:e15–21. doi: 10.1016/j.jaad.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Alsaleh QA, Dvorak R, Nanda A. Ketoconazole in the treatment of cutaneous leishmaniasis in Kuwait. Int J Dermatol. 1995;34:495–7. doi: 10.1111/j.1365-4362.1995.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 17.al-Fouzan AS, al Saleh QA, Najem NM, Rostom AI. Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole. Int J Dermatol. 1991;30:519–21. doi: 10.1111/j.1365-4362.1991.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 18.Dogra J, Saxena VN. Itraconazole and leishmaniasis: A randomised double-blind trial in cutaneous disease. Int J Parasitol. 1996;26:1413–5. doi: 10.1016/s0020-7519(96)00128-2. [DOI] [PubMed] [Google Scholar]
- 19.Goyonlo VM, Vosoughi E, Kiafar B, Nahidi Y, Momenzadeh A, Taheri AR. Efficacy of intralesional amphotericin B for the treatment of cutaneous leishmaniasis. Indian J Dermatol. 2014;59:631. doi: 10.4103/0019-5154.143571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madke B, Kharkar V, Chikhalkar S, Mahajan S, Khopkar U. Successful treatment of multifocal cutaneous leishmaniasis with miltefosine. Indian J Dermatol. 2011;56:587–90. doi: 10.4103/0019-5154.87165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]


