Abstract
Background:
Herpes zoster (HZ) is a dermatomal viral infection, caused by reactivation of varicella zoster virus (VZV) that persists in the posterior root ganglion. HZ is uncommonly reported in immunocompetent children. It may be due to intrauterine VZV infection or secondary to postnatal exposure to VZV at an early age.
Aims:
Our study was to review clinico-epidemiological data for HZ in children for early diagnosis and treatment to prevent complications.
Materials and Methods:
A prospective observational study was conducted from January 2013 to December 2014. Consecutive cases clinically diagnosed as HZ in the pediatric age group were taken up.
Results:
We report the clinico-epidemiological study of 26 cases of HZ, their benign course and recovery among children.
Conclusions:
HZ is a rare disease in childhood. Varicella in early childhood is a risk factor of HZ in immunocompromised and immunocompetent children. Childhood zoster occurs in either healthy or underlying immunodeficient children. The appearance of HZ in a young child does not always imply an underlying immunodeficiency or malignancy. But the identification of HZ with or without immunodeficiency is of prime importance from the treatment and prognostic point of view and should be considered in the differential diagnosis of vesicular eruptions. The prognosis is generally good in healthy children.
Keywords: Children, herpes zoster, varicella zoster virus
Introduction
What was known?
HZ in children implies immunodefficiancy or malignancy. Anti viral therapy depends on the age and immune status of the child. The prognosis is generally good both in healthy and in HIV-reactive children with CD 4 count above 350/cumm.
Varicella zoster virus (VZV) has a high level of infectivity and has a worldwide prevalence. Herpes zoster is a re-activation of latent VZV infection. Herpes zoster (HZ) can occur at any time after varicella infection or varicella vaccination. But the incidence of zoster is less after varicella vaccination than after natural infection. The incidence of zoster increases with age, although children who had varicella during the first year of life (or in utero) are at increased risk of developing zoster. It is diagnosed clinically by unilateral vesicular eruption involving a dermatome or dermatomes.
Materials and Methods
A prospective observational study was conducted from January 2013 to December 2014 in Hyderabad city. Consecutive cases clinically diagnosed as HZ, in pediatric age group, attending DVL OPD were taken up. Inclusion criteria: All clinically diagnosed cases of HZ in children were taken up for the study. Exclusion criteria: All above 18 years of age were excluded from the study. Objectives: Our study is to review clinico-epidemiological data for HZ for early diagnosis and treatment to minimize complications.
Results
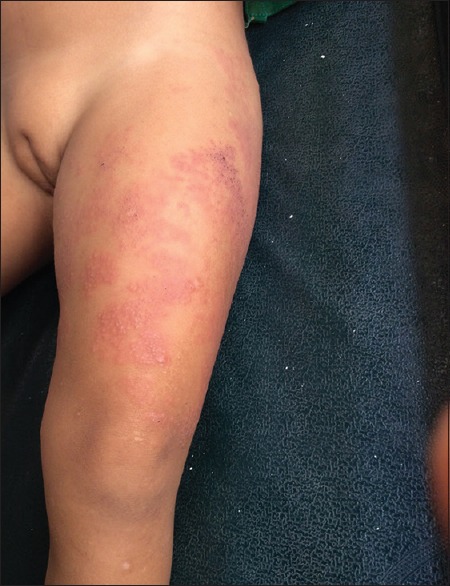
A total number of 26 cases of HZ presented to our OPD over a period of 2 years. There were no symptoms and signs of primary immunodeficiency disorders like recurrent or atypical microbial infections in any case. There was no history of taking any immunosuppressive medication like systemic steroids or anti-cancer drugs. Out of 26 cases, 54% (14) were females and 46% (12) were male children [Chart 1]. 69% (18 children) were between the age group of 2 and 12 years and 31% (8) were above the age of 12 years. The lowest age was 3 years [Figure 1] and the highest was 17 years [Chart 2]. Out of 26, 30% (8) children were vaccinated, 54% (14) were not vaccinated and in 16% (4) children, history of vaccination was not known [Chart 3]. In 54% (14) children, there was a history of varicella infection and in 46% (12), there was no history of infection in early age [Chart 4]. There was no history of prodromal symptoms or mild prodromal symptoms like fever and lassitude between the age group of 2 and 12 years (18 cases), whereas prominent prodromal symptoms were seen in above 12 years (8 cases) of age [Chart 5]. The lesions were multiple, grouped vesicles on erythematous base involving unilateral, single dermatome. Mild pain and burning sensation were observed in the age group of 2 to 12 years whereas moderate pain and burning sensation were seen in above 12 years of age [Chart 6]. Thoracic dermatomal involvement [Figure 3] was observed in 54% (14) cases [Chart 7]. In remaining 12 cases, there is involvement of head 15.3% (4), upper limbs [Figure 2] 15.3% (4) and lower limbs [Figure 4] 15.3% (4). In a 14-year-old child, there was involvement of left external ear and face but there were no symptoms and signs of 7th and 8th cranial nerve involvement [Figure 5]. In an another case, a 17-year-old child presented with vesicular lesions on the nose and left side of the face but it was not associated with ophthalmic complications [Figure 6]. The illness was of short duration and resolved in less than two to three weeks. Tzanck test revealed multinucleated giant cells [Figure 7]. HSV1 serology for IgM was negative in all cases. HIV 1 was reactive in four cases and HIV 1 and 2 were non-reactive in remaining 22 cases. CD 4 count was above 350/cumm in HIV-reactive cases. All cases were diagnosed as HZ based on detailed history and clinical examination. They were treated symptomatically with antipyretics and calamine lotion. Above 12 years of age acyclovir 20 mg/kg/qid for 7 days was given in 8 children who reported within 72 hours [Chart 8]. There was complete resolution of the lesions without any sequelae in 2 to 3 weeks in all the children. Post herpetic neuralgia was not observed in any child during follow up.
Chart 1.
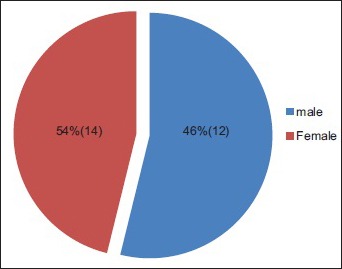
Out of 26 cases, 14 were females, 12 were males
Figure 1.
Multiple, grouped vesicles on erythematous base involving unilateral, single dermatome in a 3-year-old child
Chart 2.
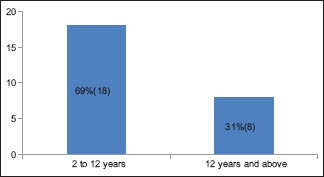
Age distribution – the youngest patient was 3 years and the oldest was 17 years. Above 12 years were 8 children
Chart 3.
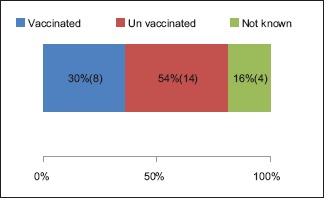
Status of vaccination
Chart 4.
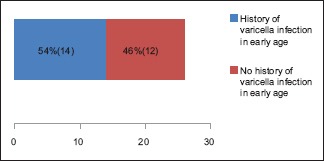
History of postnatal varicella infection in early age was seen in 54%
Chart 5.
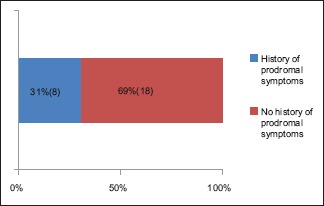
Intraherpetic burning pain and constitutional symptoms
Chart 6.
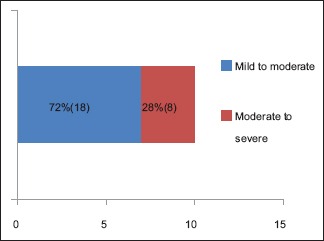
Severity of symptoms based on age - Mild to moderate between the age group of 2 to 12 years and Moderate to severe in above 12 years of age
Figure 3.
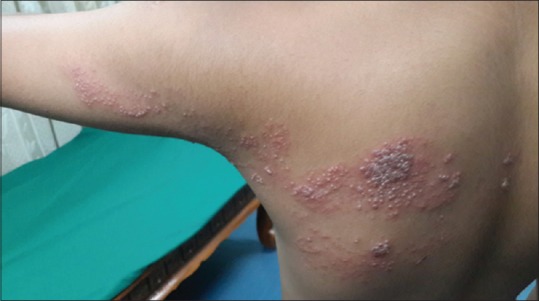
HZ on trunk in a 10-year-old male child
Chart 7.
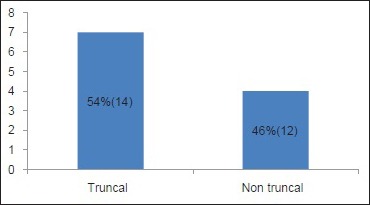
Site of involvement
Figure 2.
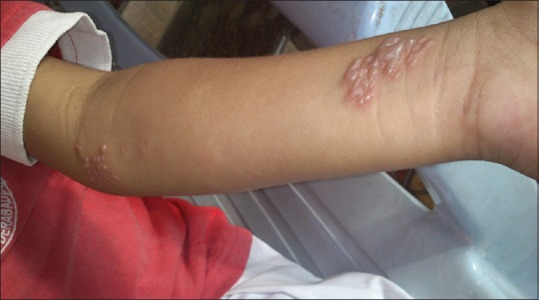
HZ on upper limb in a 5-year-old female child
Figure 4.
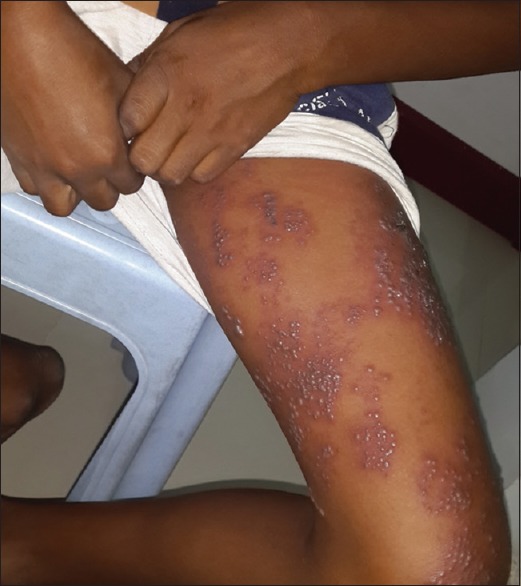
HZ on left thigh in an 11-year-old male child
Figure 5.
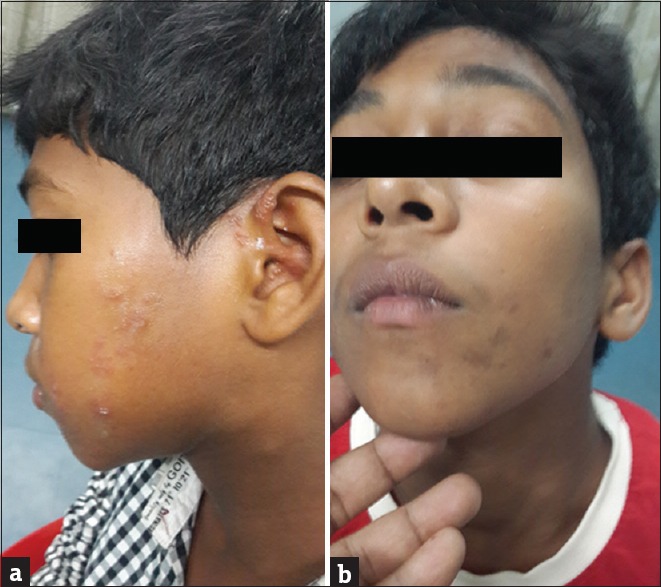
(a) HZ on face with external ear involvement in a 14-year-old male child. (b) After 2 months of treatment only post inflammatory hyperpigmentation was seen
Figure 6.
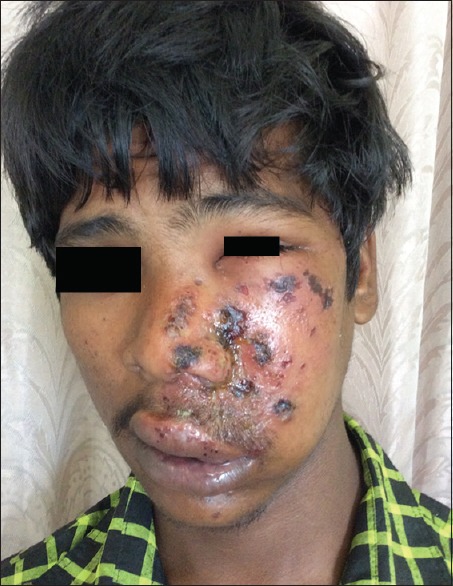
HZ on face with involvement on nose in a 17-year-old male child
Figure 7.
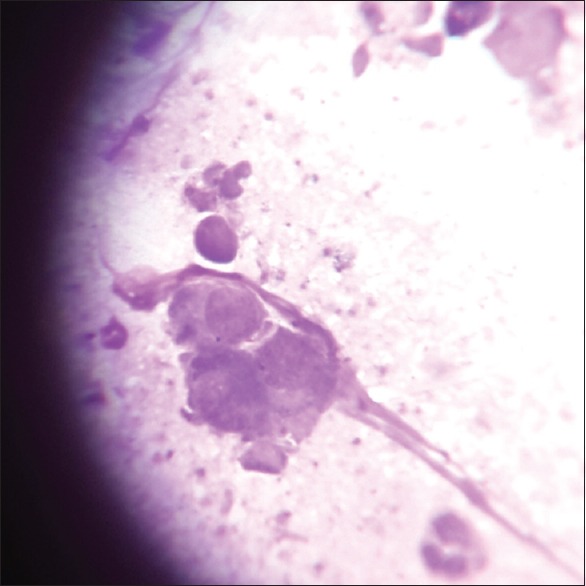
Tzanck test showed multinucleated giant cells (Giemsa stain, ×100)
Chart 8.
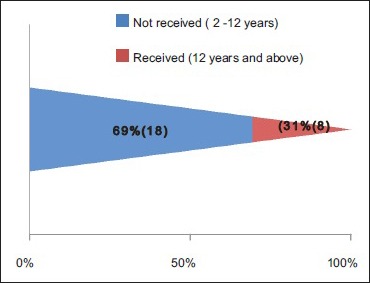
Antiviral therapy: Supportive and symptomatic treatment- in all children. Children between 2 to 12 years of age- Not had given antiviral therapy. Children above 12 years of age- Acyclovir 20 mg/kg/qid for 7 days
Discussion
Primary varicella is usually a disease of childhood, whereas its reactivation (HZ) occurs in adults. The incidence of HZ is only 0.45 per 1000 in children below 14 years of age. The incidence of HZ ranges from 1.2 to 3.4 cases per 1000 healthy individuals which are increasing to 3.9–11.8 per year per among those older than 65 years.[1,2,3] HZ in the elderly is associated with loss of varicella-zoster virus (VZV)-specific cellular immunity, whereas in chemotherapy, suppression of cellular immunity occurs. Viral destruction of T cells may occur in HIV-infected individuals.[4]
Historically, childhood HZ was thought to be an indicator of an underlying malignancy, especially acute lymphatic leukemia, whereas recent studies have shown no increase in the incidence of malignancy in children with HZ as reported in our study. Approximately 3% of HZ cases occur in children with malignancies.[5] Rising incidence of HZ in healthy children may be due to acquiring primary varicella infection in utero, or in infancy, wherein the immunity is not fully developed, as seen in our study where 54% were not vaccinated and in 16% children history of vaccination was not known. Vaccination with live attenuated virus may also contribute to development of HZ. Tereda et al. stated that the immunological status at the time of acquiring the primary infection is the most important factor in childhood HZ. A low level of lymphocytes, natural killer (NK) cells and cytokines are seen in infants along with virus-specific immunoglobulins that may result in an inability to maintain the latency of VZV, leading to early appearance of zoster in children.[6]
A diagnosis of HZ is made by detailed clinical examination and confirmed by lab diagnosis by doing a Tzanck smear of scrapings from the floor of the vesicles that reveal multinucleated giant cells on direct microscopy. The other methods are by direct fluorescent antibody tests, presence of high or rising titers to VZV, or by culture studies.[7] Direct fluorescent monoclonal antibody test or detection of serum specific IgM by the indirect fluorescent antibody method is also used to confirm HZ. Ideally, in childhood HZ, lymphocyte counts, CD4/CD8 ratio, and serum immunoglobulin levels have also to be estimated to rule out undetected concurrent immunosuppression. The severity of clinical manifestations of HZ is mainly dependent on the age of the child and CD 4 count even though HIV serology is positive as seen in our study where the CD 4 count (immune status) was above 350/cumm. HIV infection may not lead to AIDS by taking care of one's immune system from the beginning by changing lifestyle such as fresh air, protected water, balanced diet, sound sleep and positive attitude. Malnutrition is one of the earliest and major complications of HIV infection and a significant factor in advanced disease. If we prevent NAIDS (nutritionally acquired immunodeficiency syndrome), we can postpone AIDS though they are HIV reactive.
In general, the course of the disease is milder in children, the mean duration being 1–3 weeks. In children between the age group of 2 and 12 years, acute neuropathic pain was not observed which the hallmark of HZ in adults. Though lesional pruritus and pain may be present, the incidence of post herpetic neuralgia is negligible which the most common complication of HZ in adults. So far, almost all the reported series and isolated case reports have stressed upon the fact that childhood zoster is a relatively mild disease with negligible prodromal symptoms, post herpetic neuralgia or other significant complications.
It is imperative that HZ be differentiated from zosteriform herpes simplex by monomorphic vesicular lesions in the latter which is more common in children. The other common differentials of dermatomal vesicular eruptions in children are bullous impetigo and bullous insect bite reaction.
Treatment
Treatment options are based on the patient's age, immune status, duration of symptoms, and presentation. Several studies indicate that antiviral medications decreased the duration of symptoms and the likelihood of post herpetic neuralgia, especially when initiated within 3 days of the onset of rash. In children between the age group of 2 and 12 years, who are otherwise healthy, oral acyclovir need not be prescribed. An important study by Kubeyinje suggested that the use of acyclovir in healthy young children with zoster is not clearly justified, especially in situ ations of limited economic resources.[8]
The aims of treatment are to limit the severity and duration of pain, shorten the duration of a HZ episode, and reduce complications. Symptomatic treatment is often needed for the complication of post herpetic neuralgia. However, a study on untreated HZ shows that, once the rash has cleared, post herpetic neuralgia is very rare.[9]
Antiviral drugs may reduce the severity and duration of HZ.[10] However, they do not prevent post herpetic neuralgia.[11] Of these drugs, acyclovir has been the standard treatment, but the new drugs valaciclovir and famciclovir demonstrate similar or superior efficacy and good safety and tolerability.[9] The drugs may be used both for prevention (for example in HIV/AIDS) and as therapy during the acute phase. Complications in immunocompromised individuals with HZ may be reduced with intravenous acyclovir. In people who are at a high risk for repeated attacks of shingles, five daily oral doses of acyclovir are usually effective.[12]
The first line of therapy in childhood HZ is oral acyclovir, given at a dose of 20–40 mg/kg body weight, four times a day.[13] Patients with HIV infection are at risk of developing severe illness from either varicella or zoster. Progressive primary varicella, a syndrome with persistent new lesion formation and visceral dissemination, may occur in HIV-infected patients and may be life threatening. Though many studies have been done in adult HIV patients, so far there are only few case reports of childhood HIV patients acquiring zoster.
Prevention
There is a live vaccine for VZV.[14] It must be maintained at a temperature not exceeding –15 °C during storage, although it can be stored and transported at refrigerator temperature for up to 72 continuous hours before reconstitution. The incidence of side effects is low with the vaccine. The lower age limit for vaccination is after first year of age but there is no recommended upper age limit.[15] The vaccine reduced incidence of persistent, severe pain after shingles (i.e., PHN) by 66% in people who contracted shingles despite vaccination.[15] Duration of protection was not known as on 2013. Evidence suggested that protection persists for up to 7 years. The need for revaccination had not been defined.[15] An episode of HZ has an immunizing effect, greatly reducing the probability of a subsequent recurrence.[15] It has been recommended that people with primary or acquired immunodeficiency should not receive the vaccine.[15] The likelihood of vaccination causing a case of HZ appears to be very low.[15]
Prognosis
The rash and pain usually subside within 2 to 3 weeks, but about one in five patients develop a painful condition called post herpetic neuralgia, which is often difficult to manage. In some patients, HZ can reactivate presenting as zoster sine herpete: Pain radiating along the path of a single spinal nerve but without an accompanying rash. This condition may involve complications that affect several levels of the nervous system and cause many cranial neuropathies, polyneuritis, myelitis, or aseptic meningitis. Other serious effects that may occur in some cases include partial facial paralysis (usually temporary), ear damage, and/or encephalitis.[12] During pregnancy, first infections with VZV, causing chickenpox, may lead to infection of the fetus and complications in the newborn, but chronic infection or reactivation in shingles are not associated with fetal infection.[16,17]
There is a slightly increased risk of developing cancer after a HZ infection. However, the mechanism is unclear and mortality from cancer did not appear to increase as a direct result of the presence of the virus.[18] Instead, the increased risk may result from the immune suppression that allows the reactivation of the virus.[19]
Although HZ typically resolves within 2 to 3 weeks, there are certain complications of secondary bacterial infection, motor nerve involvement. Eye involvement and trigeminal nerve involvement may arise. They should be treated early and aggressively as it may lead to blindness or paralysis. Involvement of the tip of the nose in the zoster rash is a strong predictor of herpes ophthalmicus.[20]
Conclusion
Herpes zoster is an uncommon disease in childhood. Varicella in early childhood is a risk factor for herpes zoster either in immunocompromised or immunocompetent children. Childhood zoster occurs in either healthy or underlying immunodeficient children. The appearance of herpes zoster in a young child does not always imply an underlying immunodeficiency or malignancy. But the identification of herpes zoster with or without immunodeficiency is of prime importance for the treatment and prognostic point of view and should be considered in the differential diagnosis of vesicular eruptions. The prognosis was generally good both in healthy and in HIV-reactive children with CD 4 count above 350/cumm in our study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
What is new?
Appearance of herpes zoster in children does not always imply an underlying immunodeficiency or malignancy. HZ though uncommon in children, needs high index of suspicion and may be considered in the differential diagnosis of vesicular eruptions.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 2.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 3.Araújo LQ, Macintyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes. 2007;14(Suppl 2):40A–4A. [PubMed] [Google Scholar]
- 4.Kurlan JG, Connelly BL, Lucky AW. Herpes zoster in the first year of life following postnatal exposure to Varicella-zoster virus: Four case reports and a review of infantile herpes zoster. Arch Dermatol. 2004;140:1268–72. doi: 10.1001/archderm.140.10.1268. [DOI] [PubMed] [Google Scholar]
- 5.Janniger CK, Driano NA. E-medicine article. [Last accessed on 2007 Jan 16]. Available from: http://www.emedicine.com/ped/topic996.htm .
- 6.Bhushan P, Sardana K, Mahajan S. Dermatomal vesicular eruption in an asymptomatic infant. Dermatol Online J. 2005;11:26. [PubMed] [Google Scholar]
- 7.Solomon AR. New diagnostic tests for herpes simplex and varicella zoster infection. J Am Acad Dermatol. 1988;18:218–21. doi: 10.1016/s0190-9622(88)70032-8. [DOI] [PubMed] [Google Scholar]
- 8.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: Epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–23. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyring SK. Management of herpes zoster and postherpetic neuralgia. J Am Acad Dermatol. 2007;57(6 Suppl):S136–42. doi: 10.1016/j.jaad.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Bader MS. Herpes zoster: Diagnostic, therapeutic, and preventive approaches. Postgrad Med. 2013;125:78–91. doi: 10.3810/pgm.2013.09.2703. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2(2):D006866. doi: 10.1002/14651858.CD006866.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RW, Dworkin RH. Clinical review: Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748–50. doi: 10.1136/bmj.326.7392.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–10. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 14.Harpaz R, Ortega-Sanchez IR, Seward JF Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1. [PubMed] [Google Scholar]
- 15.Shapiro M, Kvern B, Watson P, Guenther L, McElhaney J, McGeer A. Update on herpes zoster vaccination: A family practitioner's guide. Can Fam Physician. 2011;57:1127–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Paryani SG, Arvin AM. Intrauterine infection with varicella-zoster virus after maternal varicella. N Engl J Med. 1986;314:1542–6. doi: 10.1056/NEJM198606123142403. [DOI] [PubMed] [Google Scholar]
- 17.Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: Prospective study of 1739 cases. Lancet. 1994;343:1548–51. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen HT, Olsen JH, Jepsen P, Johnsen SP, Schønheyder HC, Mellemkjaer L. The risk and prognosis of cancer after hospitalisation for herpes zoster: A population-based follow-up study. Br J Cancer. 2004;91:1275–9. doi: 10.1038/sj.bjc.6602120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Risk of cancer after herpes zoster: A population-based study. N Engl J Med. 1982;307:393–7. doi: 10.1056/NEJM198208123070701. [DOI] [PubMed] [Google Scholar]
- 20.Herpes Zoster Ophthalmicus. Merck Manual (Merk.com) 2008. Oct, [Last retrieved on 2010 Jun]. pubmed.


