Abstract
Lymphoepithelioma-like carcinomas are distinctive epithelial derived malignant neoplasms that have a syncytial growth pattern and lymphoid stroma. The majority of tumors with this appearance are Epstein Barr virus (EBV)-associated. We report a patient with a clinical presentation concerning for lymphoma who was diagnosed with an EBV-associated pancreatic carcinoma with a lymphoepithelioma-like pattern. Targeted sequencing analysis showed a molecular profile distinct from conventional ductal adenocarcinoma of the pancreas.
Keywords: Epstein Barr Virus, Pancreas carcinoma, Lymphoepithelial-like carcinoma, lymphoepithelioma-like carcinoma, EBV-associated carcinoma
Introduction
Lymphoepithelioma-like carcinomas (LELC) have a distinctive syncytial growth pattern with associated non-neoplastic lymphoid stroma. The majority of LELC are Epstein Barr virus (EBV)-associated. To our knowledge, three cases of EBV-associated carcinomas have been reported to originate in the pancreas.1-3 We report a patient with an EBV-associated pancreatic carcinoma showing a LELC pattern with targeted molecular analysis.
Case report
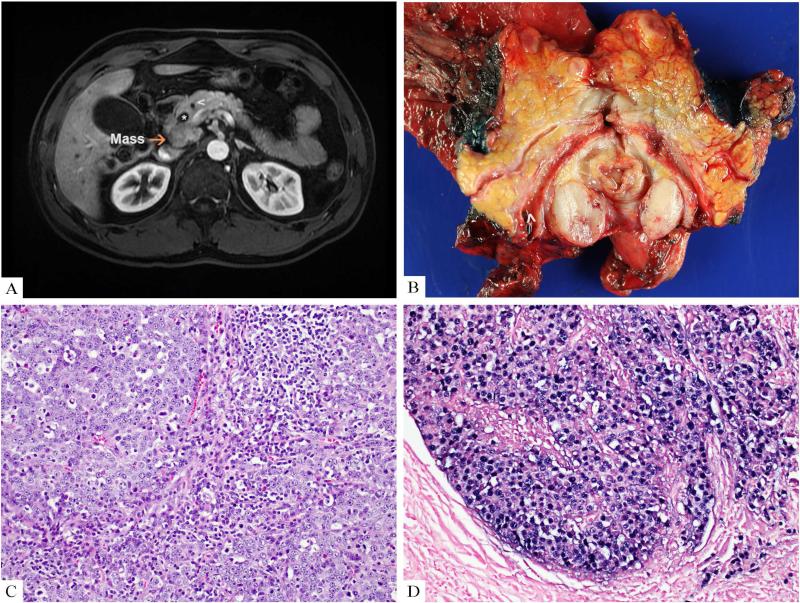
A 45-year-old man presented with four months of fatigue and abdominal pain. Abdominal MRI showed a 3.2 cm mass in the pancreatic uncinate process, dilatation of the main pancreatic and extrahepatic biliary ducts, and peripancreatic lymphadenopathy (Figure 1A). Fine needle aspiration (FNA) of a peri-pancreatic lymph node favored carcinoma but due to a prominent population of small lymphoid cells intimately admixed with tumor cells concern for concomitant lymphoma was raised. Concurrently, he developed fever and palpable neck lymphadenopathy with a differential diagnosis including infection, metastasis or lymphoma. FNA of a submandibular lymph node showed atypical lymphoid cells and granulomatous inflammation. A pylorus preserving pancreatoduodenectomy was performed, and massive lymphadenopathy extending along course of the superior mesenteric artery was identified intraoperatively. The resected tumor was a 3.2 cm circumscribed mass in the head of pancreas (Figure 1B). Histologic findings included a marked lymphocytic infiltrate surrounding and within a syncytial mass of tumor cells with prominent nucleoli and amphophilic cytoplasm (Figure 1C). The tumor involved peripancreatic soft tissue, duodenal mucosa, and 29 lymph nodes. Immunohistochemical stains were positive for pancytokeratin while negative for synaptophysin, chromogranin, and trypsin. Staining for DNA mismatch repair proteins MLH1, MSH2, MSH6, and PMS2 was retained. In situ hybridization for EBV encoded RNA (EBER-ISH) was positive in the tumor cells and absent in CD3 positive T cells (Figure 1D). These findings supported a LELC with associated EBV infection. An elevated EBV viral load was detected. Next generation sequencing via a custom hybrid capture assay (MSK-IMPACT) targeting all exons of 341 genes was performed against matched normal finding somatic mutations in the following genes: BARD1, BCOR, FAT1, HIST1H1C, INPP4, and PARP1. 4
Figure.
1A) MRI of the abdomen revealed a 3 cm mass in the uncinate of the pancreas with a dilated bile duct (*) and a dilated pancreas duct (^). 1B) The radiologic lesion corresponded to a 3.2 cm mass in the resected pancreas (Hematoxylin and eosin stain, 20x). 1C) The histologic findings included sheets of tumor cells with a prominent lymphoid stroma. 1D) In situ hybridization for EBV encoded RNA was positive in the malignant epithelial cells.
Discussion
LELC was initially used to describe undifferentiated, non-keratinizing carcinomas with dense non-neoplastic lymphocytic stroma arising in the nasopharynx but the term has been adopted for tumors of other foregut-derived organs that exhibit similar histology and there is often an associated EBV infection. EBV is a herpes virus causing ubiquitous infection by adulthood world-wide and the gold standard for detecting the virus in tissue is EBER-ISH. In EBV-associated tumors of the nasopharynx and stomach, tumorigenesis has been shown to be related to latent EBV infection which may be facilitated by genetic alterations in the host cells.5 At both anatomic sites, EBV has been detected in adjacent dysplastic and normal epithelium and expression of EBV latent proteins can lead to genetic instability, epigenetic changes, and eventual cell transformation.5 EBV-associated gastric carcinomas occur as either as either ordinary appearing adenocarcinoma or with a LELC pattern and not all gastric carcinomas with a LELC-like pattern are associated with EBV; some are microsatellite instability high carcinomas. The two associations are mutually exclusive.6, 7 In the pancreatobiliary tract, the rate of latent EBV infection in conventional adenocarcinomas is not well understood. Histopathological features of biliary LELC are similar with and without EBV association.3, 8 In the present case, the histologic differential diagnosis included LELC, medullary carcinoma, high grade neuroendocrine carcinoma, and metastatic carcinoma. Medullary carcinoma of the pancreas exhibits poor differentiation, pushing invasion, a syncytial growth pattern, and frequent microsatellite instability.3 Features suggesting microsatellite instability are important to recognize to inform the subset of patients with germline mutations. This tumor had a medullary-like phenotype but there was retained nuclear expression of the mismatch repair proteins. A carcinoma with no evidence of neuroendocrine or acinar differentiation was supported by immunohistochemical stains. Lymphoid stroma and absent glandular differentiation supported the diagnosis of LELC-like carcinoma and EBER-ISH positivity supported latent EBV infection. Clinical imaging studies were used to establish the absence of an alternative primary.
The overall significance of the somatic mutations identified is uncertain. EBV-associated gastric cancers have been described as having a distinct genomic profile according The Cancer Genome Research Network data which includes extreme CpG-island methylation of the promoter region; frequent mutations of PIK3CA, ARID1A, JAK2, and BCOR; and amplification of ERBB2.9 This list shares with our patient a BCOR mutation. Germline mutation of BCOR results in oculo-facio-cardio-dental genetic syndrome, while somatic mutation has been described in myeloid leukemias.10, 11 There is evidence that disrupted BCOR in the context of leukemia would have similar implications as a loss of function mutation in a tumor suppressor gene.11 Mutations in the most commonly altered genes in pancreatic adenocarcinoma (KRAS, TP53, CDKN2A, and SMAD4) were not detected.12 Likewise, the assay did not detect mutations in genes implicated in nasopharyngeal carcinoma such as CCND1, LTBR, p16, RASSF1A, TSLC1 and THY1.The patient received 6 months of FOLFORINOX-based treatment chemotherapy and at 8 months, he was without disease progression and his EBV viral load decreased.
In conclusion, we report a case of EBV-associated LELC of the pancreas in a 45-year-old male. Molecular characterization suggests the tumor is distinct from conventional pancreatic ductal adenocarcinoma.
Acknowledgements
The authors have no conflict of interest or financial relationships to disclose for this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kekis PB, Murtin C, Kunzli BM, Kappler A, Buchholz B, Buchler MW, et al. Epstein-barr virus-associated lymphoepithelial carcinoma in the pancreas. Pancreas. 2004;28:98–102. doi: 10.1097/00006676-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Sessler R, Ruether U, Hornberger M, Bokemeyer C. Post-transplant ebv-associated pancreas carcinoma. European journal of cancer. 1997;33:2435–2436. doi: 10.1016/s0959-8049(97)00316-x. [DOI] [PubMed] [Google Scholar]
- 3.Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. The American journal of pathology. 2000;156:1641–1651. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Won HH, Scott SN, Brannon AR, Shah RH, Berger MF. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. Journal of visualized experiments : JoVE. 2013:e50710. doi: 10.3791/50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of npc through molecular, cytogenetic, and epigenetic approaches. Seminars in cancer biology. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: Associations with epstein-barr virus, microsatellite instability, histology, and survival. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16:641–651. doi: 10.1097/01.MP.0000076980.73826.C0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng N, Hui DY, Liu Y, Zhang NN, Jiang Y, Han J, et al. Is gastric lymphoepithelioma-like carcinoma a special subtype of ebv-associated gastric carcinoma? New insight based on clinicopathological features and ebv genome polymorphisms. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014 doi: 10.1007/s10120-014-0376-9. [DOI] [PubMed] [Google Scholar]
- 8.Ishida M, Mori T, Shiomi H, Naka S, Tsujikawa T, Andoh A, et al. Non-epstein-barr virus associated lymphoepithelioma-like carcinoma of the inferior common bile duct. World journal of gastrointestinal oncology. 2011;3:111–115. doi: 10.4251/wjgo.v3.i7.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N: Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, et al. Oculofaciocardiodental and lenz microphthalmia syndromes result from distinct classes of mutations in bcor. Nature genetics. 2004;36:411–416. doi: 10.1038/ng1321. [DOI] [PubMed] [Google Scholar]
- 11.Grossmann V, Tiacci E, Holmes AB, Kohlmann A, Martelli MP, Kern W, et al. Whole-exome sequencing identifies somatic mutations of bcor in acute myeloid leukemia with normal karyotype. Blood. 2011;118:6153–6163. doi: 10.1182/blood-2011-07-365320. [DOI] [PubMed] [Google Scholar]
- 12.Yachida S, White CM, Naito Y, Zhong Y, Brosnan JA, Macgregor-Das AM, et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6339–6347. doi: 10.1158/1078-0432.CCR-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]