Abstract
Hippocampal place cells encode information about an animal’s spatial world. A study now finds that these same neurons envisage a future journey moments before a rat sets off.
Navigation is a cognitive process that depends on more than a paper map; much like a modern GPS, it requires the ability to plan routes using the map. In a paper published on Nature’s website today, Pfeiffer and Foster1 show that sequences of activity in neurons called place cells, located in the hippocampus of the rat brain, transiently predict (plan) the journey that the animal is about to take. This report provides direct evidence for the future-focused navigational activity of place cells in a realistic two-dimensional environment.
In the 1940s, the psychologist Edward Tolman proposed that mammals (including rats and humans) have a ‘cognitive map’ that represents the spatial environment. He also proposed that animals could use the map to plan future trajectories2. In the 1970s, two neuroscientists, John O’Keefe and Lynn Nadel, suggested that the hippocampus was a key component of this cognitive map, with its place cells representing locations within the environment3. In the intervening years, evidence has mounted that place cells can be used cognitively — that is, they play out information about both the environment (during rest and sleep)4–7 and potential options before starting off on a journey along a track or making a decision at a choice point in a T-shaped track8–10.
These previous studies used one-dimensional, limited paths and so could not determine whether the hippocampus was checking specific options or actually planning future paths. Pfeiffer and Foster overcome this problem by bringing together a 40-tetrode microdrive and an elegant experimental task, which allowed them to decode sequences of two-dimensional position representations from sequences of hippocampal electrical firing. The microdrive permitted simultaneous recordings of 250 place cells, providing sufficient coverage to decode the location represented by this cell ensemble, even at short time scales (20 milliseconds). In the experimental task, rats alternately foraged for food rewards between randomly distributed locations and a stationary ‘home’ location that changed daily, but remained constant within each day. This combination of daily changes but consistency within a day meant that rats could learn the general task of alternately foraging and returning home, but would take novel routes that could be studied in two dimensions.
Hippocampal place cells express most of their activity within a specific small area (called the place field) while the rat is in that area. But they also typically fire a small number of ‘extra-field’ spikes at other locations in the environment. In non-linguistic animals, proving that the extra-field spikes are neither part of a representation of the animal’s current location nor simply random noise is an elusive proposition11.
Pfeiffer and Foster, however, combined their large place-cell ensembles with sophisticated mathematical analyses to show that the extra-field spikes often produce a more coherent representation of the future journey than of the actual location of the rats. In the moments when the animals paused before taking a journey, the place cells fired in a sequence that predicted the journey the animal was going to take (Fig. 1). These sequences occurred during sharp-wave-ripple (SWR) events, which are well-studied irregular bursts of brief (100–200 ms), large-amplitude and high-frequency (140–200 Hz) neuronal activity in the hippocampus. And their temporal sequence represented trajectories to behaviourallyrelevant locations such as the next foraging location or the home base.
Figure 1. What is the best way home?
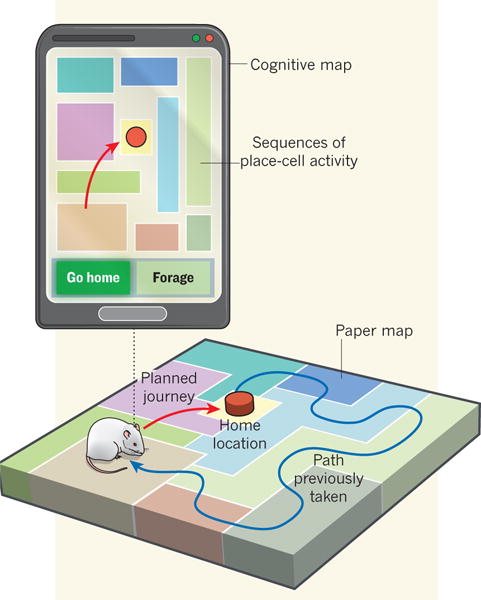
An ordinary paper map encodes location only, whereas a cognitive map is also involved in planning a route. Pfeiffer and Foster report that, just before a rat takes a journey, hippocampal place cells in its brain play out sequences predicting the animal’s future path. This suggests that the hippocampus functions much like a GPS unit that not only shows where you are, but also how to get home.
As an elegant control, the authors found that the representations were unrelated to the journey just completed (the past). Moreover, these trajectories were not simply straight-line paths in front of the rat. Instead, excitingly, they represented the future path taken regardless of the rat’s orientation, which implies that the sequences reflected not the animal’s spatial view but rather its intentions.
Future-trajectory planning was not simply a product of experience either, as it was seen even before novel traversals to the home location. As with previous examples in which untaken paths have been found to play out during waking SWR events (see ref. 7, for example), the sequences in Pfeiffer and Foster’s study occurred in situations in which the map was known (the animals had a lot of experience with the environment) but the specific path to be taken was not — the home location changed every day. This is when maps are most useful, when they allow one to attach new significance to old locations2,12.
Place-cell sequences during SWR events were originally seen during sleep and are believed to facilitate memory consolidation, which involves information transfer from the hippocampus to the cortex of the brain4. Indeed, disruption of SWR events during sleep impairs memory consolidation13,14. Increasing evidence suggests, however, that when SWR events occur during waking states they encode different information5,15. For instance, disruption of SWR events during wakefulness impairs only hippocampal-dependent spatial navigation, suggesting that SWR events facilitate cognitive processes during wakefulness16. In their two-dimensional set-up, Pfeiffer and Foster show that the sequences during waking states reflect future plans rather than past experiences.
Functional connectivity within the hippocampal formation changes during distinct behavioural states. Whereas SWR events occur during sleep or quiet wakefulness, large-amplitude, low-frequency theta oscillations (4–12 Hz) characterize neuronal activity when an animal moves and during attentive wakefulness. Hippocampal firing during these theta states have been found to encode potential future options. For example, animals making decisions at a choice point on a T-shaped maze also show future-representing sequences, but these sequences occur during theta oscillations rather than SWR events9,17. A fascinating question is, what is the relationship between these two planning phenomena? Does one negate the need for the other?
It also remains unclear what triggers the hippocampal neural sequences associated with future-trajectory planning and how these sequences interact with other neural circuits. The hippocampus is only part of a complex neural network that involves several related brain structures. In humans, for example, planning processes entail an interaction of multiple structures, including prefrontal cortex18,19. What are these other structures doing during the planning events observed by Pfeiffer and Foster? In light of their remarkable results, researchers must now explore what processes generate these place-cell sequences, and how they are used in recalculating the journey home.
Contributor Information
Brandy Schmidt, Email: schmidtb@umn.edu.
A. David Redish, Email: redish@umn.edu.
References
- 1.Pfeiffer BE, Foster DJ. Nature. 2013 doi: 10.1038/nature12112. http://dx.doi.org/10.1038/nature12112. [DOI] [PMC free article] [PubMed]
- 2.Tolman EC. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon; 1978. [Google Scholar]
- 4.Sutherland GR, McNaughton B. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Foster DJ, Wilson MA. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 6.Davidson TJ, Kloosterman F, Wilson MA. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diba K, Buzsáki G. Nature Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson A, Redish AD. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer AC, Carr MF, Karlsson MP, Frank LM. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson A, Fenton AA, Kentros C, Redish AD. Trends Cogn Sci. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tse D, et al. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 13.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Nature Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 14.Ego-Stengel V, Wilson MA. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wikenheiser AM, Redish AD. Hippocampus. 2013;23:22–29. doi: 10.1002/hipo.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadhav SP, Kemere C, German PW, Frank LM. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Nature Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiers HG, Maguire EA. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Voss JL, et al. Proc Natl Acad Sci USA. 2011;108:E402–E409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]


