Abstract
Background
Behavioral models relevant to stroke research seek to capture important aspects of motor skill typically impaired in human patients, such as coordination of distal musculature. Such models may focus on mice since many genetic tools are available for use only in that species, and since the training and behavioral demands of mice can differ from rats even for superficially similar behavioral readouts. However, current mouse tests are time consuming to train and score, especially in a manner producing continuous quantification. An automated assay of mouse forelimb function may provide advantages for quantification and speed, and may be useful for many applications including stroke research.
New Method
We present an automated assay of distal forelimb function. In this task, mice reach forward, grip and pull an isometric handle with a prescribed force. The apparatus partially automates the training process so that mice can be trained quickly and simultaneously.
Results
Using this apparatus, it is possible to measure long-lasting impairment in success rate, force pulled, latency to pull, and latency to success up to 22 weeks following photothrombotic cortical strokes in mice.
Comparison with Existing Method(s)
This assessment measures forelimb function as do pellet reach tasks, however it utilizes a different motion and provides automatic measures that can ease and augment the research process.
Conclusions
This high-throughput behavioral assay can detect long-lasting motor impairments, eliminates the need for subjective scoring, and produces a rich, continuous data set from which many aspects of the reach and grasp motion can be automatically extracted.
Keywords: stroke, ischemic stroke, behavior, forelimb, mouse
1 Introduction
About 80% of people who suffer ischemic strokes incur motor deficits that interfere with quality of life1. Developing and refining measures of functional motor impairment and recovery after stroke in mouse models could therefore contribute to improved relevance of mouse research. The most promising current motor assays require extensive scoring and subjective evaluation that makes efficient, high-throughput, flexible research challenging.
Since functional impairment of the distal forearm is an important cause of disability in stroke patients, an ideal rodent assay will capture the important aspects of such movements2. It is advantageous to develop such assays particularly for mice given the extensive availability of genetic and pharmacological mouse models. However, assays for mice need to be developed independently from those utilized in rats since neither training nor performance patterns necessarily overlap between the species. Existing tests meeting these criteria such as skilled pellet retrieval reaching tasks 3-5 can be time consuming to train and score, especially in a manner producing continuous quantification. When behavior is automatically measured rather than scored visually, it can be more easily quantified to a finer degree than is practical with visual scoring. We have developed such an automated assay of skilled forelimb use for mice based on versions published for use in rats6, 7. Here, we describe this assay, which requires the coordination of several forearm muscles. Like existing skilled reach tasks, this assay requires a mouse to reach through a slit to grasp an object in a manner amenable to automated or hand-scored motion analysis3, however unlike existing tests the subsequent force exertion on the object is highly constrained and isometric. The task precludes behavioral compensation, shows lasting deficits as a result of photothrombotic cortical stroke, allows for flexibility in different aspects of behavioral measurement, can be trained in a partially automated fashion without close attention, and can be consistently applied in large numbers of mice with efficiency and precision. We present and validate this assay primarily for use in a photothrombotic mouse model of stroke and demonstrate its sensitivity to that injury. However, this assay could be useful for any application that requires a sensitive assay of forelimb function.
2 Methods
2.1 Apparatus and Procedure
2.1.1 Enclosure, Behavior, Measurement and Analysis
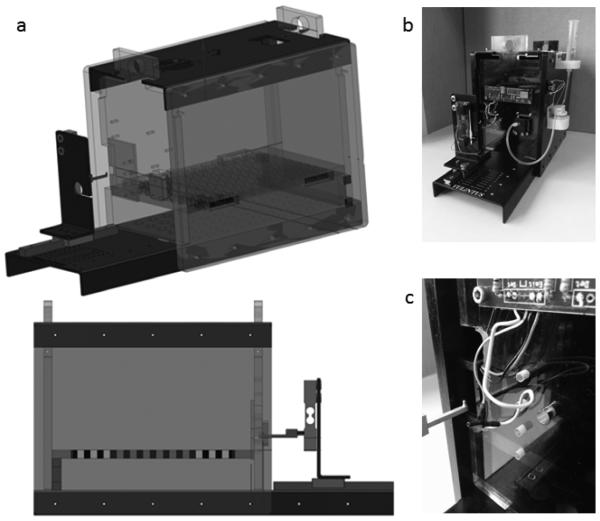
The apparatus has been developed in collaboration with Vulintus, Inc. (Dallas, TX) and resembles a similar design optimized for rats6 (Figure 1). It consists of a plexiglass enclosure 5.5” high × 5” wide × 8” long. A pattern of square holes in the floor allow waste to fall to the level below. A slot in the right side of the front wall provides access to a vertical handle 3 mm tall, 1 mm wide and 1 mm thick, connected to a force transducer that measures unidirectional horizontal force exerted in the direction of the mouse. The position of the handle and the directionality of the required force constrain the behavior; the mouse cannot succeed by pushing the handle from the sides, top or bottom and must use paw musculature to grasp around the back of it. Additionally, the handle is most easily grasped from the side since a grasp from the top would allow fewer digits to exert force on the back of the handle, providing a natural constraint to the top-down raking motion normally considered compensatory in similar assays. The calibrated transducer measures up to ~70 g with 1 g precision. Accuracy of the force signal is assured through regular calibration and testing with precision weights. Mice can generally pull a maximum of about 35 g on this apparatus. The front edge of the handle is positioned 1 cm from the inner edge of the chamber. Between that edge and the handle, an infrared (IR) slot detector is positioned vertically across the slit to detect reach attempts. Adjacent to the slot, a bracket recessed in the plexiglass wall presents the blunt tip of a feeding needle controlled by a pinch valve. Following a successful pull, the pinch valve emits an audible click and releases approximately 2 μl of peanut oil at the end of the feeding needle. Signals from the infrared beam and the force transducer are sampled every 10 ms using a custom control board and recorded permanently during adjustable trial windows. Trials are initiated by either a break of the IR beam or by a force exerted on the handle greater than an adjustable initiation threshold of 2 g. A trial ends upon the longer of two seconds (also adjustable) or when the IR beam has been unbroken and less than 2 g has been exerted on the handle for at least 1 second. Trial data is written continually during a behavioral session, preventing incidental data loss. Data is streamed by custom MATLAB software, which displays and stores the data as continuous traces. The raw data is used to derive five different measurements, summarized in Table 1 and Figure 2. Only the first 50 responses of a session are considered in this analysis.
Figure 1 – 1 or 1.5 column.
A pparatus a. Schematic representation without peanut oiIdispenser. b. Wide-angle pictures of complete apparatus. c. Close up of handle and positioning relative to apparatus.
Table 1.
Derived Behavioral Measures. This validation experiment used these five transformations of the raw data returned by the apparatus, though many others are possible.
| Derived Measure | Description |
|---|---|
| Success Rate | Number of successful trials divided by number of total trials analyzed |
|
| |
| Highest Force in Trial | Highest force measurement within each trial, averaged over trials |
|
| |
| Latency to Pull | Time between the first moment that the IR beam was broken until the moment that the force reading exceeded the initiation threshold, averaged over trials |
|
| |
| Latency to Success | Time between the first moment that the IR beam was broken until the moment that the force reading first exceeded 20 g, averaged over trials |
|
| |
|
Attempts Before
Success |
Number of local force peaks above initiation threshold within a trial before the force reading first exceeded 20 g. These “attempts” are caused by grasp-and-pull motions that fall below force requirements. |
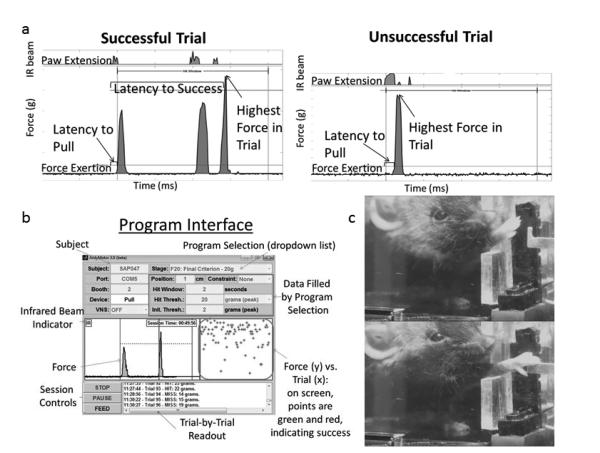
Figure 2 – 1 or 1.5 column.
a. Example Trials. Data traces for sample trials showing raw measurements (paw extension and force) and derived measurements (latency to success, highest force in trial, latency to pull). Both signals are stable while the mouse is not interacting with the slot or handle, and both signals change upon behavioral performance with a clear signal relative to noise. The left trace shows 3 attempts before success. b. Program Interface. An experimenter can control and monitor a session via this GUI. Individual trails can be seen in a list on the lower right, and a maximum trial force through time for both hits and misses can be seen in a graph at the middle right. Raw data for the past several seconds can be seen in the middle left, and settings for the session at the top. Sessions are controlled by buttons on the lower left. The GUI is displayed in color on computer screens. c. Photos of a mouse reaching for the handle. The mouse first brings its nose close to the opening then extends the right forepaw with the long axis of the wrist close to vertical. The first digits then wrap around the handle and exert force in the direction of the mouse’s body.
2.1.2 Program
The apparatus is controlled by custom MATLAB software. This software presents a user interface as seen in Figure 2. A drop-down menu allows the user to select from variable, customizable program settings. The program specifies the initiation force required to begin a logged pull, the force that must be exceeded to trigger reinforcement (peanut oil delivery), and the manner in which the force required to trigger reinforcement changes throughout the session. “Static” sessions retain a constant force requirement. Adaptive “Linear” sessions increase the requirement by a customizable increment every time a successful pull occurs. Adaptive “Median” sessions set a force criterion as the lower half or quartile of the previous n pulls. For experiments reported here, “Linear” was used for training and “Static” for baseline and post-stroke sessions. While a session is running, the MATLAB interface provides a real time list of logged pulls and a plot of trial vs. grams of force, with successful trials in green and unsuccessful in red (Figure 2 shows a black-and-white image; the on-screen graphical user interface is in color).
2.2 Validation of Apparatus in the Context of Stroke
2.2.1 Subjects
Thirteen adult C57-Bl6 mice weighing approximately 20-30 g were used to assess the behavioral effects of photothrombotic stroke on this forelimb task. Mice ranged in age from 25 to 35 weeks old at the time of stroke; four were female and nine were male. All mice were housed in a temperature and humidity maintained facility on a reverse light cycle to assure that their high-activity periods would occur during working hours. All mice had food and water available to them ad-libitum in their home cage and also in their reach chambers if subjected to long sessions. All procedures involving these mice were approved by the UT Southwestern Institutional Animal Care and Use Committee.
2.2.2 Training and Baseline Procedure
Mice for this validation were trained using automated sessions to pull the handle with a force greater than 20 g. First, mice were exposed to peanut oil in the home cage. After acclimation to the reach chamber, they received random deliveries of peanut oil every 3-8 minutes until they responded to the sound of the pinch valve by approaching the feeding needle. The handle was then introduced through the slit in the chamber wall, baited at first with a small drop of peanut oil. At first, any detectable force exerted on the handle triggered delivery of peanut oil. The handle was slowly removed from the chamber until located at its final position relative to the opening, at which point the force criteria for peanut oil delivery slowly increased until it reached 20 g. Baseline sessions then began and continued until three consecutive sessions showed stability in all measures; stability was defined as a variance that was equal to or less than the average variance of the final three sessions of three mice who had run for 2-3 months without injury and no longer showed any performance trends. Overall, this procedure takes ~10-20 sessions (one session/day) for training and another ~5-10 for baseline. The range in duration for the final three baseline sessions for these subjects was .48 hours to 9.75 hours (mean 3.06, standard deviation 2.39). The longest durations were due to logistics and not due to slow response rates; during baseline animals were often allowed to pull well more than 50 times if it was not convenient to check on them often. Training sessions were usually longer, ranging from approximately 5 to 24 hours, also depending on logistics. In this group 4 of 17 mice were eliminated because they were taking too many training sessions to progress due to human error or unknown factors. Mice were safely left alone in the chambers for spans of several hours with food and water available in longer sessions. However, ideally the mice were monitored every hour or two in order to assure that sessions ended at approximately the target number of pull attempts or to assure ideal training progress.
2.2.3 Stroke
After training and baseline, mice were anesthetized with a mixture of 30° O2, 70° NO, and 1-4° isoflourine. They were then affixed to a stereotaxic apparatus, their scalp injected with lidocane, and a small incision made along the midline of the scalp. The skin was pulled aside and the scalp dried. A Coherent 561nm laser was aimed at the forepaw representation of the motor cortex, 1.7 mm directly left of bregma (derived from Tennant, et al.8). Mice then received an intraperitoneal injection of 1.5 mg of Rose Bengal suspended in 0.3 ml of saline. The laser was activated one minute after the injection, delivering 55 mW of light to an area of skull roughly 3 mm in diameter for a total of 15 minutes. The mouse’s eyes were shielded with a piece of aluminum foil to avoid damage from reflections. The incision was then stitched shut and 0.2 cc subcutaneous saline was administered to restore any lost body fluids. Buprenorphine was used during and after surgery to control postoperative pain.
2.2.4 Behavioral Recovery, Sacrifice, and Perfusion
Sessions of automated reach occurred three and seven days after stroke, then weekly for another 21 weeks. Post-stroke session duration varied to allow the mouse to reach at least 50 pulls. Due to low pull rates, some mice were given very long sessions or multiple chances through a period of days to reach their minimum pull count. Of 299 sessions, 88 were multi-day sessions (35 of these were eliminated from analysis, see below), 41 were overnight, and non-overnight sessions ranged in duration from 0.18 to 11.45 hours (mean = 3.94, SD = 2.52). The average amount of practice that an individual mouse experienced in one week was 134 pulls, though this varied (SD = 138). Variation in pull rate tended to be higher between mice than within mice; mice who required longer sessions did so consistently (5 of the 13 mice accounted for 65 of the 88 multi-day sessions). The first three pilot mice continued to be tested weekly after their 22nd week in order to probe possible longer-term patterns of recovery before being sacrificed and perfused at 5 ml/min with 20 ml chilled PBS and 0.1° heparin and then 40 ml of chilled paraformaldehyde. Other mice were sacrificed and perfused after 22 weeks. Three mice, after an extended period of recovery, ceased to pull at a sufficiently high rate to confidently quantify their performance. This may have been due to apparatus repairs (and thus potential accidental environmental changes) that loosely coincided with these performance disruptions. Since these mice produced viable data until the point of their disruption, their data is included in graphs. However, for any week where less than 20 pulls occurred (a total of 35 sessions between the three of them), their data has been removed and they are not included in recovery statistics.
2.2.5 Histology
Brains from perfused mice were extracted and stored for one day in paraformaldehyde at 4°C and for at least two days in 30° sucrose at 4°C. Coronal slices 30 μm thick were collected with a freezing microtome and stained with cresyl violet to visualize and quantify the stroke size and location. Six sections from each mouse were first mounted onto slides; the first section was located at approximately 1.7 mm anterior of bregma, and subsequent sections were 720 μm apart. Slides were incubated for 20 minutes in cresyl violet, developed for 5 minutes in 70° EtOH, 3 minutes in 95° EtOH, 3 minutes in 100° EtOH, and 5 minutes in Xylene. Slides were then coverslipped with permount and visualized with a Hammamatsu Nanozoommer bright field slide scanning microscope after drying.
2.2.6 Analysis and Statistics
For behavior data, each session’s success rate, average highest force within trial, and average latency to pull measurement were calculated using only the first 50 pulls of each session. For average latency to success, analysis was restricted to the first 50 successes. If a session yielded no successes, a value for latency to success was determined by averaging the previous two non-baseline sessions and the subsequent two sessions for that mouse; the same method applied to rare sessions in which equipment malfunction yielded inaccurate latency measures. Impairment on each derived measure was assessed using a two-tailed paired t-test between the average of the last three stable baseline sessions and the 7-day post-stroke time point. Recovery data was normalized to individual baseline averages before analysis. To assess global recovery, each behavioral measure was evaluated using a two-tailed paired t-test between day 7 post-stroke and day 154 post-stroke. One way ANOVAs were used to assess changes over time, and each time point was assessed individually by performing a Fisher’s LSD analysis comparing each post-stroke time point to the final time point of baseline.
To determine stroke volumes, the healthy area of each hemisphere of cresyl-violet stained sections was determined using Image J, excluding ventricles. A mouse’s stroke volume was calculated by subtracting the area of the ipsilesional hemisphere of each section from the contralesional, then multiplying the sum of the differences by the space between sections.
3 Results
3.1 General
The apparatus and software produced a reliable force signal with a resolution of 1 g of force and an easily discernable infrared break signal due to paw extension (Figure 2). Mice performed the reach task in the desired physical form, reaching and grasping with the right paw from the right side of the handle (Figure 2). Three mice without a stroke performed the task for three months and showed no trends in performance.
3.2 Stroke impairment
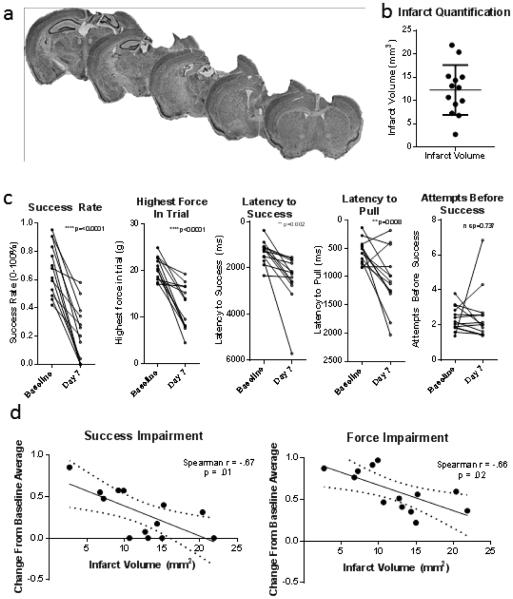
After baseline, thirteen mice were subjected to photothrombotic stroke of the forepaw representation of the left motor cortex. Most mice had lesions larger than 10 mm3, and all but one had lesions larger than 5 mm3 (Figure 3). The infarct included the forelimb representation for all mice, and the subcortical white matter below the target was eliminated in all but two. The variability of photothrombotic stroke volumes (coefficient of variation .43) falls within the range of some of the most recent examples of mouse photothrombotic stroke experiments in the literature (coefficitions of variation .459, .2410, .0511, .2212) despite the fact that we did not eliminate subjects based on stroke volume. Variability in stroke volume can be due to slight differences in laser scattering, rose Bengal uptake, or individual differences in physiology.
Figure 3 – 2 column.
a. Cresyl Violet stains of 6 serial sections 720μm apart from one mouse who received photothrombotic stroke. Stroke included the forelimb representation of the motor cortex and disrupted subcortical white matter tracts in all but two animals. b. Infarct volumes between 1.7 mm anterior to bregma to 2.6 mm posterior to bregma. c. Baseline and post-stroke performance in each of 5 measures, p values from paired two-tailed t-tests. After stroke, the average success rate and maximum force were nearly two standard deviations below baseline, while latency measures were one standard deviation below baseline. d. Decrease from baseline average in success and force on day 7 following stroke is correlated with infarct volume.
After stroke, measures of success rate, highest force within trial, latency to pull, and latency to success all showed significant impairment (2-tailed paired t-tests between the average of the final 3 baseline sessions and the 7 day post-stroke session p=0.001, 0.002, 0.002, and 0.008, respectively). The number of attempts before success did not change after stroke (p=0.737). Impairment was not equal among all mice, and impairment in success rate and highest force in trial was correlated with stroke volume (Spearman’s correlation success rate r = .67, p = .01, highest force in trial r = .66, p = .02) (Figure 3). While mice with smaller stroke volumes had less impairment, all animals had statistically significant impairment at day 7 following stroke as compared to baseline.
3.3 Recovery
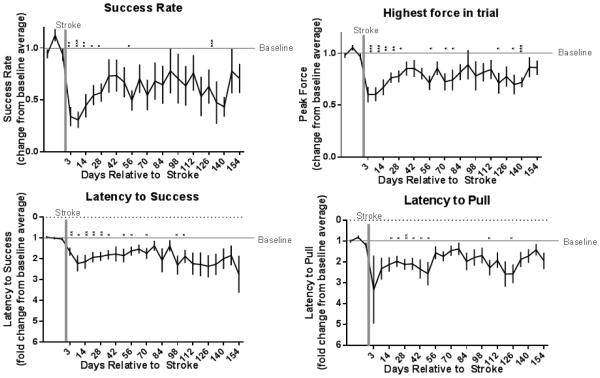
Success rate and highest force in trial improved significantly through the course of 154 days of recovery (paired two-tailed t-tests between day 7 post-stroke and day 154, each normalized to individual baselines p= .04, .02, respectively), but latency to pull and latency to success did not (p= .83, .36, respectively). Though variance in the latter two measures was too high to show statistical differences between days 7 and 154, averages returned to approximate baseline performance around week 8 and Fisher’s LSD comparisons to baseline no longer showed consistent differences. Performance in success rate and highest force in trial continued to show averages below baseline and statistically significant differences from baseline throughout the 22 week period (Figure 4). One-way ANOVAs show significant changes over time for these measures (p = .05, .03) but not for latency measures (p = .17, .22), indicating recovery in the latter. Individual recovery data (Supplementary Figure 1) shows that reliable baseline performance, defined as returning to the individual’s baseline 95° confidence interval at least twice, was never reacquired in the 22 week period for 4 mice in this group (31°). Standard deviation of performance at week 28 (a typical recovery period in the literature) indicates that for some effect sizes, fewer than 10 mice should be needed to determine differences in improvement when using this assay to test variables affecting functional recovery (Table 2).
Figure 4 – 2 column.
Recovery data (n=13) collected at days 3, 7, and weekly until day 154 after stroke: error bars represent SEM. Stars above individual time points represent Fishers LSD individual comparisons between that time point and the last baseline time point: p .05 (*), p .05 (**), p .005 (***),and p .0005 (****).
Table 2.
Power Analysis. These are estimates of sample sizes needed to distinguish a difference of certain magnitudes in each of four measures at day 28 after stroke. Power analysis used 5% alpha, 50% beta and the standard deviation and mean of day 28 data normalized to baseline.
| Effect Size | Approximate Sample Size (# of Mice) | |||
|---|---|---|---|---|
|
| ||||
| Success Rate | Highest Force in Trial |
Latency to Success |
Latency to Pull | |
| 50% | 9 | 2 | 3 | 3 |
|
| ||||
| 40% | 15 | 3 | 5 | 5 |
|
| ||||
| 30% | 26 | 5 | 8 | 8 |
|
| ||||
| 20% | 59 | 12 | 18 | 18 |
|
| ||||
| 10% | 236 | 48 | 72 | 72 |
3.4 Age and sex differences
Mice ranged in age from 25 to 35 weeks at the time of stroke, and these age differences did not correlate with either impairment or recovery after 22 weeks (all Spearman’s correlations p>0.2). This experiment included both sexes, but not in the numbers necessary to determine potential sex differences.
4 Discussion
This study establishes a valid measure of functional forelimb impairment and recovery for this mouse model of ischemic stroke. A relatively small, 5-20 mm3 cortical stroke produced an impairment of one to two standard deviations in four of the five derived measures examined here. Impairments in these measures were long-lasting, and 4 of 13 mice never returned reliably to baseline performance in a 22 week period. Attempts before success did not change as a result of stroke, even though a similar measure in rats did show impairment6.
Some researchers are interested not only in outcome measures of forelimb behavior (such as success rate) but also in the physical form of the motion. Video scoring remains the primary methods for performing this kind of analysis. While our setup allows for video, it also captures near-continuous quantitative measurements that permit many more derived measures than those directly examined here, some of which may serve as indices of physical motion. For example, particular movements could influence the shape of the force waveform, slope, local maxima, duration or could limit rate or latency. Investigators could also easily modify aspects of the behavior itself via reinforcement contingency, requiring different particular forms of force, timing, etc. for reinforcement. The apparatus could even easily be adapted to measure different forms of motion, requiring the mouse to push, displace, or even twist the handle13.
Many aspects of this apparatus provide benefits for the research process. Most trained behavioral assays require an experimenter to closely shape the initial behavior in each animal and sometimes to individually monitor subsequent sessions, which can be prohibitively time-consuming when many mice are needed for statistical analysis. In this apparatus, numerous mice can be trained and evaluated concurrently. Shaping is largely automated, which eliminates the need to closely monitor, permits the simultaneous training of multiple mice and decreases the time to run experiments. Shaping and training require ~15-30 sessions. Overnight sessions are possible but not necessary. Two derived behavioral measures are directly related to the operant requirements of the task (success rate, highest force) and two are not (latencies); thus, indirect training effects can be evaluated along with overtly trained/rehabilitated motor patterns without extra data collection requirements. The long-lasting deficits determined here present the potential to test interventions that change the extent as well as the speed of recovery. Finally, a dynamic range of one to two standard deviations enables the clear evaluation of experimental impacts with manageable group sizes (Table 2).
This task and pellet reach tasks are designed to measure forelimb motor function, although they involve different mechanical motions and reinforcement parameters; thus they are not directly comparable. Pellet reach tasks also show long-lasting deficits following ischemic injury. In one case those deficits lasted up to three months in rats14; however in that study the stroke was considerably larger. Photothrombotic strokes in mice of a size comparable to that reported here produce faster recovery of approximately 16 days3, 14 days4, or at least 28 days5 whereas our task detected deficits up to 154 days. The performance change resulting from injury measured via single-pellet reach tasks in these studies was approximately 30°3, 25°4 and 65°5 of baseline; success, peak force, pull and success latency measures of this assay compare well at approximately 69°, 40°, 132° and 122° of baseline, respectively.
This task, along with most operant tasks, is taught and maintained using food reinforcement. Unlike many tasks, this assay requires no food restriction for either acquisition or maintenance; the novel innovation of using peanut oil as a reinforcer maintains high levels of responding without dietary constraints. Since caloric restriction influences stroke impairment and recovery15, and since most patients are not calorically restricted, the possibility of training and maintaining this task without deprivation adds strength to the model and could potentially help with translational validity. However, in scenarios where this consideration is not paramount, this task still could be maintained using food restriction. Such restriction could potentially decrease training time or session time, which could increase throughput and efficiency even further.
Key genetic manipulations are often possible or readily available only in mice; this assay of forelimb function should be particularly useful for studies utilizing models such as gene knockouts, modifications, or insertions. Genetically encoded tools such as activity sensors and optogenetically controllable ion channels may prove especially valuable for the study of stroke and stroke recovery.
Mice in this study were 25-35 weeks old at the time of stroke; while age was not a factor in impairment or recovery in this study, potential differences between wider age ranges remain possible. It’s also possible that strains other than the C57/BL6 mice examined here may differ in training, performance and post-injury performance.
In summary, we have developed a new task for assessing upper forelimb function in mice. The task requires mice to grasp a small handle and pull it in the direction of their body while the extension of the paw and the force exerted on the handle are automatically measured. The task setup is mechanically constrained to minimize compensation. Mice can be trained quickly and simultaneously, with most or all of the shaping process unattended. This high throughput behavioral assay is capable of quantifying multiple aspects of the reach and grasp motion such as success rate, force dynamics, and more that may be important in the context of stroke research. The test can be administered efficiently, analyzed automatically, and produces reliable, precise, and richly informative data while requiring relatively little time investment.
Recovery from stroke is ultimately a functional behavioral issue; any relevant biological phenomenon will be accompanied by behavioral effects. Rodent models of ischemic stroke recovery have produced many promising approaches to stroke treatment that failed to show similar effects in humans16. While we don’t know the exact reason for this trend, one potential approach to improving the translatability of mouse models is to develop behavioral assays that model the details of problems experienced in the clinical setting more closely without imposing prohibitive logistical strain on the research process. The automated reach task described here therefore provides one potential step toward increasing the clinical impact of rodent stroke research.
Supplementary Material
Supplementary Figure 1 – 1 column
Individual Recovery. Cumulative plot showing percent of mice at each timepoint that had performed within that individual’s baseline 95° confidence interval at least twice.
Highlights.
We present an automated assay of distal forelimb function.
This assay precludes compensation and quantifies multiple aspects of the reach and grasp motion. Training, assessment, and analysis on this assay are automated to produce reliable, precise, and richly informative data while requiring relatively little time investment.
We show that photothrombotic stroke of the cortical motor forelimb representation causes long-term impairment in multiple aspects of this task through 22 weeks of weekly practice and assessment.
5 Acknowledgements
This study was financially supported by a National Institute of Health training grant (T32 NS069562 Cellular Biophysics of the Neuron Training Program, AMB), the Patrick and Beatrice Haggerty Foundation (MPG), the Beatrice Menne Haggerty Center for Research on Brain Injury and Repair in Stroke (MPG), the Texas Institute for Brain Injury and Repair, and National Institute of Health research project grants R01NS085167 and R44NS086344 (MK). We are grateful for help provided by the Neurorepair lab at UTSW and the Rennaker lab at UTD’s School of Behavioral and Brain Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–54. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 2.Klein A, Sacrey LA, Whishaw IQ, Dunnett SB. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neuroscience and biobehavioral reviews. 2012;36:1030–42. doi: 10.1016/j.neubiorev.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Lai S, Panarese A, Spalletti C, Alia C, Ghionzoli A, Caleo M, Micera S. Quantitative kinematic characterization of reaching impairments in mice after a stroke. Neurorehabilitation and neural repair. 2015;29:382–92. doi: 10.1177/1545968314545174. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:716–23. doi: 10.1038/jcbfm.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, Kim JH, Kim SH. Photochemically induced cerebral ischemia in a mouse model. Surgical neurology. 2007;67:620–5. doi: 10.1016/j.surneu.2006.08.077. discussion 625. [DOI] [PubMed] [Google Scholar]
- 6.Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, Kilgard MP, Rennaker RL. The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. Journal of neuroscience methods. (2nd) 2013;212:329–37. doi: 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Wong CC, Ramanathan DS, Gulati T, Won SJ, Ganguly K. An automated behavioral box to assess forelimb function in rats. Journal of neuroscience methods. 2015;246:30–7. doi: 10.1016/j.jneumeth.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral cortex. 2011;21:865–76. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liguz-Lecznar M, Zakrzewska R, Kossut M. Inhibition of Tnf-alpha R1 signaling can rescue functional cortical plasticity impaired in early post-stroke period. Neurobiology of aging. 2015 doi: 10.1016/j.neurobiolaging.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Toda T, Ishida K, Kiyama H, Yamashita T, Lee S. Down-regulation of KCC2 expression and phosphorylation in motoneurons, and increases the number of in primary afferent projections to motoneurons in mice with post-stroke spasticity. PloS one. 2014;9:e114328. doi: 10.1371/journal.pone.0114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierbower SM, Choveau FS, Lechleiter JD, Shapiro MS. Augmentation of M-type (KCNQ) potassium channels as a novel strategy to reduce stroke-induced brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:2101–11. doi: 10.1523/JNEUROSCI.3805-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quattromani MJ, Cordeau P, Ruscher K, Kriz J, Wieloch T. Enriched housing down-regulates the Toll-like receptor 2 response in the mouse brain after experimental stroke. Neurobiology of disease. 2014;66:66–73. doi: 10.1016/j.nbd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Meyers E, Sindhurakar A, Hays S, Sloan A, Carmel J, Kilgard M, Rennaker R. A novel automated method for isolating and quantifying supination performance in a rat model of ischemic stroke. 2014 Neuroscience Meeting Planner; Washington, DC. Society for Neuroscience; 2014. Program No.715.16/CC16. Online. [Google Scholar]
- 14.Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–895. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- 15.Manzanero S, Gelderblom M, Magnus T, Arumugam TV. Calorie restriction and stroke. Experimental & translational stroke medicine. 2011;3:8. doi: 10.1186/2040-7378-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freret T, Schumann-Bard P, Boulouard M, Bouet V. On the importance of long-term functional assessment after stroke to improve translation from bench to bedside. Experimental & translational stroke medicine. 2011;3:6. doi: 10.1186/2040-7378-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – 1 column
Individual Recovery. Cumulative plot showing percent of mice at each timepoint that had performed within that individual’s baseline 95° confidence interval at least twice.