Abstract
IMPORTANCE
The use of anticholinergic (AC) medication is linked to cognitive impairment and an increased risk of dementia. To our knowledge, this is the first study to investigate the association between AC medication use and neuroimaging biomarkers of brain metabolism and atrophy as a proxy for understanding the underlying biology of the clinical effects of AC medications.
OBJECTIVE
To assess the association between AC medication use and cognition, glucose metabolism, and brain atrophy in cognitively normal older adults from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Indiana Memory and Aging Study (IMAS).
DESIGN, SETTING, AND PARTICIPANTS
The ADNI and IMAS are longitudinal studies with cognitive, neuroimaging, and other data collected at regular intervals in clinical and academic research settings. For the participants in the ADNI, visits are repeated 3, 6, and 12 months after the baseline visit and then annually. For the participants in the IMAS, visits are repeated every 18 months after the baseline visit (402 cognitively normal older adults in the ADNI and 49 cognitively normal older adults in the IMAS were included in the present analysis). Participants were either taking (hereafter referred to as the AC+ participants [52 from the ADNI and 8 from the IMAS]) or not taking (hereafter referred to as the AC− participants [350 from the ADNI and 41 from the IMAS]) at least 1 medication with medium or high AC activity. Data analysis for this study was performed in November 2015.
MAIN OUTCOMES AND MEASURES
Cognitive scores, mean fludeoxyglucose F 18 standardized uptake value ratio (participants from the ADNI only), and brain atrophy measures from structural magnetic resonance imaging were compared between AC+ participants and AC− participants after adjusting for potential confounders. The total AC burden score was calculated and was related to target measures. The association of AC use and longitudinal clinical decline (mean [SD] follow-up period, 32.1 [24.7] months [range, 6–108 months]) was examined using Cox regression.
RESULTS
The 52 AC+ participants (mean [SD] age, 73.3 [6.6] years) from the ADNI showed lower mean scores on Weschler Memory Scale–Revised Logical Memory Immediate Recall (raw mean scores: 13.27 for AC+ participants and 14.16 for AC− participants; P = .04) and the Trail Making Test Part B (raw mean scores: 97.85 seconds for AC+ participants and 82.61 seconds for AC− participants; P = .04) and a lower executive function composite score (raw mean scores: 0.58 for AC+ participants and 0.78 for AC− participants; P = .04) than the 350 AC− participants (mean [SD] age, 73.3 [5.8] years) from the ADNI. Reduced total cortical volume and temporal lobe cortical thickness and greater lateral ventricle and inferior lateral ventricle volumes were seen in the AC+ participants relative to the AC− participants.
CONCLUSIONS AND RELEVANCE
The use of AC medication was associated with increased brain atrophy and dysfunction and clinical decline. Thus, use of AC medication among older adults should likely be discouraged if alternative therapies are available.
Anticholinergic (AC) medications have been linked to impaired cognition1–16 primarily in nondemented older adults10,17 and an increased risk for cognitive impairment and dementia in older adults.1,3,4,18–20 The biological basis for the cognitive effects of AC medications is unknown. However, given the importance of the cholinergic system in cognition, researchers speculate that direct impairment of cholinergic neurons may underlie these effects. In fact, previous studies21,22 using scopolamine hydrobromide, a cholinergic antagonist, have shown transient cognitive impairment in young and older adults. A recent study23 suggested that administration of AC medications modulates the association between brain volume and cognition. However, to our knowledge, no studies have examined the effects of regular AC medication use on neuroimaging measures of brain structure and function in cognitively normal (CN) older adults.
The goal of the present study was to assess AC medication use in CN older adults from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). In particular, we sought to evaluate whether cognitive performance, brain glucose hypometabolism, structural brain atrophy, and clinical progression to mild cognitive impairment (MCI) and/or Alzheimer disease (AD) were associated with the use of AC medication. We also completed a similar analysis in an independent cohort of CN older adults from the Indiana Memory and Aging Study(IMAS). We hypothesized that participants taking AC medications (hereafter referred to as AC+ participants) would show poorer cognition, reduced glucose metabolism, brain atrophy, and increased clinical decline relative to those not taking AC medications (hereafter referred to as AC− participants) and that these effects would be greatest in those with the highest total AC burden score.
Methods
Alzheimer’sDisease Neuroimaging Initiative
Data used in the preparation of this article were obtained from the ADNI (http://adni.loni.usc.edu; for more information, see the eAppendix in the Supplement,http://www.adni-info.org, and previous reports24–29). Written informed consent was obtained according to the Declaration of Helsinki.30
Indiana Memory and Aging Study
The IMAS includes CN participants, participants with subjective cognitive decline, participants with MCI, and participants with AD, but only data from CN participants and participants with subjective cognitive decline were used for this analysis. Participants provided written informed consent according to the Declaration of Helsinki,30 and the procedures were approved by the Indiana University Committee for the Protection of Human Subjects.
AC Medications
Medication logs from the ADNI and the IMAS were manually curated to identify medications with low, medium, or high AC effects as defined by the Anticholinergic Cognitive Burden (ACB) scale and other reports.18,31–33 See eTable 1 in the Supplement for all medications identified. To be defined as an AC+ participant, participants had to have been taking the medication at the baseline visit for a minimum of 1 month. The total AC burden score was also calculated using the ACB scale, which uses the literature to guide an expert-based determination of the adverse cognitive AC activities (low effect = 1, medium effect = 2, and high effect = 3). The total AC burden score was the sum of ACB scores of all applicable medications taken by a participant.4,6,8 See eTable 2 in the Supplement for medications included in calculating the total AC burden score.
Participants
A total of 402 CN participants from ADNI 1, ADNI Grand Opportunity, and ADNI 2, including 301 CN participants without significant memory concerns and 101 CN participants with significant memory concerns, were included in the present analysis. A diagnosis was made as previously described34,35 and as in the ADNI 2 manual (http://www.adni-info.org/Scientists/doc/ADNI2_Procedures_Manual_20130624.pdf). Participants were divided by AC medication use into those taking 1 or more medications with medium or high AC activity (AC+ participants) and those not taking any such medications (AC− participants), resulting in 52 AC+ participants and 350 AC− participants. There was no significant difference in the rates of AC use between CN participants with significant memory concerns and those without, nor was there a significant effect of diagnosis (with or without significant memory concerns) or of the interaction between diagnosis and AC use on clinical progression.
The CN participants with or without subjective cognitive decline from the IMAS were also evaluated as an independent replication sample. Participants were CN if they had normal cognition relative to demographically adjusted norms and no significant self- or informant-based cognitive complaints. Participants had subjective cognitive decline if they had normal cognition and self- and/or informant-based complaints. From the IMAS, there were 8 AC+ participants and 41 AC− participants.
Cognitive Testing
The ADNI participants underwent a comprehensive cognitive and clinical battery. We assessed the effect of AC use on executive function (Trail Making Test Part B [TMT-B], a composite executive function score36) and memory (Weschler Memory Scale–Revised Logical Memory Immediate and Delayed, a composite memory score37).
Participants from the IMAS received a battery of neuropsychological tests and cognitive concern questionnaires, most of which have been previously described.38 After pread-justing for age, sex, and education, combined z scores (relative to the complete IMAS CN group) were generated for 3 domains: executive function, memory, and general cognition. We then assessed the effect of AC use on the z scores of these 3 domains.
Fluorodeoxyglucose F 18 Positron Emission Tomography
Preprocessed fluorodeoxyglucose F 18–positron emission tomographic (FDG-PET) scans (coregistered, averaged, standardized image and voxel size, and uniform resolution) were downloaded from the ADNI Laboratory of Neuroimaging (LONI) site (http://adni.loni.usc.edu) and processed as previously described.25,34 Mean standardized uptake value ratios (SUVRs) were extracted from 2 regions of interest, including a bilateral hippocampal region of interest39 and an overall cortical region of interest representing regions where CN participants show greater glucose metabolism than participants with AD from the full ADNI 1 cohort. Seventy-three participants were excluded from FDG-PET analyses for missing data. The IMAS participants did not undergo FDG PET.
Structural Magnetic Resonance Imaging
Baseline structural 3-T magnetic resonance imaging (MRI) scans were downloaded from LONI for ADNI 2 participants; ADNI 1 participants were excluded because their scans were collected on 1.5-T scanners using a different protocol. Scans were corrected prior to downloading as previously described.24 After downloading, we processed the scans using FreeSurfer version 5.134,35 to extract target measures of atrophy selected for known relevance in cognitive function and AD (temporal lobe, ventricle volume, and total cortex). If 2 MRI scans were available, the values from both scans were averaged. A total of 116 ADNI participants were excluded from this analysis owing to missing data. The IMAS participants underwent structural magnetization-prepared rapid acquisition gradient-echo scans on a Siemens 3T Tim Trio using the ADNI sequence. Similar to ADNI, scans were processed using FreeSurfer version 5.1 to extract the same atrophy measures. Two participants were excluded owing to missing data.
Confounding Effects of Medical History and Medication Use
Because the observed effects may potentially be caused by overall morbidity in AC+ participants, we evaluated the effect of the total number of medications, the total number of common comorbid conditions, and the presence or absence of each comorbid condition. The comorbid conditions tested included transient ischemic attack, myocardial infarction, cardiac surgery, hypertension, hyperlipidemia, diabetes, sleep apnea, other vascular disorders, insomnia, depression, anxiety, attention-deficit/hyperactivity disorder, and other psychiatric disorders. First, we determined whether there was a difference between AC+ participants and AC− participants regarding medical history and medication use (Table). Next, we determined whether these variables were associated with the outcome variables. Finally, we included those variables that were either different between AC+ participants and AC− participants or associated with an outcome in the general linear model assessing the effect of AC medication use on cognitive and imaging measures. Only those that were significant within the final general linear model were included (covariates reported in the Results). Furthermore, we randomly selected samples matched on medical history variables (52 AC− participants and 52 AC+ participants) and ran similar analyses.
Table.
Demographic Characteristics and Medical Histories of 402 Participants From the ADNI
| Characteristic | Participants, No. | P Value | |
|---|---|---|---|
| AC− (n = 350) |
AC+ (n = 52) |
||
| Age, mean (SD), y | 73.3 (5.8) | 73.3 (6.6) | .96 |
| Sex | |||
| Male | 171 | 18 | .06 |
| Female | 179 | 34 | |
| Education, mean (SD), y | 16.4 (2.6) | 16.1 (2.7) | .40 |
| Handedness | |||
| Right | 318 | 50 | .20 |
| Left | 32 | 2 | |
| APOE ε4 positive, % of participants | 28.0 | 25.0 | .65 |
| Non-Hispanic white, % of participants | 84.6 | 94.2 | .06 |
| Medications, mean (SD), Total No. | 4.2 (2.8) | 6.7 (3.1) | <.001 |
| Comorbid conditions, mean (SD), Total No. | 1.8 (1.3) | 2.2 (1.5) | .03 |
| Transient ischemic attack | |||
| No | 341 | 51 | .78 |
| Yes | 9 | 1 | |
| Myocardial infarction | |||
| No | 325 | 51 | .15 |
| Yes | 25 | 1 | |
| Cardiac surgery | |||
| No | 330 | 50 | .58 |
| Yes | 20 | 2 | |
| Hypertension | |||
| No | 193 | 24 | .23 |
| Yes | 157 | 28 | |
| Hyperlipidemia | |||
| No | 181 | 29 | .59 |
| Yes | 169 | 23 | |
| Diabetes | |||
| No | 324 | 49 | .67 |
| Yes | 26 | 3 | |
| Sleep apnea | |||
| No | 334 | 49 | .70 |
| Yes | 16 | 3 | |
| Other vascular conditions (eg, atrial fibrillation) | |||
| No | 327 | 47 | .42 |
| Yes | 23 | 5 | |
| Anxiety | |||
| No | 342 | 47 | .01 |
| Yes | 8 | 5 | |
| Depression | |||
| No | 306 | 37 | .002 |
| Yes | 44 | 15 | |
| Insomnia | |||
| No | 338 | 46 | .01 |
| Yes | 12 | 6 | |
| ADD or ADHD | |||
| No | 348 | 52 | .59 |
| Yes | 2 | 0 | |
| Other psychiatric condition (eg, posttraumatic stress disorder) | |||
| No | 348 | 52 | .56 |
| Yes | 2 | 0 | |
| Concussion | |||
| No | 331 | 48 | .51 |
| Yes | 19 | 4 | |
Abbreviations: AC+, participant taking anticholinergic medication with medium or high anticholinergic activity; AC−, participant not taking anticholinergic medication; ADD, attention-deficit disorder; ADHD, attention-deficit/ hyperactivity disorder; ADNI, Alzheimer’s Disease Neuroimaging Initiative.
Statistical Analysis
In the ADNI, cross-sectional measures of cognitive performance, glucose metabolism, and brain atrophy were compared between AC+ participants and AC− participants using a general linear model preadjusted for age, sex, and Aβ positivity (yes/no; defined using previously established cutoffs on either cerebrospinal fluid sample [Aβ1–42 < 192 mg/mL]40 or cortical florbetapir F-18 SUVR [SUVR≥1.1],41 years of education [included in analyses of cognitive variables only], and total intracranial volume [included in analyses of MRI variables only] using the residuals of a linear regression model). After checking normality, we determined that the TMT-B score, the FDG SUVR in the cortical region of interest, and the inferior lateral ventricle and lateral ventricle volumes were skewed. Using log transformations, we normalized the TMT-B scores and FDG SUVR in the overall cortical region-of-interest variables, while the ventricular volumes were normalized using a square root transformation. These transformed variables were used in the statistical analyses to test the effect of AC medication use. All other variables were normally distributed, so untransformed values are reported. The statistical threshold for significance was set at P < .05.
Associations between the total AC burden score and cognitive performance, glucose metabolism, and brain atrophy measures were evaluated using Spearman correlation models. Target cognitive and imaging variables were preadjusted for age, sex, Aβ positivity, education, medical history variables (see Results), and total intracranial volume as appropriate.
Finally, a Cox regression model was used to determine whether AC medication use was associated with clinical progression from CN to MCI and/or AD in the ADNI cohort (mean [SD] follow-up period, 32.1 [24.7] months [range, 6–108 months]), covaried for age, sex, medical history variables (see Results), and Aβ positivity. We also looked at the interaction of AC medication use and Aβ positivity on clinical progression.
Cognitive performance and brain atrophy measures were compared between AC+ participants and AC− participants in the IMAS to replicate the results observed in the ADNI. All measures showed a normal distribution. A general linear model was used to assess the effect of AC medication use in the IMAS, co-varied for age, sex, education, and total intracranial volume as appropriate. Associations between the total AC burden score and cognitive performance and brain atrophy measures were also evaluated using Spearman correlation models. No medical history variables were found to be significant covariates in the IMAS.
Results
Cognitive Performance
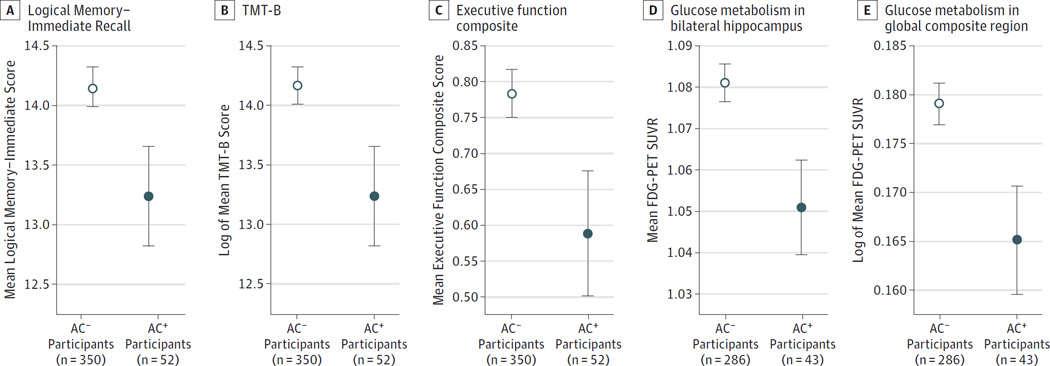
No significant differences in age, sex, education, ethnicity/ race, or APOE ε4 genotype were observed between AC+ participants and AC− participants in either sample (Table; see eTable 3 in the Supplement for the demographic characteristics of the IMAS participants). Of the medical variables examined, only the total number of medications, the total number of comorbid conditions, anxiety, and depression were different between AC+ participants and AC− participants (P < .05). Significant effects of AC medication use on the mean Logical Memory–Immediate score (raw mean scores: 13.27 for AC+ participants and 14.16 for AC− participants; P = .04 [Figure 1A]), the mean TMT-B score (raw mean scores: 97.85 seconds for AC+ participants and 82.61 seconds for AC− participants; P = .04 [Figure 1B], with transient is-chemic attack as an additional covariate), and the mean composite executive function score (raw mean scores: 0.58 for AC+ participants and 0.78 for AC− participants; P = .04 [Figure 1C], with transient ischemic attack, myocardial infarction, and diabetes as additional covariates) were found, with AC+ participants showing lower scores than AC− participants. The mean Logical Memory–Delayed Memory score (raw mean scores: 12.40 for AC+ participants and 13.24 for AC− participants; P = .07) and the mean memory composite score (raw mean scores: 0.85 for AC+ participants and 0.93 for AC− participants; P = .11 [data not shown]) trended toward significance, with AC+ participants showing lower scores than AC− participants. In the IMAS, the general mean cognition z score was significantly reduced for the AC+ participants relative to the AC− participants (raw mean scores: −1.27 for AC+ participants and −0.34 for AC− participants; P = .03 [eFigure 1 in the Supplement]).
Figure 1. Association of Anticholinergic (AC) Medication Use With Cognition and Glucose Metabolism Among Participants From the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Cognitively normal older adults taking 1 or more medications with medium or high AC activity (referred to as AC+ participants [n = 52]) showed poorer cognition than those not taking these medications (referred to as AC− participants [n = 350]), including a lower score on the Weschler Memory Scale–Revised Logical Memory Immediate Recall (P = .04 [A]), the Trail Making Test Part B (TMT-B) (P = .04 [B]), and an executive function composite (P = .04, with transient ischemic attack, myocardial infarction, and diabetes as additional covariates [C]). Glucose hypometabolism, as measured by the fluorodeoxyglucose F 18–positron emission tomographic (FDG-PET) standardized uptake value ratio (SUVR), was also observed in the bilateral hippocampus (P = .02, with anxiety as an additional covariate [D]) and in a global cortical region of interest of AC+ participants (n = 43) relative to AC− participants (n = 286), generated from an analysis of cognitively normal participants who show greater glucose metabolism than participants with AD from the full ADNI 1 cohort (P = .03, with other vascular conditions and concussion as additional covariates [E]). Error bars indicate SD.
FDG Positron Emission Tomography
Differences in glucose metabolism between AC+ participants and AC− participants were observed, with the AC+ participants showing reduced glucose metabolism in the hippocampus (raw mean values: 1.06 for the AC+ participants and 1.08 for AC− participants; P = .02 [Figure 1D], with anxiety as an additional covariate) and the global FDG-PET region of interest (raw mean values: 1.48 for AC+ participants and 1.52 for AC− participants; P = .03 [Figure 1E], with concussion and other vascular diseases as additional covariates) relative to AC− participants.
Structural MRI
A significant effect of AC medication use on brain structure was also observed. The AC+ participants demonstrated reduced total cortical volume (raw mean values: 406134.21 mm3 for AC+ participants and 423107.01 mm3 for AC− participants; P = .02 [Figure 2A]) and larger lateral ventricle (raw mean values: 17880.19 mm3 for AC+ participants and 15620.22 mm3 for AC− participants; P = .01 [Figure 2B]) and inferior lateral ventricle volumes (raw mean values: 757.25 mm3 for A C+ participants and 571.49 mm3 for AC− participants; P < .001 [Figure 2C]) relative to the AC− participants. Regional effects were also observed in the temporal lobe, with AC+ participants showing a reduced temporal lobe cortical thickness (raw mean values: 2.80 mm for AC+ participants and 2.84 mm for AC− participants; P = .02 [Figure 2D], with concussion as an additional covariate) and a reduced medial temporal lobe (MTL) cortical thickness (raw mean values: 3.10 mm for AC+ participants and 3.15 mm for AC− participants; P = .02 [Figure 2E], with concussion and cardiac surgery as additional covariates) relative to AC− participants. In the IMAS, the AC+ participants had a reduced MTL cortical thickness (raw mean values: 2.91 mm for AC+ participants and 3.10 mm for AC− participants; P = .01 [eFigure 2A in the Supplement]) and showed a trend toward thinner bilateral temporal lobe cortices (raw mean values: 2.69 mm for AC+ participants and 2.81 mm for AC− participants; P = .05 [eFigure 2B in the Supplement]) compared with the AC− participants.
Figure 2. Effect of Anticholinergic (AC) Medication Use on Brain Atrophy Measures.
Cognitively normal older adults taking 1 or more medications with medium or high anticholinergic activity (referred to as AC+ participants [n = 35]) showed more brain atrophy than participants not taking these medications (referred to as AC participants [n = 251]). Reduced total cortex volume (P = .02 [A]), increased bilateral lateral ventricle volume (P = .01 [B]), and increased inferior lateral ventricle volume (P < .001 [C]) were observed in AC+ participants relative to AC participants. Furthermore, reduced bilateral temporal lobe (P = .02, with concussion as an additional covariate [D]) and medial temporal lobe (P = .02, with concussion and cardiac surgery as additional covariates [E]) cortical thicknesses were also observed. Error bars indicate SD.
Association of Total AC Burden Score With Cognition and Brain Atrophy
Significant associations of the total AC burden score with cognition and brain atrophy were observed. Specifically, a higher total AC burden score was associated with a poorer TMT-B performance (r = 0.137; P = .01 [Figure 3A], with transient ischemic attack and total number of medications additional as co-variates) and greater inferior lateral ventricle (r = 0.126; P = .03 [Figure 3B]) and lateral ventricle volumes (r = 0.154; P = .01 [Figure 3C]). The inferior lateral ventricle volume remained significantly associated with the total AC burden score after excluding participants with a total AC burden score of 0 (r = 0.331; P < .001 [Figure 3E]). The TMT-B score (r = 0.146; P = .06 [Figure 3D]) and the lateral ventricle volume (r = 0.152; P = .10 [Figure 3F]) showed nonsignificant trend associations with the total AC burden score after excluding those participants with a total AC burden score of 0.
Figure 3. Association of Total Anticholinergic (AC) Burden Score and Brain Atrophy.
The total AC burden score was significantly associated with both cognition and brain atrophy. Specifically, a higher total AC burden score was associated with poorer performance on the Trail Making Test Part B (TMT-B) (r = 0.137; P = .01, with transient ischemic attack and total number of medications as additional covariates [A]) and greater inferior lateral ventricle (r = 0.126; P = .03 [B]) and lateral ventricle volumes (r = 0.145; P = .01 [C]). Inferior lateral ventricle volume was still significantly associated with the total AC burden score after excluding participants with a total AC burden score of 0 (r = 0.331; P < .001 [E]). The TMT-B score (r = 0.146; P = .06 [D]) and lateral ventricle volume showed nonsignificant trend associations with the total AC burden score after excluding those with a total AC burden score of 0 (r = 0.152; P = .10 [F]).
In the IMAS, the pattern of results was similar, although mostly nonsignificant trends were observed owing to attenuated power. Specifically, a higher total AC burden score was associated with reduced general cognition and atrophy (eFigure 3 in the Supplement). A trend for a negative association between the total AC burden score and general cognition across all participants (r = −0.239; P = .10 [eFigure 3A in the Supplement]) was observed, which was significant after excluding those with a total AC burden score of 0 (r = −0.625; P = .004 [eFigure 3C in the Supplement]). A negative association was observed between the total AC burden score and MTL cortical thickness (r = −0.313; P = .03 [eFigure 3B in the Supplement]), which only trended toward significant after excluding those with a total AC burden score of 0 (r = −0.428; P = .07 [eFigure 3D in the Supplement]).
Association of AC Use With Future Progression
A significant association between AC medication use and future progression of ADNI participants to MCI and/or AD was observed (P = .01; hazard ratio, 2.47 [Figure 4A]; with total number of medications, cardiac surgery, total number of comorbid conditions, and other psychiatric conditions as additional covariates). After evaluating the interaction between AC medication use and Aβ positivity, we observed that AC+ participants who are Aβ positive showed the highest risk of conversion relative to AC− participants who are Aβ negative (P < .001; hazard ratio, 7.73 [Figure 4B]; with cardiac surgery and other psychiatric conditions as additional covariates) or those who are positive for either AC medication use or Aβ (P = .001; hazard ratio, 4.24 [Figure 4B]).
Figure 4. Effect of Anticholinergic (AC) Medication Use on Clinical Conversion.
A, A significant association between AC use and future progression of Alzheimer’s Disease Neuroimaging Initiative participants to mild cognitive impairment and/or Alzheimer disease was observed (P = .01; hazard ratio [HR], 2.47; with total number of medications, cardiac surgery, total number of comorbid conditions, and other psychiatric conditions as additional covariates).
B, When evaluating the interaction between AC use and Aβ positivity, we found that participants taking 1 or more medications with medium or high AC activity who are positive for Aβ on florbetapir F-18–positron emission tomographic (PET) scans or cerebrospinal fluid (CSF) samples (referred to as AC+ and Aβ+ participants) showed a higher risk of conversion relative to participants not taking these medications who are negative for Aβ on florbetapir F-18–PET scans or CSF samples (referred to as AC and Aβ participants) (P < .001; HR, 7.73; with cardiac surgery and other psychiatric conditions as additional covariates) and participants who are positive for either AC use or Aβ (P = .001; HR, 4.24).
Matched Sample
In the matched sample, the AC+ participants showed reduced total cortex volumes (raw mean values: 406134.21 mm3 for AC+ participants and 417770.60 mm3 for AC− participants; P = .01), increased inferior lateral ventricle volumes (raw mean values: 757.25 mm3 for AC+ participants and 583.62 mm3 for AC− participants; P = .02), and an increased likelihood for clinical conversion (P = .01; hazard ratio, 3.87 [data not shown]) compared with the AC− participants. The AC+ participants also showed a trend toward poorer Logical Memory–Immediate performance (raw mean values: 13.27 for AC+ participants and 14.42 for AC− participants; P = .08) and increased lateral ventricle volumes (raw mean values: 17880.19 mm3 for AC+ participants and 15164.28 mm3 for AC− participants; P = .10 [data not shown]) compared with the AC− participants.
Discussion
Use of medications with medium or high AC effects in the ADNI cohort was associated with poorer cognition (particularly in immediate memory recall and executive function), reduced glucose metabolism, whole-brain and temporal lobe atrophy, and clinical decline. The effect appeared additive because an increased burden of AC medications was associated with poorer executive function and increased brain atrophy. Similar effects were seen in an independent cohort of older adults. These results suggest that medications with AC properties may be detrimental to brain structure and function, as well as cognition.
The observed findings support previous reports1–16 regarding the association between AC medication use and cognitive impairments, with a significant effect of AC medication use on executive and immediate, rather than delayed, memory. We also found that the increased clinical progression from CN to MCI and/or AD was associated with AC medication use.
This study is one of the first, to our knowledge, to examine in vivo brain structural and functional differences between CN participants taking medications with medium or high AC activity and CN participants not taking these medications. We observed that AC+ participants had reduced brain glucose metabolism and increased brain atrophy compared with AC− participants. Furthermore, those with the highest total AC burden scores showed the most atrophy.
The increased brain atrophy and decreased brain function that we observed may be linked to the central effects of AC medications on cholinergic pathways within the brain. Cholinergic pathways, especially those extending from the basal fore-brain, are important for cognition.42 Studies have suggested that AC medications may affect cognition by altering cholinergic inputs, with a study23 showing that AC medication administration leads to an uncoupling between brain structure and cognition in older adults. The process by which AC medications might lead to neurodegeneration is less clear. Cholinergic receptor antagonists have been shown to induce cell death,43 while increased cholinergic neurotransmission reduces neurodegeneration in an AD mouse model.44 Decreased cholinergic activity due to AC medications may induce synaptic loss and neurodegeneration in regions with significant cholinergic innervation, namely the MTL and cortex.45
In mice, lesioning or damaging cholinergic neurons in the basal forebrain has been shown to cause degeneration of the septal-hippocampal and basalo-cortical projections and neurons in the hippocampus and cortex.46 Another possibility is that participants taking AC medications may be more sensitive to neuronal damage in response to stress. This hypothesis centers around the interaction of cholinergic systems and stress because MTL cholinergic neurons have been shown to regulate the hypothalamic-pituitary-adrenal axis.47 Reduced cholinergic activity has been linked to increased plasma corticosterone levels, which in turn are linked to increased hippocampal cell death.47 Furthermore, chronic stress has been associated with increased Aβ levels, tau hyperphosphorylation and aggregation, and neurodegeneration in mouse models through dysregulation of the hypothalamic-pituitary-adrenal axis.48
Overall, the findings in this study provide a potential biological basis for the reduced cognition associated with the use AC medications through the functional and structural changes in the brain. However, future longitudinal studies with imaging and other brain biomarkers, as well as in animal models, are needed to more fully understand the mechanism underlying the effect of AC medications on the brain.
There are a few notable limitations to this study. First, the information on medication use was based on self-report rather than directly ascertained through medical/prescription records. Self-report could be inaccurate because participants may forget to report specific medication use. However, given the normal cognitive status of the participants at baseline, it is unlikely that they would have reported taking medications that they were, in fact, not taking. Thus, the observed effect is potentially underestimated because some AC− participants may in fact have been taking an AC medication. Future studies using medical/ pharmacy records, along with imaging and biomarker measures, would help to confirm the findings of the present study.
A second limitation is the relatively small sample size of AC+ participants. Future studies using larger samples are warranted. A third limitation is the inability to determine the causality of the findings because the results may be due to poor health rather than AC medication use.49 We did include common comorbid health conditions (eg, vascular and psychiatric conditions), total number of medications, and total number of comorbid conditions as covariates. However, the only way to determine true causality would be by use of a well-controlled prospective longitudinal study.
Another limitation may be the variability in the duration of AC medication use among participants. Furthermore, a participant who had taken an AC medication for many years but ceased shortly before the baseline visit would not be captured as an AC+ participant. Future studies with a better-controlled medication history assessment (ie, using medical/pharmacy records and patient self-report) are warranted, as well as studies on the effect of the duration of AC medication use on the target outcomes. Finally, only structural MRI and FDGPET were assessed in the present report. Future studies examining changes on more advanced imaging measures (ie, diffusion tensor imaging and resting-state or task-based functional MRI) would provide additional evidence about the selective effect of AC medications on the brain structure and function in specific circuits.
Conclusions
In summary, we observed that CN older adults taking medications with medium or high AC activity showed poorer cognition, reduced cerebral glucose metabolism, increased brain atrophy, and increased clinical decline compared with those not taking these medications and that these symptoms were greatest in CN older adults with the highest total AC burden scores. These findings highlight the importance of considering the cognitive adverse effects of AC medications before using them to treat older adults at risk for cognitive decline in a clinical setting, as well as in therapeutic trials.
Supplementary Material
Key Points.
Question
Is use of anticholinergic medication associated with poorer cognition, brain hypometabolism, brain atrophy, and/or increased risk of clinical decline in cognitively normal older adults?
Findings
In this longitudinal study of 2 cohorts of cognitively normal older adults, use of medications with medium or high anticholinergic activity was associated with poorer memory and executive function, brain hypometabolism, brain atrophy, and increased risk of clinical conversion to cognitive impairment. This finding was greatest for those taking drugs with the most anticholinergic activity.
Meaning
Use of medication with significant anticholinergic activity should likely be discouraged in older adults if alternative therapies are available.
Acknowledgments
Funding/Support: Data collection and sharing for this project were funded by the ADNI (NIH grant U01 AG024904) and Department of Defense ADNI (Department of Defense award W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering, and through the generous contributions from the Alzheimer’s Association, the Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica Inc, Biogen Idec Inc, Bristol-Myers Squibb Company, Eisai Inc, Elan Pharmaceuticals Inc, Eli Lilly and Company, EuroImmun, F. Hoffmann–La Roche Ltd and its affiliated company Genentech Inc, Fujirebio, GE Healthcare, IXICO Ltd, Janssen Alzheimer Immunotherapy Research and Development LLC, Johnson and Johnson Pharmaceutical Research and Development LLC, Medpace Inc, Merck and Company Inc, Meso Scale Diagnostics LLC, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc, Piramal Imaging, Servier, Synarc Inc, and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research are providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (http://www.fnih.org/). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. The ADNI data are disseminated by the LONI at the University of Southern California. The ADNI was also supported by NIH grants P30 AG010129 and K01 AG030514 and the Dana Foundation. Additional support for analyses included in the present report was provided by the following sources: the National Institute on Aging (grants R01 AG19771, P30 AG10133, and K01 AG049050), the Alzheimer’s Association, the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative, and the Indiana Clinical and Translational Sciences Institute.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Appendix
Group Information: The ADNI investigators were as follows: I. ADNI 1, Grand Opportunity, and 2: Part A: Leadership and Infrastructure: Michael W. Weiner, MD, University of California–San Francisco (UCSF), principal investigator (PI). Paul Aisen, MD, University of California, San Diego (UC San Diego), Alzheimer’s Diseases Cooperative Study PI and Director of Coordinating Center Clinical Core. Executive Committee: Michael Weiner, MD, UCSF; Paul Aisen, MD, UC San Diego; Ronald Petersen, MD, PhD, Mayo Clinic, Rochester; Clifford R. Jack Jr, MD, Mayo Clinic, Rochester; William Jagust, MD, UC Berkeley; John Q. Trojanowki, MD, PhD, University Pennsylvania; Arthur W. Toga, PhD, University of Southern California (USC); Laurel Beckett, PhD, University of California, Davis (UC Davis); Robert C. Green, MD, MPH, Brigham and Women’s Hospital/ Harvard Medical School; Andrew J. Saykin, PsyD, Indiana University; John Morris, MD, Washington University in St Louis (WUSTL); Leslie M. Shaw, University of Pennsylvania. ADNI External Advisory Board: Zaven Khachaturian, PhD, Prevent Alzheimer’s Disease 2020 (Chair); Greg Sorensen, MD, Siemens; Maria Carrillo, PhD, Alzheimer’s Association; Lew Kuller, MD, University of Pittsburg; Marc Raichle, MD, WUSTL; Steven Paul, MD, Cornell University; Peter Davies, MD, Albert Einstein College of Medicine of Yeshiva University; Howard Fillit, MD, AD Drug Discovery Foundation; Franz Hefti, PhD, Acumen Pharmaceuticals; Davie Holtzman, MD, WUSTL; M. Marcel Mesulam, MD, Northwestern University; William Potter, MD, National Institute of Mental Health; Peter Snyder, PhD, Brown University. ADNI 2 Private Partner Scientific Board: Adam Schwartz, MD, Eli Lilly (Chair). Data and Publication Committee: Robert C. Green, MD, MPH, Brigham and Women’s Hospital/ Harvard Medical School (Chair). Resource Allocation Review Committee: Tom Montine, MD, PhD, University of Washington (Chair). Clinical Core Leaders: Ronald Petersen, MD, PhD, Mayo Clinic, Rochester (core PI); Paul Aisen, MD, UC San Diego. Clinical Informatics and Operations: Ronald G. Thomas, PhD, UC San Diego; Michael Donohue, PhD, UC San Diego; Sarah Walter, MSc, UC San Diego; Devon Gessert, UC San Diego; Tamie Sather, MA, UC San Diego; Gus Jiminez, MBS, UC San Diego; Archana B. Balasubramanian, PhD, UC San Diego; Jennifer Mason, MPH, UC San Diego; Iris Sim, UC San Diego. Biostatistics Core Leaders and Key Personnel: Laurel Beckett, PhD, UC Davis (core PI); Danielle Harvey, PhD, UC Davis; Michael Donohue, PhD, UC San Diego. MRI Core Leaders and Key Personnel: Clifford R. Jack Jr, MD, Mayo Clinic, Rochester (core PI); Matthew Bernstein, PhD, Mayo Clinic, Rochester; Nick Fox, MD, University of London; Paul Thompson, PhD, UCLA School of Medicine; Norbert Schuff, PhD, UCSF MRI; Charles DeCarli, MD, UC Davis; Bret Borowski, RT, Mayo Clinic; Jeff Gunter, PhD, Mayo Clinic; Matt Senjem, MS, Mayo Clinic; Prashanthi Vemuri, PhD, Mayo Clinic; David Jones, MD, Mayo Clinic; Kejal Kantarci, Mayo Clinic; Chad Ward, Mayo Clinic. PET Core Leaders and Key Personnel: William Jagust, MD, UC Berkeley (core PI); Robert A. Koeppe, PhD, University of Michigan; Norm Foster, MD, University of Utah; Eric M. Reiman, MD, Banner Alzheimer’s Institute; Kewei Chen, PhD, Banner Alzheimer’s Institute; Chet Mathis, MD, University of Pittsburgh; Susan Landau, PhD, UC Berkeley. Neuropathology Core Leaders: John C. Morris, MD, WUSTL; Nigel J. Cairns, PhD, MRCPath, WUSTL; Erin Householder, WUSTL; Lisa Taylor-Reinwald, BA, HTL (ASCP), past investigator, WUSTL. Biomarkers Core Leaders and Key Personnel: Leslie M. Shaw, PhD, University of Pennsylvania School of Medicine; John Q. Trojanowki, MD, PhD, University of Pennsylvania School of Medicine; Virginia Lee, PhD, MBA, University of Pennsylvania School of Medicine; Magdalena Korecka, PhD, University of Pennsylvania School of Medicine; Michal Figurski, PhD, University of Pennsylvania School of Medicine. Informatics Core Leaders and Key Personnel: Arthur W. Toga, PhD, USC (core PI); Karen Crawford, USC; Scott Neu, PhD, USC. Genetics Core Leaders and Key Personnel: Andrew J. Saykin, PsyD, Indiana University; Tatiana M. Foroud, PhD, Indiana University; Steven Potkin, MD, UC Irvine; Li Shen, PhD, Indiana University; Kelley Faber, MS, CCRC, Indiana University; Sungeun Kim, PhD, Indiana University; Kwangsik Nho, PhD, Indiana University. Initial Concept Planning and Development: Michael W. Weiner, MD, UCSF; Lean Thal, MD, UC San Diego; Zaven Khachaturian, PhD, Prevent Alzheimer’s Disease 2020. Early Project Proposal Development: Leon Thal, MD, UC San Diego; Neil Buckholtz, National Institute on Aging; Michael W. Weiner, MD, UCSF; Peter J. Snyder, PhD, Brown University; William Potter, MD, National Institute of Mental Health; Steven Paul, MD, Cornell University; Marylyn Albert, PhD, Johns Hopkins University; Richard Frank, MD, PhD, Richard Frank Consulting; Zaven Khachaturian, PhD, Prevent Alzheimer’s Disease 2020. National Institute on Aging: John Hsiao, MD, National Institute on Aging.
Part B: Investigators by Site. Oregon Health and Science University: Jeffrey Kaye, MD; Joseph Quinn, MD; Lisa Silbert, MD; Betty Lind, BS; Raina Carter, BA; Sara Dolen, BS, past investigator. USC: Lon S. Schneider, MD; Sonia Pawluczyk, MD; Mauricio Beccera, BS; Liberty Teodoro, RN; Bryan M. Spann, DO, PhD, past investigator. UC San Diego: James Brewer, MD, PhD; Helen Vanderswag, RN; Adam Fleisher, MD, past investigator. University of Michigan: Judith L. Heidebrink, MD, MS; Joanne L. Lord, LPN, BA, CCRC. Mayo Clinic, Rochester: Ronald Petersen, MD, PhD; Sara S. Mason, RN; Colleen S. Albers, RN; David Knopman, MD; Kris Johnson, RN, past investigator. Baylor College of Medicine: Rachelle S. Doody, MD, PhD; Javier Villanueva-Meyer, MD; Munir Chowdhury, MBBS, MS; Susan Rountree, MD; Mimi Dang, MD. Columbia University Medical Center: Yaakov Stern, PhD; Lawrence S. Honig, MD, PhD; Karen L. Bell, MD. WUSTL: Beau Ances, MD; John C. Morris, MD; Maria Carroll, RN, MSN; Mary L. Creech, RN, MSW; Erin Franklin, MS, CCRP; Mark A. Mintun, MD, past investigator; Stacy Schneider, APRN, BC, GNP, past investigator; Angela Oliver, RN, BSN, MSG, past investigator. University of Alabama–Birmingham: Daniel Marson, JD, PhD; Randall Griffith, PhD, ABPP; David Clark, MD; David Geldmacher, MD; John Brockington, MD; Erik Roberson, MD; Marissa Natelson Love, MD. Mount Sinai School of Medicine: Hillel Grossman, MD; Effie Mitsis, PhD. Rush University Medical Center: Raj C. Shah, MD; Leyla deToledo-Morrell, PhD, past investigator. Wien Center: Ranjan Duara, MD; Daniel Varon, MD; Maria T. Greig, MD; Peggy Roberts, CAN, past investigator. Johns Hopkins University: Marilyn Albert, PhD; Chiadi Onyike, MD; Daniel D’Agostino II, BS; Stephanie Kielb, BS, past investigator. New York University: James E. Galvin, MD, MPH; Brittany Cerbone; Christina A. Michel, past investigator; Dana M. Pogorelec, past investigator; Henry Rusinek, PhD, past investigator; Mony J de Leon, EdD, past investigator; Lidia Glodzik, MD, PhD, past investigator; Susan De Santi, PhD, past investigator. Duke University Medical Center: P. Murali Doraiswamy, MBBS, FRCP; Jeffrey R. Petrella, MD; Salvador Borges-Neto, MD; Terence Z. Wong, MD, past investigator; Edward Coleman, past investigator. University of Pennsylvania: Steven E. Arnold, MD; Jason H. Karlawish, MD; David Wolk, MD; Christopher M. Clark, MD. University of Kentucky: Charles D. Smith, MD; Greg Jicha, MD; Peter Hardy, PhD; Partha Sinha, PhD; Elizabeth Oates, MD; Gary Conrad, MD. University of Pittsburgh: Oscar L. Lopez, MD; MaryAnn Oakley, MA; Donna M. Simpson, CRNP, MPH. University of Rochester Medical Center: Anton P. Porsteinsson, MD; Bonnie S. Goldstein, MS, NP; Kim Martin, RN; Kelly M. Makino, BS, past investigator; M. Saleem Ismail, MD, past investigator; Connie Brand, RN, past investigator. UC Irvine: Ruth A. Mulnard, DNSc, RN, FAAN; Gaby Thai, MD; Catherine McAdams-Ortiz, MSN, RN, A/GNP. University of Texas Southwestern Medical School: Kyle Womack, MD; Dana Mathews, MD, PhD; Mary Quiceno, MD. Emory University: Allan I. Levey, MD, PhD; James J. Lah, MD, PhD; Janet S. Cellar, DNP, PMHCNS-BC. University of Kansas, Medical Center: Jeffrey M. Burns, MD; Russell H. Swerdlow, MD; William M. Brooks, PhD. UCLA: Liana Apostolova, MD; Kathleen Tingus, PhD; Ellen Woo, PhD; Daniel H. S. Silverman, MD, PhD; Po H. Lu, PsyD, past investigator; George Bartzokis, MD, past investigator. Mayo Clinic, Jacksonville: Neill R Graff-Radford, MBBCH, FRCP (London); Francine Parfitt, MSH, CCRC; Tracy Kendall, BA, CCRP; Heather Johnson, MLS, CCRP, past investigator. Indiana University: Martin R. Farlow, MD; Ann Marie Hake, MD; Brandy R. Matthews, MD; Jared R. Brosch, MD; Scott Herring, RN, CCRC, past investigator; Cynthia Hunt, BS, CCRP, past investigator. Yale University School of Medicine: Christopher H. van Dyck, MD; Richard E. Carson, PhD; Martha G. MacAvoy, PhD; Pradeep Varma, MD. McGill University, Montreal-Jewish General Hospital: Howard Chertkow, MD; Howard Bergman, MD; Chris Hosein, MEd. Sunnybrook Health Sciences, Ontario: Sandra Black, MD, FRCPC; Bojana Stefanovic, PhD; Curtis Caldwell, PhD. University of British Columbia Clinic for AD and Related Disorders: Ging-Yuek Robin Hsiung, MD, MHSc, FRCPC; Howard Feldman, MD, FRCPC; Benita Mudge, BS; Michele Assaly, MA, past investigator. Cognitive Neurology–St Joseph’s, Ontario: Elizabeth Finger, MD; Stephen Pasternack, MD, PhD; Irina Rachisky, MD; Dick Trost, PhD, past investigator; Andrew Kertesz, MD, past investigator. Cleveland Clinic Lou Ruvo Center for Brain Health: Charles Bernick, MD, MPH; Donna Munic, PhD. Northwestern University: Marek-Marsel Mesulam, MD; Kristine Lipowski, MA; Sandra Weintraub, PhD; Borna Bonakdarpour, MD; Diana Kerwin, MD, past investigator; Chuang-Kuo Wu, MD, PhD, past investigator; Nancy Johnson, PhD, past investigator. Premiere Research Institute (Palm Beach Neurology): Carl Sadowsky, MD; Teresa Villena, MD. Georgetown University Medical Center: Raymond Scott Turner, MD, PhD; Kathleen Johnson, NP; Brigid Reynolds, NP. Brigham and Women’s Hospital: Reisa A. Sperling, MD; Keith A. Johnson, MD; Gad Marshall, MD. Stanford University: Jerome Yesavage, MD; Joy L. Taylor, PhD; Barton Lane, MD; Allyson Rosen, PhD, past investigator; Jared Tinklenberg, MD, past investigator. Banner Sun Health Research Institute: Marwan N. Sabbagh, MD; Christine M. Belden, PsyD; Sandra A. Jacobson, MD; Sherye A. Sirrel, CCRC. Boston University: Neil Kowall, MD; Ronald Killiany, PhD; Andrew E. Budson, MD; Alexander Norbash, MD, past investigator; Patricia Lynn Johnson, BA, past investigator. Howard University: Thomas O. Obisesan, MD, MPH; Saba Wolday, MSc; Joanne Allard, PhD. Case Western Reserve University: Alan Lerner, MD; Paula Ogrocki, PhD; Curtis Tatsuoka, PhD; Parianne Fatica, BA, CCRC. UC Davis–Sacramento: Evan Fletcher, PhD; Pauline Maillard, PhD; John Olichney, MD; Charles DeCarli, MD, past investigator; Owen Carmichael, PhD, past investigator. Neurological Care of Central New York: Smita Kittur, MD, past investigator. Parkwood Hospital: Michael Borrie, MB, ChB; T.-Y. Lee, PhD; Rob Bartha, PhD. University of Wisconsin: Sterling Johnson, PhD; Sanjay Asthana, MD; Cynthia M. Carlsson, MD, MS. UC Irvine, Brain Imaging Center: Steven G. Potkin, MD; Adrian Preda, MD; Dana Nguyen, PhD. Banner Alzheimer’s Institute: Pierre Tariot, MD; Anna Burke, MD; Nadira Trncic, MD, PhD, CCRC; Adam Fleisher, MD, past investigator; Stephanie Reeder, BA, past investigator. Dent Neurologic Institute: Vernice Bates, MD; Horacio Capote, MD; Michelle Rainka, PharmD, CCRP. Ohio State University: Douglas W. Scharre, MD; Maria Kataki, MD, PhD; Anahita Adeli, MD. Albany Medical College: Earl A. Zimmerman, MD; Dzintra Celmins, MD; Alice D. Brown, FNP. Hartford Hospital, Olin Neuropsychiatry Research Center: Godfrey D. Pearlson, MD; Karen Blank, MD; Karen Anderson, RN. Dartmouth-Hitchcock Medical Center: Laura A. Flashman, PhD; Marc Seltzer, MD; Mary L. Hynes, RN, MPH; Robert B. Santulli, MD, past investigator. Wake Forest University Health Sciences: Kaycee M. Sink, MD, MAS; Leslie Gordineer; Jeff D. Williamson, MD, MHS, past investigator; Pradeep Garg, PhD, past investigator; Franklin Watkins, MD, past investigator. Rhode Island Hospital: Brian R. Ott, MD; Henry Querfurth, MD; Geoffrey Tremont, PhD. Butler Hospital: Stephen Salloway, MD, MS; Paul Malloy, PhD; Stephen Correia, PhD. UCSF: Howard J. Rosen, MD; Bruce L. Miller, MD; David Perry, MD. Medical University South Carolina: Jacobo Mintzer, MD, MBA; Kenneth Spicer, MD, PhD; David Bachman, MD. St Joseph’s Health Care: Elizabeth Finger, MD; Stephen Pasternak, MD; Irina Rachinsky, MD; John Rogers, MD; Andrew Kertesz, MD, past investigator; Dick Drost, MD, past investigator. Nathan Kline Institute: Nunzio Pomara, MD; Raymundo Hernando, MD; Antero Sarrael, MD. University of Iowa College of Medicine: Susan K. Schultz, MD; Laura L. Boles Ponto, PhD; Hyungsub Shim, MD; Karen Ekstam Smith, RN. Cornell University: Norman Relkin, MD, PhD; Gloria Chaing, MD; Michael Lin, MD; Lisa Ravdin, PhD. University of South Florida (USF), USF Health Byrd Alzheimer’s Institute: Amanda Smith, MD; Balebail Ashok Raj, MD; Kristin Fargher, MD, past investigator.
A Study of Brain Aging in Vietnam War Veterans; Department of Defense ADNI; Part A: Leadership and Infrastructure: Michael W. Weiner, MD, UCSF, PI; Paul Aisen, MD, UC San Diego, Alzheimer’s Diseases Cooperative Study PI and Director of Coordinating Center Clinical Core. Executive Committee: Michael Weiner, MD, UCSF; Paul Aisen, MD, UC San Diego; Ronald Petersen, MD, PhD, Mayo Clinic, Rochester; Robert C. Green, MD, MPH, Brigham and Women’s Hospital/Harvard Medical School; Danielle Harvey, PhD, UC Davis; Clifford R. Jack Jr, MD, Mayo Clinic, Rochester; William Jagust, MD, UC Berkeley; John C. Morris, MD, WUSTL; Andrew J. Saykin, PsyD, Indiana University; Leslie M. Shaw, PhD, Perelman School of Medicine, University of Pennsylvania; Arthur W. Toga, PhD, USC; John Q. Trojanowki, MD, PhD, Perelman School of Medicine, University of Pennsylvania. Psychological Evaluation/Posttraumatic Stress Disorder Core: Thomas Neylan, MD, UCSF. Traumatic Brain Injury Core: Jordan Grafman, PhD, Rehabilitation Institute of Chicago, Feinberg School of Medicine, Northwestern University. Data and Publication Committee: Robert C. Green, MD, MPH, Brigham and Women’s Hospital/Harvard Medical School (Chair). Resource Allocation Review Committee: Tom Montine, MD, PhD, University of Washington (Chair). Clinical Core Leaders: Michael Weiner MD (core PI); Ronald Petersen, MD, PhD, Mayo Clinic, Rochester (core PI); Paul Aisen, MD, UC San Diego. Clinical Informatics and Operations: Ronald G. Thomas, PhD, UC San Diego; Michael Donohue, PhD, UC San Diego; Devon Gessert, UC San Diego; Tamie Sather, MA, UC San Diego; Melissa Davis, UC San Diego; Rosemary Morrison, MPH, UC San Diego; Gus Jiminez, MBS, UC San Diego. San Francisco Veterans Affairs Medical Center: Thomas Neylan, MD, UCSF; Jacqueline Hayes, UCSF; Shannon Finley, UCSF. Biostatistics Core Leaders and Key Personnel: Danielle Harvey, PhD, UC Davis (core PI); Michael Donohue, PhD, UC San Diego. MRI Core Leaders and Key Personnel: Clifford R. Jack Jr, MD, Mayo Clinic, Rochester (core PI); Matthew Bernstein, PhD, Mayo Clinic, Rochester; Bret Borowski, RT, Mayo Clinic; Jeff Gunter, PhD, Mayo Clinic; Matt Senjem, MS, Mayo Clinic; Kejal Kantarci, Mayo Clinic; Chad Ward, Mayo Clinic. PET Core Leaders and Key Personnel: William Jagust, MD, UC Berkeley (core PI); Robert A. Koeppe, PhD, University of Michigan; Norm Foster, MD, University of Utah; Eric M. Reiman, MD, Banner Alzheimer’s Institute; Kewei Chen, PhD, Banner Alzheimer’s Institute; Susan Landau, PhD, UC Berkeley. Neuropathology Core Leaders: John C. Morris, MD, WUSTL; Nigel J. Cairns, PhD, FRCPath, WUSTL; Erin Householder, MS, WUSTL. Biomarkers Core Leaders and Key Personnel: Leslie M. Shaw, PhD, Perelman School of Medicine, University of Pennsylvania; John Q. Trojanowki, MD, PhD, Perelman School of Medicine, University of Pennsylvania; Virginia Lee, PhD, MBA, Perelman School of Medicine, University of Pennsylvania; Magdalena Korecka, PhD, Perelman School of Medicine, University of Pennsylvania; Michal Figurski, PhD, Perelman School of Medicine, University of Pennsylvania. Informatics Core Leaders and Key Personnel: Arthur W. Toga, PhD, USC (core PI); Karen Crawford, USC; Scott Neu, PhD, USC. Genetics Core Leaders and Key Personnel: Andrew J. Saykin, PsyD, Indiana University; Tatiana M. Foroud, PhD, Indiana University; Steven Potkin, MD, UC Irvine; Li Shen, PhD, Indiana University; Kelley Faber, MS, CCRC, Indiana University; Sungeun Kim, PhD, Indiana University; Kwangsik Nho, PhD, Indiana University. Initial Concept Planning and Development: Michael W. Weiner, MD, UCSF; Karl Friedl, Department of Defense (retired).
Part B: Investigators by Site: USC: Lon S. Schneider, MD, MS; Sonia Pawluczyk, MD; Mauricio Beccera. UC San Diego: James Brewer, MD, PhD; Helen Vanderswag, RN. Columbia University Medical Center: Yaakov Stern, PhD; Lawrence S. Honig, MD, PhD; Karen L. Bell, MD. Rush University Medical Center: Debra Fleischman, PhD; Konstantinos Arfanakis, PhD; Raj C. Shah, MD. Wien Center: Ranjan Duara, MD, PI; Daniel Varon, MD, co-PI; Maria T Greig, HP Coordinator. Duke University Medical Center: P. Murali Doraiswamy, MBBS; Jeffrey R. Petrella, MD; Olga James, MD. University of Rochester Medical Center: Anton P. Porsteinsson, MD (director); Bonnie Goldstein, MS, NP (coordinator); Kimberly S. Martin, RN. UC Irvine: Ruth A. Mulnard, DNSc, RN, FAAN; Gaby Thai, MD; Catherine McAdams-Ortiz, MSN, RN, A/GNP. Medical University South Carolina: Jacobo Mintzer, MD, MBA; Dino Massoglia, MD, PhD; Olga Brawman-Mintzer, MD. Premiere Research Institute (Palm Beach Neurology): Carl Sadowsky, MD; Walter Martinez, MD; Teresa Villena, MD. UCSF: William Jagust, MD; Susan Landau, PhD; Howard Rosen, MD; David Perry. Georgetown University Medical Center: Raymond Scott Turner, MD, PhD; Kelly Behan; Brigid Reynolds, NP. Brigham and Women’s Hospital: Reisa A. Sperling, MD; Keith A. Johnson, MD; Gad Marshall, MD. Banner Sun Health Research Institute: Marwan N. Sabbagh, MD; Sandra A. Jacobson, MD; Sherye A. Sirrel, MS, CCRC. Howard University: Thomas O. Obisesan, MD, MPH; Saba Wolday, MSc; Joanne Allard, PhD. University of Wisconsin: Sterling C. Johnson, PhD; J. Jay Fruehling, MA; Sandra Harding, MS. University of Washington: Elaine R. Peskind, MD; Eric C. Petrie, MD, MS; Gail Li, MD, PhD. Stanford University: Jerome A. Yesavage, MD; Joy L. Taylor, PhD; Ansgar J. Furst, PhD; Steven Chao, MD. Cornell University: Norman Relkin, MD, PhD; Gloria Chaing, MD; Lisa Ravdin, PhD.
Additional Information: Data used in preparation of this article were obtained from the ADNI database (http://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in analysis or writing of this report.
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Dr Risacher had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Saykin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Risacher.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Risacher, Gao.
Obtained funding: Jagust, Saykin.
Administrative, technical, or material support: Tallman, West, Boustani, Jack, Jagust, Weiner.
Study supervision: Weiner.
Conflict of Interest Disclosures: Dr Farlow reports grant research support from Accera, Biogen, Eisai, Eli Lilly, Genentech, Roche, Lundbeck, Chase Pharmaceuticals, and Boehringer-Ingelheim and being a consultant for or on the advisory or data and safety monitoring boards of Accera, Alltech, Avanir, Biogen, Eisai Medical Research Inc, FORUM Pharmaceuticals, Genentech Inc, Grifols, Helicon Inc Research, Lundbeck, Medavante, Medivation Inc, Merck and Company Inc, Medtronic, Neurotrope Biosciences, Novartis, Pfizer, QR Pharma, Axovant Sciences Inc, Roche, Sanofi-Aventis, Schering-Plough, Takeda, Toyama Pharmaceutical, Pharm, Eli Lilly and Company, and UCB Pharma. Dr Petersen serves on the advisory boards of Pfizer Inc and Janssen Alzheimer Immunotherapy and is a consultant for Roche Incorporated, Merck, Genentech, and Biogen. He receives support from the National Institute on Aging (grants U01-AG006786, P50-AGG016574, R01-AG011378, and U01-AG024904) and publishing royalties from Oxford University Press. Dr Jack serves as a consultant for Eli Lilly and Company and receives research support from the National Institute on Aging (grants R01 AG11378 and R01 AG041851) and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. Dr Jagust serves on the scientific advisory boards of Genentech and Banner/ Genentech and was a consultant for Synarc, Siemens Medical, Genentech, F. Hoffman–La Roche, and Novartis. He receives research support from Avid Radiopharmaceuticals, the Rainwater Foundation, and the National Institutes of Health (NIH) (grants AG034570, AG025303, AG044292, AG012345, AG021028, AG031563, AG019724, AG030048, AG032306, and AG024904). Dr Aisen serves on the scientific advisory boards of Novartis and Biogen and has been a consultant for Elan Corporation, Wyeth, Eisai Inc, Schering-Plough Corp, Bristol-Myers Squibb, Eli Lilly and Company, Roche, Amgen, Genentech Inc, Abbott, Pfizer Inc, Novartis, Bayer, Medivation Inc, Daiichi Sankyo, Astellas, Bainippon, Biomarin, Solvay, Ostuka, AstraZeneca, and Janssen. He receives research support from Baxter, Pfizer, Janssen, and Eli Lilly and Company, as well as from the National Institute on Aging (grants U01-AG10483, U01 AG024904, R01 AG030048, and R01 AG16381). Dr Weiner serves on the scientific advisory boards for Pfizer, BOLT International, Neurotrope Bioscience, Eli Lilly and Company, the University of Pennsylvania’s Neuroscience of Behavior Initiative, the National Brain Research Centre, India, and the ADNI and is a consultant for Synarc, Pfizer, Janssen, KLJ Associates, Easton Associates, Harvard University, the University of California, Los Angeles (UCLA), the Alzheimer’s Drug Discovery Foundation, Avid Radiopharmaceuticals, Clearview Healthcare Partners, Perceptive Informatics, Smartfish AS, Decision Resources Inc, Neurotrope Bioscience, Araclon, Merck, Defined Health, and Genentech. He receives research support from Avid Radiopharmceuticals, Eli Lilly and Company, the NIH, the US Department of Defense, and the Veterans Administration. He also has received travel funding or speaker honoraria from Pfizer, Paul Sabatier University, the MCI Group France, eDreams, the Neuroscience School of Advanced Studies, Danone Trading, BV, CTAD ANT Congrès, Kenes International, the Aging and Disability Resource Center, UCLA, the University of California, San Diego, the Sanofi-Aventis Group, University Center Hospital, Toulouse, Araclon, AC Immune, Eli Lilly and Company, the New York Academy of Sciences, the National Brain Research Center, Indiana for Johns Hopkins Medicine, the Consortium for Multiple Sclerosis Centers, Northwestern University, and the University of Pennsylvania. Dr Saykin serves on scientific advisory boards of Siemens Healthcare and Eli Lilly and Company and was a consultant for Baxter Bioscience, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer Inc, Siemens Healthcare, Dartmouth Medical School, the University of Michigan, the University of Vermont, Vanderbilt University, and Brigham and Women’s Hospital/ Massachusetts General Hospital. He has received research support from Siemens Medical Solutions, Welch Allyn Inc, the Foundation for the NIH, and the NIH (grants R01 AG19771, R01 CA101318, R01 LM011360, RC2 AG036535, P30 AG10133, and U01 AG032984). He has also received travel support and/or speaker honoraria from Siemens Healthcare. No other disclosures are reported.
REFERENCES
- 1.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–459. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottiggi KA, Salazar JC, Yu L, et al. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14(11):980–984. doi: 10.1097/01.JGP.0000224619.87681.71. [DOI] [PubMed] [Google Scholar]
- 3.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. [Google Scholar]
- 4.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013;9(4):377–385. doi: 10.1016/j.jalz.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrière I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317–1324. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap M, Belleville S, Mulsant BH, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. J Am Geriatr Soc. 2014;62(2):336–341. doi: 10.1111/jgs.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konishi K, Hori K, Uchida H, et al. Adverse effects of anticholinergic activity on cognitive functions in Alzheimer’s disease. Psychogeriatrics. 2010;10(1):34–38. doi: 10.1111/j.1479-8301.2010.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Lampela P, Lavikainen P, Garcia-Horsman JA, Bell JS, Huupponen R, Hartikainen S. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging. 2013;30(5):321–330. doi: 10.1007/s40266-013-0063-2. [DOI] [PubMed] [Google Scholar]
- 12.Lechevallier-Michel N, Molimard M, Dartigues JF, Fabrigoule C, Fourrier-Réglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59(2):143–151. doi: 10.1111/j.1365-2125.2004.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah RC, Janos AL, Kline JE, et al. Cognitive decline in older persons initiating anticholinergic medications. PLoS One. 2013;8(5):e64111. doi: 10.1371/journal.pone.0064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sittironnarit G, Ames D, Bush AI, et al. AIBL research group. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31(3):173–178. doi: 10.1159/000325171. [DOI] [PubMed] [Google Scholar]
- 15.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639–658. doi: 10.1007/BF03262280. [DOI] [PubMed] [Google Scholar]
- 16.Uusvaara J, Pitkala KH, Kautiainen H, Tilvis RS, Strandberg TE. Detailed cognitive function and use of drugs with anticholinergic properties in older people: a community-based cross-sectional study. Drugs Aging. 2013;30(3):177–182. doi: 10.1007/s40266-013-0055-2. [DOI] [PubMed] [Google Scholar]
- 17.Fox C, Livingston G, Maidment ID, et al. The impact of anticholinergic burden in Alzheimer’s dementia-the LASER-AD study. Age Ageing. 2011;40(6):730–735. doi: 10.1093/ageing/afr102. [DOI] [PubMed] [Google Scholar]
- 18.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessen F, Kaduszkiewicz H, Daerr M, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S111–S115. doi: 10.1007/s00406-010-0156-4. [DOI] [PubMed] [Google Scholar]
- 20.Kashyap M, Mulsant BH, Tannenbaum C. Small longitudinal study of serum anticholinergic activity and cognitive change in community-dwelling older adults. Am J Geriatr Psychiatry. 2015;23(3):326–329. doi: 10.1016/j.jagp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Flicker C, Ferris SH, Serby M. Hypersensitivity to scopolamine in the elderly. Psychopharmacology (Berl) 1992;107(2–3):437–441. doi: 10.1007/BF02245172. [DOI] [PubMed] [Google Scholar]
- 22.Molchan SE, Martinez RA, Hill JL, et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev. 1992;17(3):215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- 23.Teipel SJ, Bruno D, Grothe MJ, Nierenberg J, Pomara N. Hippocampus and basal forebrain volumes modulate effects of anticholinergic treatment on delayed recall in healthy older adults. Alzheimers Dement. 2015;1(2):216–219. doi: 10.1016/j.dadm.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Jr, Bernstein MA, Borowski BJ, et al. Alzheimer’s Disease Neuroimaging Initiative. Update on the magnetic resonance imaging core of the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2010;6(3):212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagust WJ, Bandy D, Chen K, et al. Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6(3):221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Alzheimer’s Disease Neuroimaging Initiative. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saykin AJ, Shen L, Foroud TM, et al. Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner MW, Aisen PS, Jack CR, Jr, et al. Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative: progress report and future plans. Alzheimers Dement. 2010;6(3):202–211. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 31.Aging Brain Care. [Accessed March 24, 2016];Anticholinergic Cognitive Burden scale: 2012 update. http://www.agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf.
- 32.Drugs on the Anitcholinergic Burden (ACB) scale. [Accessed March 24, 2016]; http://www.bmedreport.com/wp-content/uploads/2011/06/Drugs-on-the-Anticholinergic-Burden-ACB-scale.jpg.
- 33.Anticholinergics and the elderly. [Accessed March 24, 2016];Pharmacist’s Letter website. http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=5&fpt=2&dd=271223&pb=PRL.
- 34.Risacher SL, Kim S, Nho K, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI). APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11(12):1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risacher SL, Kim S, Shen L, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI)†. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbons LE, Carle AC, Mackin RS, et al. Alzheimer’s Disease Neuroimaging Initiative. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crane PK, Carle A, Gibbons LE, et al. Alzheimer’s Disease Neuroimaging Initiative. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6(4):502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Presented at: Eighth International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. [Google Scholar]
- 40.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s Disease Neuroimaging Initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landau SM, Mintun MA, Joshi AD, et al. Alzheimer’s Disease Neuroimaging Initiative. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 43.Del Pino J, Zeballos G, Anadón MJ, et al. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels [published online May 31, 2015] Arch Toxicol. doi: 10.1007/s00204-015-1540-7. [DOI] [PubMed] [Google Scholar]
- 44.Capsoni S, Giannotta S, Stebel M, et al. Ganstigmine and donepezil improve neurodegeneration in AD11 antinerve growth factor transgenic mice. Am J Alzheimers Dis Other Demen. 2004;19(3):153–160. doi: 10.1177/153331750401900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geula C. Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology. 1998;51(1)(suppl 1):S18–S29. doi: 10.1212/wnl.51.1_suppl_1.s18. discussion S65-S67. [DOI] [PubMed] [Google Scholar]
- 46.Moreau PH, Cosquer B, Jeltsch H, Cassel JC, Mathis C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus. 2008;18(6):610–622. doi: 10.1002/hipo.20422. [DOI] [PubMed] [Google Scholar]
- 47.Paul S, Jeon WK, Bizon JL, Han JS. Interaction of basal forebrain cholinergic neurons with the glucocorticoid system in stress regulation and cognitive impairment. Front Aging Neurosci. 2015;7:43. doi: 10.3389/fnagi.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carroll JC, Iba M, Bangasser DA, et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci. 2011;31(40):14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.