Abstract
Endometriosis is a major cause of chronic pelvic pain and infertility. Activation of STAT3 appears central to the inflammatory phenotype of eutopic endometrium in women with endometriosis. However, the molecular mechanism by which this occurs remains unknown. Our objective is to determine how STAT3 activity is regulated in endometriosis. Protein inhibitor of activated STAT3 (PIAS3) is a negative regulator of STAT3 activity. We examined the levels of PIAS3 in endometrium from women with and without endometriosis using Western blot analysis and immunohistochemistry. Levels of PIAS3 are significantly lower, in contrast with phosphorylation of STAT3, in women with endometriosis compared to women without endometriosis. Furthermore, induction of endometriosis in the baboon showed a significant reduction of PIAS3 expression during the progression of the disease. Interferon-γ (INFγ) reduces PIAS3 protein levels and increases phospho-STAT3 levels through CXCL10 in endometrial cells, Ishikawa, and 12Z cells. These results suggest that attenuation of PIAS3 causes aberrant activation of STAT3 in endometriosis, leading to inflammatory changes that may impair fertility or cause pain.
Keywords: cytokine, endometriosis, endometrium, PIAS3, STAT3
INTRODUCTION
Endometriosis, defined as the presence of endometrial cells outside of the uterine cavity, is a major cause of infertility and pelvic pain. It affects >5% of reproductive age women [1]. Endometriosis results in significant health care costs, upwards of $22 billion per year in the United States [2]. Endometriosis likely contributes significantly to other common disorders, including irritable bowel syndrome, interstitial cystitis, and chronic fatigue syndrome [1, 3]. Endometriosis is strongly associated with infertility, but only 50% of women with endometriosis are infertile [4, 5]. Such heterogeneity among women with endometriosis makes it difficult to assign a true cause-and-effect relationship between endometriosis and infertility. The distribution of ectopic endometriosis tissue is most often within the pelvic peritoneum but can include the ovary, pelvic viscera, rectovaginal septum, pleura, abdominal wall, and, rarely, the brain [6, 7]. Pelvic endometriosis lesions are thought to derive predominantly from retrograde menstruation, which occurs in nearly all women [8, 9]. Endometriosis is treated by surgical removal of lesions and/or hormonal suppression focused on reducing estrogen, such as progestins, androgens, gonadotropin-releasing hormone (GnRH) agonists, and aromatase inhibitors. However, both approaches are associated with various side effects and a high incidence of relapse [1, 10]. Therefore, identification of mechanisms involved in the pathogenesis of endometriosis and strategic therapies for treatment are critical.
Endometriosis is an estrogen-dependent inflammatory condition, and inflammatory components play key roles in the pathogenesis of endometriosis [1]. A variety of cytokines, including leukemia inhibitory factor, interleukin-6 (IL-6), IL-11, and epidermal growth factor, can activate the signal transducer and activator of transcription (STAT) family of transcription factors, specifically STAT3 [8–11]. STAT3 is localized in the cytoplasm until activated by phosphorylation. On activation, STAT3 are phosphorylated by Janus kinase (JAK) and then form homo- or heterodimers that translocate to the cell nucleus, where they act as transcription activators [12]. STAT3 has an important role as signal transducer and regulator of gene expression critical to normal cellular processes, including cell development, differentiation, proliferation, survival, angiogenesis and immune function [13]. However, excessive activation of STAT3 leads to a number of tumor-promoting processes, including maintenance of the stem cell, block differentiation, promotion of growth and angiogenesis, and regulation of the immune response and tumor microenvironment [14–16]. STAT3 has been used as a cancer therapeutic target because it plays a pivotal role in oncogenic functions and immunosuppression [17]. Additionally, levels of phosphorylation of STAT3 are significantly higher in eutopic endometrium from women with endometriosis compared to women without endometriosis, and aberrant activation of STAT3 signaling plays an important role in pathogenesis of endometriosis [18, 19]. However, STAT3 signaling has not been studied enough as a therapeutic target for endometriosis.
In normal cells, STAT activation is rapid and transient because it is negatively regulated by regulator proteins, such as SOCS and protein inhibitor of activated STAT3 (PIAS3) [20, 21]. PIAS3 is identified as a primary inhibitor of STAT3.
PIAS3 binds to the STAT3 DNA binding domain and inhibits the STAT3 transcriptional activity by physical prevention [21, 22]. PIAS3 is also found to be a regulator protein of other key transcription factors, including microphthalmia-associated transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells, and SMADs [23–25]. Most of the proteins that are known to be modulated by PIAS3 play an essential role in the immune system. However, the function of PIAS3 has not been studied in the endometrium and endometriosis.
In this study, we examined the levels of STAT3 regulators in endometrium from women with and without endometriosis. The levels of PIAS3 were significantly lower in endometrium from women with endometriosis compared to the control. We showed that interferon-γ (INFγ) suppressed the expression of PIAS3 and phosphorylated the STAT3 through CXCL10 in endometrial cells, Ishikawa, and 12Z cells. Therefore, our results suggest that attenuation of PIAS3 causes aberrant activation of STAT3 in the pathogenesis of endometriosis.
MATERIALS AND METHODS
Human Subjects
The human endometrial samples used to examine PIAS3 expression patterns were obtained from Michigan State University's Center for Women's Health Research Female Reproductive Tract Biorepository, the University of North Carolina, and the Greenville Hospital System in accordance with the guidelines set by the Institutional Review Boards of Michigan State University (Grand Rapids, MI), the University of North Carolina (Chapel Hill, NC), and the Greenville Health System (Greenville, SC), respectively. Written informed consent was obtained from all participants. For experiments examining PIAS3 expression patterns in the eutopic endometrium of women with and without endometriosis at secretory stage, control samples were compared to endometriotic samples obtained. The human endometrial samples were used for Western blot (10 samples per group) and immunohistochemistry analysis (16 samples per group).
Animals and Tissue Collection
The endometriosis baboon animal model is reviewed and approved by the Institutional Animal Care and Use Committees of both the University of Illinois at Chicago and Michigan State University. Endometriosis is induced by intraperitoneal inoculation of menstrual endometrium on two consecutive menstrual cycles and harvested using laparotomy via endometriectomy from five female baboons as previously described [26]. Laparotomies were performed at 3, 6, and 9 mo postinoculation to harvest the eutopic endometrial tissues. Eutopic endometrial tissues were used for immunohistochemistry analysis.
Western Blot Analysis
Western blot analyses were performed as described previously [27]. Proteins were extracted using lysis buffer (150 mM NaCl, 0.125% Nonidet P-40 [vol/vol]), 10 mM Tris-HCl (pH 7.4), 2.5 mM EDTA, a protease inhibitor cocktail (Roche, Indianapolis, IN) and a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO). Equal amounts of total protein (20 μg) were resolved via SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membrane was blocked with 0.5% Casein for 2 h at room temperature followed by overnight incubation with 1:1000 diluted anti-PIAS3 (ab22856; Abcam, Cambridge, MA), anti-SOCS1 (ab3691; Abcam), anti-SOCS2 (48-584; Prosci Inc., Poway, CA), anti-SOCS3 (ab16030; Abcam), anti-SOCS4 (ab118530; Abcam), anti-SOCS5 (ab97283; Abcam), anti-SOCS6 (ab13950; Abcam), anti-pSTAT3 (9131; Cell Signaling, Danvers, MA), anti-STAT3 (4904; Cell Signaling), or anti-HIF1A (61275; Active Motif, Carlsbad, CA) antibodies. Immunoreactivity was visualized by incubation with a horseradish peroxidase-linked secondary antibody and developed by ECL reagents (GE Healthcare Biosciences, Piscataway, NJ). To control for loading, the membrane was stripped, probed with anti-Actin (sc1616; Santa Cruz Biotechnology, Santa Cruz, CA), and developed. The band intensity was determined by relative densitometry using ImageJ (National Institutes of Health) and normalized against the bands obtained for Actin.
Immunohistochemistry
Immunohistochemistry analysis was performed as previously described [27]. The paraffin-embedded uterine tissues were cut into 6-μm sections, mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were blocked with 10% normal goat serum in PBS (pH 7.5) and then incubated with anti-PIAS3 (ab22856; Abcam; dilutions: 1:1000), anti-pSTAT3 (9131; Cell Signaling; dilutions: 1:100), or anti-STAT3 (4904; Cell Signaling; dilutions: 1:1000) antibodies in PBS supplemented with 10% normal goat serum overnight at 4°C. The next day, sections were incubated with secondary antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Immunoreactivity was detected using diaminobenzidine (DAB; Vector Laboratories) and then counterstained with hematoxylin and coverslipped with permount. Immunostaining was analyzed using microscopy software from NIS Elements, Inc. (Nikon, Melville, NY).
Statistical Analysis
Statistical analyses were performed using the Student t-test for data with two groups. For data containing more than two groups, we performed an analysis of variance test and analyzed by Tukey or Bonferroni test for pairwise t-test. All data are presented as means ± SEM; P < 0.05 was considered statistically significant. All statistical analyses were performed using the Instat package from GraphPad (San Diego, CA).
RESULTS
Levels of PIAS3 in Eutopic Endometrial Tissue from Women with Endometriosis
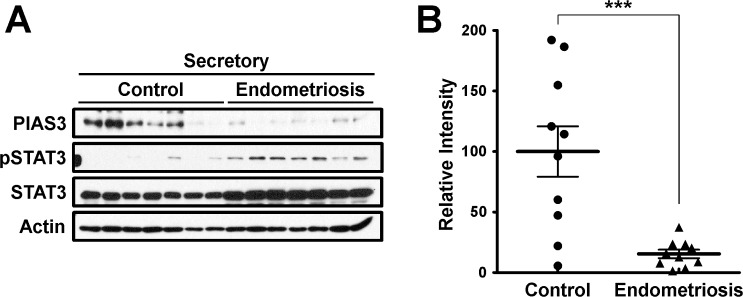
Phosphorylation of STAT3 appeared central to the inflammatory phenotype of eutopic endometrium in women with endometriosis [18, 19]. To determine how the regulation of phosphorylation of STAT3 in endometriosis contributes to infertility, we examined the levels of STAT3 regulators, including six suppressors of cytokine signaling (SOCS) and protein inhibitor of activated STAT3 (PIAS3), phospho-STAT3, and hypoxia inducible factor 1 α (HIF1A), a STAT3 target protein, in endometrium from women with and without endometriosis at secretory stage. As it is known [28], the levels of phospho-STAT3 (pSTAT3) and HIF1A proteins were increased in endometrium from women with endometriosis compared to controls. The levels of SOCS1-3 protein were not different between the endometrium with endometriosis, and those without endometriosis but SOCS4 levels were lower in endometriosis (Supplemental Fig. S1; Supplemental Data are available online at www.biolreprod.org). Interestingly, the levels of PIAS3 protein were significantly decreased in endometrium from women with endometriosis compared to controls. In contrast, the levels of pSTAT3 were increased in the endometrium of endometriosis patients compared to controls (Supplemental Fig. S1 and Fig. 1).
FIG. 1.
Levels of PIAS3 in endometrium from women with and without endometriosis. A) Representative result of PIAS3, pSTAT3, and STAT3 by Western blot analysis in endometrium from women with and without endometriosis. B) Quantification of PIAS3 protein levels by Western blot data in endometrium from women with and without endometriosis obtained by densitometric analysis. The results represent the mean ± SEM. ***P < 0.001.
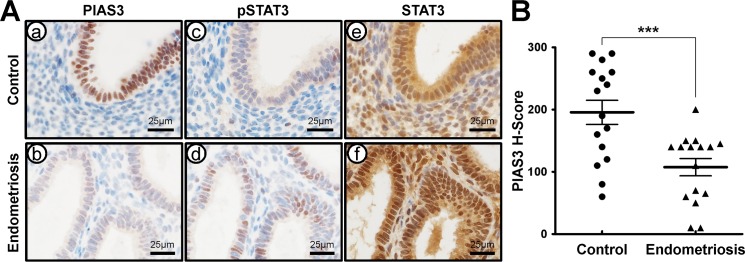
To examine the cell-specific expression of PIAS3, pSTAT3, and STAT3, we next performed immunohistochemical analysis of PIAS3, pSTAT3, and STAT3 in endometrium from women with and without endometriosis at the secretory stage using serial section. PIAS3 proteins were strongly detected in epithelial cells but weakly observed in stromal cells of endometrium without endometriosis (Fig. 2A). Levels of PIAS3 were significantly lower in the epithelial and stromal compartments in endometrium from women with endometriosis compared to women without endometriosis. In contrast, phosphorylation levels of STAT3 were higher in the endometrium from endometriosis patients compared to controls. Total levels of STAT3 protein were not different between them (Fig. 2). The IgG antibody was intended for use as a negative control with PIAS3, pSTAT3, and STAT3 proteins in the endometrium (Supplemental Fig. S2). These results suggest that attenuation of PIAS3 may play an important role in the pathogenesis of endometriosis through STAT3 signaling.
FIG. 2.
Comparison of PIAS3 expression in the endometrium between women with and without diagnosed endometriosis. A) Representative photomicrograph of immunohistochemical staining of PIAS3 (a and b), pSTAT3 (c and d), and STAT3 (e and f) in the endometrium from women without (a, c, and e) or with endometriosis (b, d, and f). Bars = 25 μm. B) The immunohistochemical H-score of PIAS3 expression in the endometrium between women with and without diagnosed endometriosis. The results represent the mean ± SEM. ***P < 0.001.
PIAS3 Expression During Progression of Endometriosis in the Baboon Model
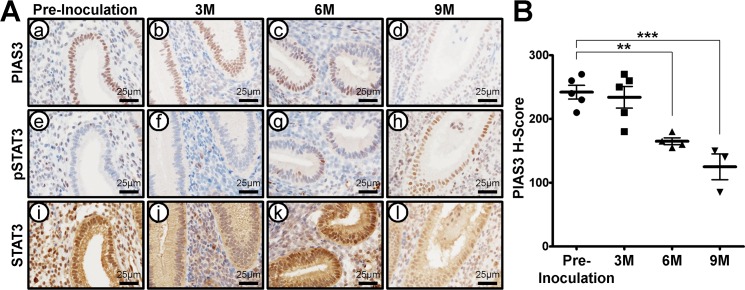
Animal models are useful for studying the temporal sequence of events involved in disease establishment and progression. The baboon model has previously been developed to study the pathophysiology of endometriosis [29–31]. To determine if aberrant expression of PIAS3 was also evident in the baboon model, we examined PIAS3 expression in the eutopic endometrium of baboons at the secretory phase following experimental induction of endometriosis by immunohistochemical analysis. PIAS3 were strongly detected in the endometrium of preinoculation (control) baboons. However, levels of PIAS3 were significantly decreased at 6 and 9 mo postinoculation during endometriosis progression. In contrast, levels of pSTAT3 were strongly detected in the baboon endometrium following induction of the disease, but total levels of STAT3 protein were unchanged (Fig. 3). The IgG antibody was intended for use as a negative control with PIAS3, pSTAT3, and STAT3 proteins in the baboon endometrium (Supplemental Fig. S3). These results suggest that PIAS3 is related to the pathogenesis of endometriosis.
FIG. 3.
PIAS3 expression in endometrium in baboon endometriosis model. A) Representative photomicrograph of immunohistochemical staining of PIAS3 (a–d), pSTAT3 (e–h), and STAT3 (i–l) in endometriosis baboon models induced by intraperitoneal inoculation of menstrual endometrium during progression of endometriosis in preinoculation (a, e, and i) at 3 (b, f, and j), 6 (c, g, and k), and 9 (d, h, and l) mo. Bars = 25 μm. B) The immunohistochemical H-score of PIAS3 in the endometriosis baboon model induced by intraperitoneal inoculation of menstrual endometrium during progression of endometriosis in preinoculation at 3, 6, and 9 mo. The results represent the mean ± SEM. **P < 0.01 and ***P < 0.001.
Suppressed Expression of PIAS3 mRNA in Endometrial Cells Treated with Interferon-γ (INFγ) Through CXCL10
A number of inflammatory factors showed increases after hormone withdrawal [32]. The interferon-gamma (IFN-γ)-induced protein (CXCL10) was increased in human endometrial stromal cells after progesterone withdrawal [32]. CXCL10 is secreted by several cell types, such as monocytes, endothelial cells, and fibroblasts, in response to IFN-γ [33]. To determine if CXCL10 induces PIAS3 expression, we treated Ishikawa, human endometrial adenocarcinoma cells with CXCL10 and subsequently used quantitative PCR and Western blot analysis to examine the expression levels of PIAS3. Levels of PIAS3 mRNA expression were decreased by more than eightfold in Ishikawa cells treated with CXCL10 within 1 hour (Fig. 4A). Additionally, levels of PIAS3 protein were reduced in Ishikawa cells treated with CXCL10 after 2 h (Fig. 4B). These results suggest that CXCL10 is a negative regulator of PIAS3 expression in endometrial epithelial cells.
FIG. 4.
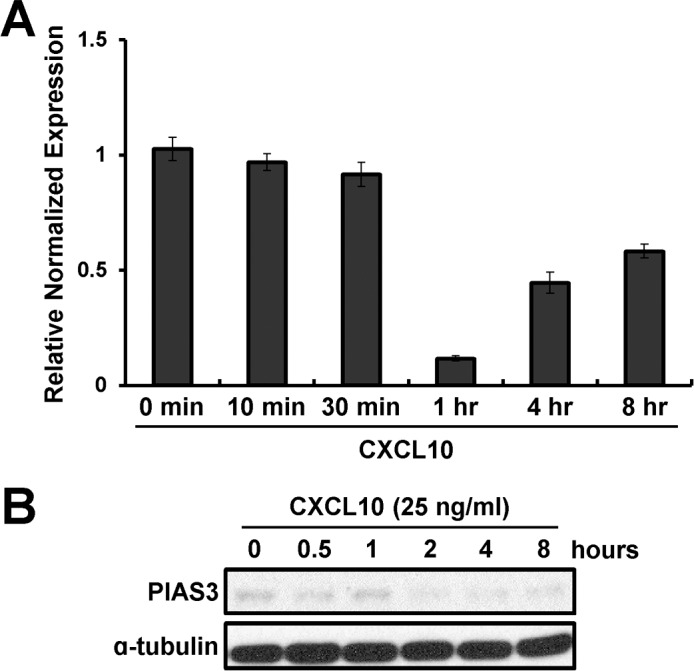
Effect of PIAS3 expression by treatment with CXCL10. RT-PCR (A) and Western blot (B) analysis for PIAS3 after treatment with CXCL10 over time in Ishikawa cells.
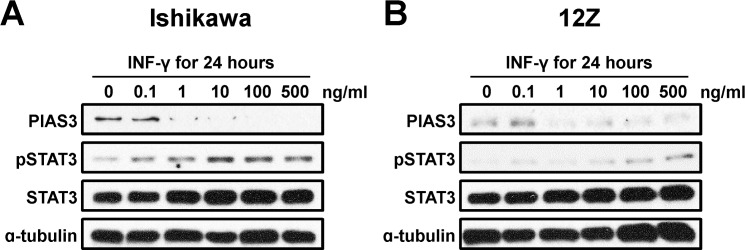
Next, we treated Ishikawa and 12Z, endometriotic epithelial cell line cells with IFN-γ for 24 h and measured PIAS3, pSTAT3, and total STAT3 protein levels by Western blot analysis. The expression of PIAS3 was reduced by IFN-γ in a dose-dependent manner in Ishikawa cells as well as 12Z cells. In contrast, phosphorylation of STAT3 protein was increased by IFN-γ in a dose-dependent manner in these cells (Fig. 5). This result demonstrates that IFN-γ appears to be a negative regulator of PIAS-3 via CXCL10 in endometrial epithelial cells.
FIG. 5.
Levels of PIAS3 protein by IFN-γ in a dose-dependent manner in Ishikawa and 12Z cells. Western blot analysis of PIAS3, pSTAT3, and STAT3 in Ishikawa (A) and 12Z (B) cells treated with IFN-γ in a dose-dependent manner for 24 h. Actin was used as loading control.
DISCUSSION
Endometriosis is an estrogen-dependent chronic inflammatory disease affecting fertility. Inflammation can lead to an anatomic disorder and plays an important role in the pathogenesis of endometriosis [1]. According to previous studies, STAT3 is significantly implicated in inflammatory responses. STAT3 can be activated by a variety of cytokines, including leukemia inhibitory factor, IL-6, IL-11, and epidermal growth factor [8, 9]. STAT3 is a key signal transducer and regulator of gene expression critical to normal cellular processes, including cell development, differentiation, proliferation, survival, angiogenesis, and immune function [13]. However, the function of STAT3 has not been studied enough in the development of endometriosis. Previously, we found that phosphorylated STAT3 is higher in endometrium of women with endometriosis compared to subjects without endometriosis. Additionally, phosphorylation of STAT3 can be remarkably increased by IL-6, and HIF1A, one of the STAT3 target genes, is elevated and significantly correlated with STAT3 phosphorylation in endometrium of women with endometriosis compared to those without endometriosis [18]. Progesterone resistance is a well-established phenomenon seen in endometriosis and the eutopic endometrium [34]. Progesterone favors the stimulation of STAT5 [35], which is an inhibitor of STAT3 [36]. We recently published a review of the central role of STAT3 signaling on the alterations in endometrial receptivity [37]. This study showing that STAT3 signaling is dysregulated by reduced PIAS3 may provide further insight into the development and progression of endometriosis but also provide information related to treatment options for women with infertility.
First, we examined levels of inhibitors of activated STAT3, such as SOCS and protein inhibitor of activated STAT3 (PIAS3), to determine how the regulation of the phosphorylation of STAT3 functions in endometriosis. PIAS3 protein levels were lower in endometrium from women with endometriosis compared to those without endometriosis. In normal conditions, activation of STAT is rapid and transient because it is regulated negatively by proteins such as SOCS and PIAS3 [20, 21]. We found that SOCS levels were not different between women with and without endometriosis, except in SOCS4. SOCS proteins inhibits STAT3 signaling by inhibiting the kinase activity of Janus kinase through direct binding [20, 38, 39]. However, PIAS3 can inhibit activity by interacting with the STAT dimer and blocking transcription [21, 22]. These results demonstrate that PIAS3 may be an important inhibitor of STAT3 signaling in the development and progression of endometriosis independent of SOCS protein changes.
We observed the novel finding that the expression level of PIAS3 was significantly reduced in the endometrium of women with endometriosis compared to women without endometriosis. We also confirmed that PIAS3 expression levels were significantly decreased in baboon endometriosis animal models during endometriosis progression. The ontogeny of inflammation in the endometrium is demonstrated in the baboon model. As we recently showed, it is difficult to remove the inflammation by surgery alone [40], suggesting that this is a systemic disease of inflammation that, once established, is perpetuated by recurrent menses or continued presence of implants. It apparently takes time after inoculation for the inflammation in the pelvis to be recognized by the immune system and then begin to be reflected in the endometrium. This model suggests it takes many months before the changes are showing up in the endometrium, of which 40% are immune cells. There are no immediate differences in the preinoculation and 3-mo images. The decrease is associated with the onset of progesterone resistance [26, 41–44]. At 3 mo, the endometrium is primarily estrogenic, and progesterone-regulated genes are not affected, so it would be expected to look like the controls. Additionally, PIAS3 proteins were strongly detected in the nucleus of human and baboon endometrial epithelial cells. However, the expression of PIAS3 was weakly detected in some stromal cells. Taken together, we suggest that PIAS3 may have an important role in the pathogenesis of endometriosis.
CXCL10 has been found to inhibit growth, angiogenesis, and metastasis in experimental tumors [45–47]. CXCL10 was reported to be elevated after progesterone withdrawal, associated with menstrual-related inflammation [32]. Several studies suggest that the down-regulated CXCL10 correlated with poor patient prognosis in human cancer and the suppression of CXCL10 expression result in drastic tumor growth and metastasis that accelerate tumor advancement [48, 49]. Additionally, levels of CXCL10 in serum and peritoneal fluid of women with advanced endometriosis are significantly lower compared to women without endometriosis [50]. Therefore, more research is needed about the role of CXCL10 in endometriosis. PIAS3 expression levels were rapidly decreased in Ishikawa cells treated with CXCL10. Furthermore, PIAS3 protein levels were reduced in Ishikawa cells and 12Z treated with dose-dependent IFN-γ upstream of CXCL10. However, we do not have direct evidence about the IFN-γ suppressed expression of PIAS3 by CXCL10 in this study. Therefore, there is a need to investigate in order to explain a molecular mechanism of IFN-γ-CXCL10 signaling to regulate PIAS3 expression. Other related factors that interfere with PIAS3 cannot be ruled out. Nitric oxide (NO) has been shown to destabilize PIAS3 in other cell types [51]. NO synthase has been shown to be elevated in women with infertility and endometriosis [52], which may be induced by STAT3 activation via IL-6-mediated mechanisms [53].
PIAS3 may be a targetable molecule for the treatment of inflammatory conditions, such as endometriosis. In published studies, curcumin suppresses activation of STAT3 in ovarian and endometrial cells through up-regulation of PIAS3 [54].
Overall, these findings show for the first time that PIAS3 is reduced in women with endometriosis. IFN-γ reduces PIAS3 and increases activity of STAT3, perhaps through CXCL10. The activation of STAT3 associated with inflammation appears to be due, in part, to aberrant regulatory pathways involving overexpression of stromal CXCL10. Activation of STAT3 and downstream events affecting endometrial receptivity appear to be a central feature of endometriosis. STAT3 and PIAS-3 represent new therapeutic targets for the treatment of endometriosis.
ACKNOWLEDGMENT
We would like to thank Mark Olson and Angela Houwing for sample preparation and Amanda Sterling for manuscript preparation.
Footnotes
This work was supported by the NIH R01 HD067721 (to S.L.Y. and B.A.L.) and NIH R01 HD084478 (to J.W.J.).
REFERENCES
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–182. doi: 10.1146/annurev-physiol-012110-142158. [DOI] [PubMed] [Google Scholar]
- Xiong J, Zeng P, Cheng X, Miao S, Wu L, Zhou S, Wu P, Ye D. Lipoxin A4 blocks embryo implantation by controlling estrogen receptor alpha activity. Reproduction. 2013;145:411–420. doi: 10.1530/REP-12-0469. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Villa ML. Endometriosis. N Engl J Med. 1994;330:70. doi: 10.1056/NEJM199401063300120. [DOI] [PubMed] [Google Scholar]
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–1769. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Kocakoc E, Dogra VS. Endometriosis: sonographic spectrum. Ultrasound Q. 2006;22:273–280. doi: 10.1097/01.ruq.0000237256.41132.fb. [DOI] [PubMed] [Google Scholar]
- Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci U S A. 2001;98:8680–8685. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- Wang Y, van Boxel-Dezaire AH, Cheon H, Yang J, Stark GR. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc Natl Acad Sci U S A. 2013;110:16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: have all roads been explored? JAKSTAT. 2013;2:e22882. doi: 10.4161/jkst.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Karin M. NF-kappaB and STAT3—key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–1078. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano Y, Kai K, Moriyama M, Narahara H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30:632–641. doi: 10.1093/humrep/deu332. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Ueki N, Seki N, Yano K, Saito T, Masuho Y, Muramatsu M. Isolation and chromosomal assignment of a human gene encoding protein inhibitor of activated STAT3 (PIAS3) J Hum Genet. 1999;44:193–196. doi: 10.1007/s100380050141. [DOI] [PubMed] [Google Scholar]
- Levy C, Nechushtan H, Razin E. A new role for the STAT3 inhibitor, PIAS3: a repressor of microphthalmia transcription factor. J Biol Chem. 2002;277:1962–1966. doi: 10.1074/jbc.M109236200. [DOI] [PubMed] [Google Scholar]
- Jang HD, Yoon K, Shin YJ, Kim J, Lee SY. PIAS3 suppresses NF-kappaB-mediated transcription by interacting with the p65/RelA subunit. J Biol Chem. 2004;279:24873–24880. doi: 10.1074/jbc.M313018200. [DOI] [PubMed] [Google Scholar]
- Long J, Wang G, Matsuura I, He D, Liu F. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3) Proc Natl Acad Sci U S A. 2004;101:99–104. doi: 10.1073/pnas.0307598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88:44. doi: 10.1095/biolreprod.112.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Yoo JY, Kim HI, Gilbert J, Ku BJ, Li J, Mills GB, Broaddus RR, Lydon JP, Lim JM, Yoon HG, Jeong JW. Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res. 2014;74:7371–7382. doi: 10.1158/0008-5472.CAN-14-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–1078. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15:577–586. doi: 10.1093/molehr/gap057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis Ann N Y Acad Sci 2002. 955 308 317 ; discussion 340, 302, 396 406 [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- Evans J, Salamonsen LA. Decidualized human endometrial stromal cells are sensors of hormone withdrawal in the menstrual inflammatory cascade. Biol Reprod. 2014;90:14. doi: 10.1095/biolreprod.113.108175. [DOI] [PubMed] [Google Scholar]
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55:795–810. doi: 10.1507/endocrj.k08e-067. [DOI] [PubMed] [Google Scholar]
- Walker SR, Nelson EA, Yeh JE, Pinello L, Yuan GC, Frank DA. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol Cell Biol. 2013;33:2879–2890. doi: 10.1128/MCB.01620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C, Morin S, Jeong JW, Scott RT, Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105:873–884. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, Lessey BA, Tayade C. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016;105:968–977. doi: 10.1016/j.fertnstert.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74:1060–1066. doi: 10.1095/biolreprod.105.049320. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75:176–182. doi: 10.1095/biolreprod.106.052852. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4((suppl 1)):S7. doi: 10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Keyser J, Schultz J, Ladell K, Elzaouk L, Heinzerling L, Pavlovic J, Moelling K. IP-10-encoding plasmid DNA therapy exhibits anti-tumor and anti-metastatic efficiency. Exp Dermatol. 2004;13:380–390. doi: 10.1111/j.0906-6705.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Iwashita Y, Ohta M, Shibata K, Ishio T, Ohmori N, Goto T, Sato S, Kitano S. Antitumor effects of the MIG and IP-10 genes transferred with poly [D,L-2,4-diaminobutyric acid] on murine neuroblastoma. Cancer Gene Ther. 2007;14:696–705. doi: 10.1038/sj.cgt.7701059. [DOI] [PubMed] [Google Scholar]
- Mei K, Wang L, Tian L, Yu J, Zhang Z, Wei Y. Antitumor efficacy of combination of interferon-gamma-inducible protein 10 gene with gemcitabine, a study in murine model. J Exp Clin Cancer Res. 2008;27:63. doi: 10.1186/1756-9966-27-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Fujimoto J, Toyoki H, Sakaguchi H, Alam SM, Jahan I, Tamaya T. Expression of IP-10 related to angiogenesis in uterine cervical cancers. Br J Cancer. 2007;96:1735–1739. doi: 10.1038/sj.bjc.6603790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Xu Y, Cai S. CXCL10 expression and prognostic significance in stage II and III colorectal cancer. Mol Biol Rep. 2010;37:3029–3036. doi: 10.1007/s11033-009-9873-z. [DOI] [PubMed] [Google Scholar]
- Galleri L, Luisi S, Rotondi M, Romagnani P, Cobellis L, Serio M, Petraglia F. Low serum and peritoneal fluid concentration of interferon-gamma-induced protein-10 (CXCL10) in women with endometriosis. Fertil Steril. 2009;91:331–334. doi: 10.1016/j.fertnstert.2007.11.075. [DOI] [PubMed] [Google Scholar]
- Qu J, Liu GH, Wu K, Han P, Wang P, Li J, Zhang X, Chen C. Nitric oxide destabilizes Pias3 and regulates sumoylation. PLoS One. 2007;2:e1085. doi: 10.1371/journal.pone.0001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorram O, Lessey BA. Alterations in expression of endometrial endothelial nitric oxide synthase and alpha(v)beta(3) integrin in women with endometriosis. Fertil Steril. 2002;78:860–864. doi: 10.1016/s0015-0282(02)03347-2. [DOI] [PubMed] [Google Scholar]
- Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J Biol Chem. 2003;278:16304–16309. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110:447–456. doi: 10.1002/jcb.22558. [DOI] [PubMed] [Google Scholar]