Abstract
Glioblastomas (GBMs) are the most common and aggressive primary human malignant brain tumors. 4-Hydroxy tamoxifen (OHT) is an active metabolite of the tamoxifen (TMX) prodrug and a well-established estrogen receptor (ER) and estrogen-related receptor antagonist. A recent study from our laboratory demonstrated that OHT induced ER-independent malignant peripheral nerve sheath tumor (MPNST) cell death by autophagic degradation of the prosurvival protein Kirsten rat sarcoma viral oncogene homolog. Because both MPNST and GBM are glial in cell origin, we hypothesized that OHT could mediate similar effects in GBM. OHT induced a concentration-dependent reduction in cell viability that was largely independent of caspase activation in a human GBM cell line and 2 patient-derived xenolines. Further, OHT induced both cytotoxic autophagy and a concentration-dependent decrease in epidermal growth factor receptor (EGFR) protein levels. A GBM cell line expressing EGFR variant III (EGFRvIII) was relatively resistant to OHT-induced death and EGFRvIII was refractory to OHT-induced degradation. Thus, OHT induces GBM cell death through a caspase-independent, autophagy-related mechanism and should be considered as a potential therapeutic agent in patients with GBM whose tumors express wild-type EGFR.
Keywords: Autophagy, Epidermal growth factor receptor (EGFR), Glioblastoma, Tamoxifen
INTRODUCTION
Glioblastoma (GBM) is the most common and aggressive human primary malignant brain tumor with a median survival of 12–15 months in optimally treated patients and an overall 5-year survival rate of <5% (1). This tumor type consists predominantly of malignant glial astrocytes (2), although a recent report suggests a potential role for neuronal dedifferentiation in maintenance of heterogeneous GBM (3). Resistance to apoptosis is considered a hallmark of all cancer types (4) and GBM is particularly resistant to this death mechanism, in large part due to altered expression or mutations of apoptosis-related proteins such as p53, BCL-2, and BCL-XL (5–7). Further, the DNA alkylating agent temozolomide (TMZ), currently the chemotherapeutic standard for GBM (8), extends patients’ median survival by only 3 months (9). The poor response of GBM to TMZ is attributed to elevated expression (via promoter hypomethylation) of the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) (10, 11), resulting in specific repair of O6-alkylguanine adducts generated by TMZ (12). Therefore, more effective chemotherapeutic options, particularly therapies that do not rely on induction of apoptosis, are needed for patients with GBM.
4-Hydroxy tamoxifen (OHT) is an active metabolite of the tamoxifen (TMX) prodrug (13) and a well-established estrogen receptor (ER) estrogen-related receptor antagonist (13, 14). Previous studies from our laboratory and others indicate a potential utility of OHT to induce tumor cell death independent of apoptosis (15, 16). An investigation into the effects of OHT on malignant peripheral nerve sheath tumors (MPNSTs) found an ER-independent inhibition of cell proliferation (15) and a subsequent study from our laboratory demonstrated and characterized OHT-induced MPNST cell death as nonapoptotic and mediated at least in part by autophagic degradation of the prosurvival protein Kirsten rat sarcoma (KRAS) (16). Given that MPNST and GBM tumors are both comprised of glial cells, we hypothesized that OHT may induce similar death mechanisms in GBM.
Autophagy is a conserved homeostatic mechanism that mediates the sequestration and removal of damaged organelles, misfolded proteins, and various other cellular components bound for lysosomal degradation (17, 18). Autophagy is also initiated in response to stress signals resulting from nutrient deprivation and/or growth factor withdrawal (19). Although normally considered to be a cell survival response, autophagy has also been shown to mediate cell death under certain circumstances (20). In addition to our published observations that OHT induces cytotoxic autophagy in MPNST cells (16), several studies have demonstrated that inhibition of autophagy in vitro (21) and in vivo (22–24) can suppress cell death induced by hypoxia/ischemia and in normal development. These studies implicate the autophagic process as a potential chemotherapeutic target for apoptosis-resistant malignancies.
Considering the pleiotropic effects of OHT, it is likely that 1 or more ER-independent mechanisms contribute to its observed cytotoxic action in hormonally insensitive neoplasms. In the context of GBM, TMX’s mechanism of action has long been considered inhibition of Ca2+ signaling through protein kinase C (PKC) (25–28). Since Ca2+ can activate both PKC and calmodulin, we examined both signaling arms and effects were compared with OHT.
In this report, we extend our previous observation that OHT induces a reduction in epidermal growth factor receptor (EGFR) levels in MPNST and established GBM cell lines (16) by demonstrating OHT-mediated caspase-independent cell death in human GBM patient-derived xenolines (PDXs). Further, OHT-induced GBM cell death was accompanied by accelerated degradation of EGFR and this effect was recapitulated by inhibition of Ca2+ signaling. Importantly, a GBM cell line expressing EGFR variant III (EGFRvIII) was relatively resistant to OHT-induced death and EGFRvIII was refractory to OHT-induced degradation, suggesting that the potential use of OHT in GBM patients should be limited to those tumors expressing wild-type (WT) EGFR.
MATERIALS AND METHODS
Antibodies and Other Reagents
Primary antibodies were obtained from the following sources: LC3 (Abgent, San Diego, CA #AM1800a), EGFR (EMD Millipore, Billerica, MA #06-847), EGFRvIII (Biorbyt, Berkeley, CA #orb47907), ATG5 (Cell Signaling, Danvers, MA #8540), GAPDH (Cell Signaling, #2118), AKT (Cell Signaling #9272), phosphorylated AKT (pAKT) S473 (Cell Signaling #9271), pAKT T308 (Cell Signaling #4056), and β-tubulin (Santa Cruz Biotechnology, Dallas, TX #sc-9104). Secondary antibodies were HRP-conjugated goat anti-rabbit (Bio-Rad, Hercules, CA #1662408) and horse anti-mouse (Cell Signaling #7076). OHT was obtained from EMD Millipore (#579002). BOC-aspartyl (Ome)-fluoromethyl ketone (BAF) was purchased from MP Biomedicals (Santa Ana, CA #03FK011). Bafilomycin A1 (Baf A1) was purchased from AG Scientific (San Diego, CA #B1183). Staurosporine was obtained from Sigma (St Louis, MO # S5921). Ro-31-8220 (Ro-31) was purchased from Tocris (Bristol, UK #125314-64-9); trifluoperazine (TFP) and cycloheximide (CHX) were purchased from Sigma (#T8516 and #C1988, respectively).
Cell Culture
U87MG cells (referred to hereafter as U87) were cultured in DMEM containing 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA), 1% L-glutamine (Sigma), and 10% fetal bovine serum (Fisher Scientific, Waltham, MA). U87MG cells stably expressing EGFRvIII (U87vIII) were provided by Dr G Yancey Gillespie of the University of Alabama at Birmingham. The origin of U87vIII cells has been previously described by Mishima et al (29). JX6 and X1016 GBM PDXs were obtained from Dr G. Yancey Gillespie (IRB approval X050415007) and cultured in neurobasal media (Fisher Scientific #21103-049) supplemented with EGF (Fisher Scientific #PHG0311, 10 ng/mL) and fibroblast growth factor (Fitzgerald, Acton, MA #30R-AF014, 10 ng/mL). The genetic characteristics of our human GBM PDXs are as follows: X1016: Classical, WT EGFR, WT CDK4, WT PTEN, WT TP53, CDK4N null, undetermined MGMT; JX6: Classical, amplified EGFRvIII, WT CDK4, WT PTEN, WT TP53, CDK4N null, unmethylated MGMT. All cells were incubated at 37 °C in a humidified 5% CO2, 95% air atmosphere. For cell viability studies and caspase 3-like enzymatic activity assays, cells were plated on 48 well-plates at a density of 25 000 cells/well- JX6 and X1016; 40 000 cells/well- U87 and U87vIII. For flow analyses and lysate collection, cells were plated on 100 mm dishes at a density of: 1.25 × 106 cells/dish- JX6 and X1016; 2 × 106 cells/dish- U87 and U87vIII. Cultures were used in experiments 24 hours postplating. Drug treatments were performed in respective media supplemented with 2% fetal bovine serum to reduce effective drug concentrations.
Cell Viability and Caspase Cleavage Assays
The calcein-AM conversion assay (Life Technologies, Carlsbad, CA C3100MP) was employed to quantify viable cell number after drug treatment. Caspase 3 cleavage was assessed using the chemical substrate DEVD-7-amino-4-methylcoumarin (AMC) (Enzo Life Sciences #ALX-260-031). Both assays were performed as previously described (30).
Calcein/Ethidium Homodimer Staining
The LIVE/DEAD Viability/Cytotoxicity Kit (Fisher Scientific, #V13241) was used to determine cell death following 24 hours of OHT treatment per the manufacturer’s instructions. Samples were analyzed with a VACalibur flow cytometer (Becton Dickinson) using a 488-nm laser for calcein and a 647-nm laser for ethidium homodimer-1. Analysis was performed using CellQuest software, version 3.3.
Western Blotting
Whole cell lysates were prepared by aspirating media, washing cells with 1X PBS, removing cells with a cell lifter, and pelleting by centrifugation at 13000g for 10 minutes. Cell pellets were resuspended in lysis buffer containing 20 mM Tris–HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol, and a protease/phosphatase inhibitor cocktail (Fisher Scientific #1861281). After 3 rounds of 10 minutes incubation on ice and 1 minute vortex, lysates were clarified by centrifugation at 13 000g for 10 minutes at 4 °C. Supernates were quantified using Pierce BCA Protein Assay Kit (Fisher Scientific #23225) and transferred to new microfuge tubes to be stored at −80 °C. Thirty-five micrograms of protein was immunoblotted per our previously described protocol (31). Primary antibodies were used at the following concentrations: LC3 (1 μg/mL), EGFR (1 μg/mL), EGFRvIII (1 μg/mL), ATG5 (0.852 μg/mL), GAPDH (0.00484 μg/mL), AKT (0.083 μg/mL), pAKT S473 (0.01 μg/mL), pAKT T308 (0.035 μg/mL), and β-tubulin (0.2 μg/mL). Secondary antibodies were HRP-conjugated goat anti-rabbit and horse anti-mouse used at 0.18 μg/mL and 0.17 μg/mL, respectively. Immunoreactive species were detected by enhanced chemiluminescence (Pierce ECL; Fisher Scientific #32106) using Classic Blue Autoradiography Film BX (MidSci #EBNU2; St. Louis, MO) and a Konica SRX-101A tabletop processor.
Immunocytochemistry
EGFR (Cell Signaling #4267) primary antibody and rabbit serum (Jackson ImmunoResearch, West Grove, PA #011-000-001) were both used at 0.00017 mg/mL. Super Picture (Invitrogen #87-9263) secondary antibody was used at 0.008 μg/mL. Immunoreactivity was detected using a tyramide signal amplification system using a Cy3 fluorophore (0.00013mg/mL) (Perkin-Elmer Life Science Products, Waltham, MA #NEL744). Hoechst (Sigma #33258, 0.02mg/mL) was used for nuclear counterstaining. Samples were examined with a Nikon A1 laser confocal microscope using a 60× plan Apo objective and a 405-nm laser for Hoechst or 561-nm laser for Cy3. Images were analyzed using NIS Elements 4.2 software.
RNAi
ATG5 siRNA (siGENOME SMARTPool) was purchased from Thermo Scientific and reconstituted according to the manufacturer’s instructions. Cells were plated in DMEM-10 and transfected 24 hours postplating using X-tremeGene siRNA transfection reagent (Roche, Indianapolis, IN #04-476-093-001) at a ratio of 5:2 (transfection reagent:siRNA). Cells were used in experiments 48 hours posttransfection. ATG5 and nontarget (NT) siRNA sequences (used as an equimolar mixture for targeting multiple transcript sequences) are as follows: (1) 5′-GGAAUAUCCUGCAGAAGAA-3′; (2) 5′-CAUCUGAGCUACCCGGAUA-3′; (3) 5′-GACAAGAAGACAUUAGUGA-3′; and (4) 5′-CAAUUGGUUUGCUAUUUGA-3′. NT siRNA sequences: (1) 5′-UAGCGACUAAACACAUCAA-3′; (2) 5′-UAAGGCUAUGAAGAGAUAC-3′; (3) 5′-AUGUAUUGGCCCUGUAUUAG-3′; and (4) 5′-AUGAACGUGAAUUGCUACAA-3′.
Statistics
All data points represent mean ± SD. All experiments were repeated at least 3 times unless otherwise stated. Statistical significance was determined by ANOVA followed by Dunnett’s post hoc test. A p value < 0.05 was considered significant.
RESULTS
OHT Mediates Caspase-Independent Cell Death in Human GBM Cells
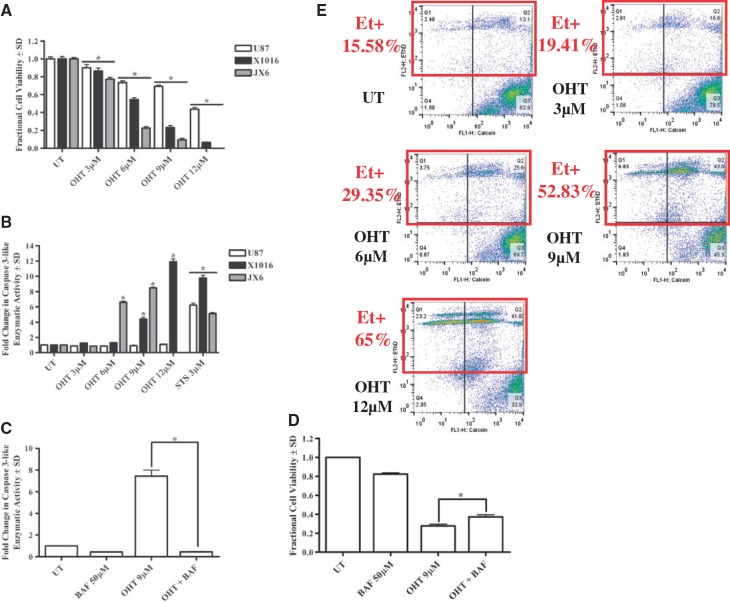
One established human GBM cell line (U87) and 2 PDXs (JX6 and X1016) were used to assess OHT’s effects on GBM cell viability. In all 3 lines tested, OHT induced a concentration-dependent decrease in viable cell number after 24 hours (Fig. 1A; Supplementary Fig. 1). To gain insight into the mechanism of OHT-induced reduction in GBM cell number, a DEVD-AMC caspase 3-like enzymatic activity assay was performed in tandem with viability studies. After 24 hours of OHT, both PDXs exhibited an induction of caspase 3-like enzymatic activity; in contrast, no increased activity was detected in U87 cells (Fig. 1B). To assess whether caspase activity was mediating the observed OHT-induced reduction in cell viability, a broad-spectrum caspase inhibitor, BAF, was used in combination with OHT and viability was assessed in JX6 cells because these exhibited the greatest caspase 3-like activity following OHT treatment. Although BAF completely suppressed OHT-induced caspase 3-like enzymatic activity (Fig. 1C), it produced only a slight but significant inhibition of OHT-induced reduction in cell viability (Fig. 1D). In keeping with the observation that OHT had no effect on caspase 3-like enzymatic activity in U87 cells, BAF had no effect on the OHT-induced loss of cell viability in U87 cells (data not shown). These data support the conclusion that caspase activity is not critical for the death promoting effects of OHT on glioma cells. To verify that the OHT-induced reduction in viable GBM cell numbers was a function of cytotoxic rather than cytostatic effects, flow cytometric analysis of calcein/ethidium homodimer double staining was performed on U87 cells after 24 hours of OHT treatment and a concentration-dependent increase in ethidium homodimer-positive (dead/dying) cells was observed (Fig. 1E). These data indicate that OHT induces a predominantly caspase-independent cell death in human GBM cells, although a statistically significant increase in caspase 3-like activity was observed.
FIGURE 1.
4-Hydroxy tamoxifen (OHT) mediates caspase-independent cell death in human Glioblastoma cells. (A) OHT-treated cells demonstrate a concentration-dependent decrease in viable cell number after 24 hours. (B) Loss of cell viability is accompanied by significant caspase 3-like enzymatic activity in JX6 and X1016 cells. (C and D) Broad caspase inhibition with BOC-aspartyl (Ome)-fluoromethyl ketone modestly but significantly attenuates OHT-mediated reduction in JX6 cell viability. (E) OHT treatment (24 hours) produces a concentration-dependent increase in ethidium homodimer-positive (Et+) U87 cells. *p < 0.05. UT, untreated.
OHT Induces Cytotoxic Autophagy in Human GBM Cells
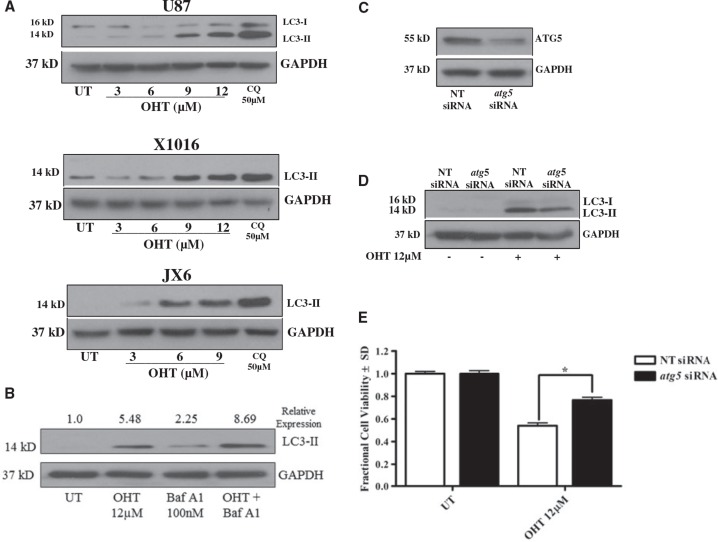
Based on our previously published observation that OHT induces cytotoxic autophagy in MPNST cells, we hypothesized that similar effects would be observed in GBM cells. During formation of autophagic vacuoles (AV) in the early stages of autophagy, microtubule-associated protein 1 light chain 3α (LC3-I) is cleaved and lipidated to form LC3-II, a molecule requisite for AV formation (32). Accordingly, we first assessed levels of LC3-II after 24 hours OHT treatment and found a concentration-dependent increase of LC3-II in all 3 cell lines tested (Fig. 2A). It is important to note that LC3I/LC3II ratios possess significant variability between cells and tumor types. Further, our lab has previously reported considerable variability in baseline autophagy and the levels of LC3I and LC3II in human GBM PDXs (33). The antibody used to detect LC3 used in this study detects both LC3I and LC3II. Low levels of LC3I in both PDXs likely reflect a high rate of baseline autophagy and rapid conversion of LC3I to LC3II. Because elevated LC3-II levels can result from induction of AV formation as well as impairment of lysosomal AV clearance, we examined the effects of OHT on LC3-II levels in U87 cells in the presence and absence of Bafilomycin A1 (Baf A1), a vacuolar ATPase inhibitor that inhibits AV fusion with lysosomes and the subsequent degradation of AV content. When AV clearance is inhibited by Baf A1, OHT imparts an additional increase in LC3-II accumulation (Fig. 2B), indicating that OHT induces AV formation rather than simply inhibiting AV clearance. Because autophagy is typically a prosurvival cellular response and can be observed in degenerating cells without being directly involved in the cell death process per se, we evaluated the ability of OHT to kill GBM cells when AV formation was genetically inhibited. Accordingly, we performed siRNA knockdown of ATG5, a protein required for conversion of LC3-I to LC3-II (34, 35) in U87 cells. A partial knockdown of ATG5 (46%) (Fig. 2C) resulted in a 60% reduction of OHT-induced LC3-II accumulation (Fig. 2D), verifying that a functional impairment of autophagy induction was achieved by ATG5 knockdown. This partial inhibition of AV formation was sufficient to produce significant inhibition of OHT-induced cell death in 2 biological replicates (Fig. 2E). Taken together, these data support the conclusion that OHT mediates GBM cell death, at least in part, via induction of cytotoxic autophagy.
FIGURE 2.
OHT-induced autophagy is cytotoxic. (A) Treatment with OHT (24 hours) yields a concentration-dependent increase in levels of LC3-II in U87, X1016, and JX6 cells. (B) OHT increases autophagic flux as indicated by increased levels of LC3-II in U87 cells treated with both OHT and Baf A1 (100 nM) relative to OHT or Baf A1 alone. (C and D) siRNA knockdown of atg5 (C) results in a 40% decrease in OHT-induced LC3-II accumulation (D), demonstrating a functional inhibition of autophagy. (E) atg5 knockdown results in significant protection from OHT-induced reduction in cell viability. Chloroquine (CQ) (50 μM) was used as a positive control for LC3-II accumulation. *p < 0.05. UT, untreated.
OHT Promotes Accelerated Degradation of EGFR
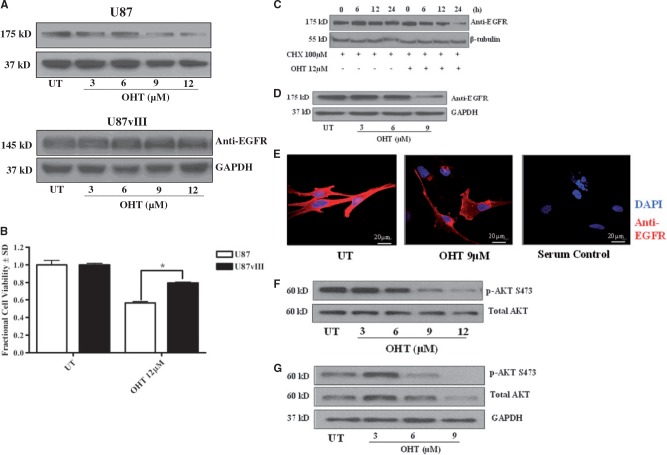
We previously showed that OHT mediates human MPNST cell death via autophagic degradation of the prosurvival protein KRAS and was additionally accompanied by a reduction in EGFR levels (16). The hypothesis that OHT induces degradation of EGFR in GBM cells is supported by both the shared lineage of MPNST and GBM cells (36, 37), and the observation that EGFR has been shown to colocalize with KRAS en route to degradation (38). Given the highly relevant contribution of EGFR to GBM growth and invasiveness (39), we evaluated the effects of OHT on this oncogenic kinase in greater detail. For these studies, we utilized the human GBM cell line U87 as well as an isogenic line stably expressing EGFRvIII (referred to hereafter as U87vIII). EGFRvIII possesses a deletion of exons 2–7, resulting in the inability to bind any currently characterized ligand due to lack of an extracellular domain (40). However, EGFRvIII confers a low level of constitutive signaling resulting from impaired internalization and increased protein stability (41). Although multiple mutant forms of EGFR exist in GBM (42–44), EGFRvIII is the most common (44, 45). We first collected total protein from U87 and U87vIII cells after 24 hours treatment with OHT. Western blot analysis revealed a concentration-dependent decrease of EGFR in U87 but not of EGFRvIII in U87vIII cells (Fig. 3A). Although EGFR in U87 cells was evaluated 3 times, we queried EGFRvIII in U87vIII cells once. The observation that EGFRvIII levels are unaffected by OHT is possibly a result of its impaired internalization (41). Importantly, U87vIII cells are relatively refractory to OHT-induced reduction in viable cell number (Fig. 3B), suggesting that degradation of EGFR contributes to the cytotoxic effects of OHT on WT EGFR-expressing GBM cells. To determine if the observed reduction of EGFR was a function of accelerated degradation rather than suppression of protein expression, we treated U87 cells with OHT with or without 100 μM CHX, a well-established protein synthesis inhibitor. Not surprisingly given the long half-life of EGFR and the relatively short time course of the experiment (46), no decrease in EGFR was observed in the presence of CHX alone whereas CHX + OHT resulted in a significant time-dependent reduction in EGFR levels in 2 separate biological replicates (Fig. 3C). To extend these results to GBM PDXs, we examined EGFR protein levels following 24 hour OHT treatment of JX6 cells via Western blot (Fig. 3D) and by immunocytochemistry (ICC) (Fig. 3E). We observed a reduction in EGFR protein levels in 3 separate replicate experiments and EGFR-like immunoreactivity in the single experiment performed. Although Western blot data was repeated at least 3 times, ICC was performed only once. Finally, we investigated the downstream effects of OHT on EGFR signaling and observed a marked decrease in pAKT (S473) after 24 hours of OHT treatment in U87 cells (Fig. 3F). This observation was extended into GBM PDXs, as JX6 cells (Fig. 3G) also exhibited reduction in pAKT in 2 separate biological replicates. These data confirm a biological response to EGFR reduction in both established human GBM cells and PDXs. Taken together these data support the hypothesis that OHT mediates cytotoxic autophagy in human GBM cells at least in part by decreasing EGFR protein levels and signaling through AKT.
FIGURE 3.
OHT accelerates degradation of epidermal growth factor receptor (EGFR). (A) Treatment with OHT (24 hours) results in a concentration-dependent decrease in EGFR protein levels in U87 but not U87vIII cells. (B) U87 cells possessing EGFRvIII (U87vIII) are significantly less sensitive to OHT’s reduction in cell viability as determined by a calcein-AM conversion assay *p < 0.001. (C) Whole cell lysates from OHT-treated U87 cells demonstrate a time-dependent decrease in EGFR in the presence of the protein synthesis inhibitor cycloheximide. (D) EGFR protein levels are reduced in JX6 cells as determined by Western blot following OHT exposure. (E) Anti-EGFR immunoreactivity is reduced in JX6 cells as determined by immunocytochemistry following 24 hours of OHT exposure. (F and G) Treatment with OHT (24 hours) mediates a marked reduction in active (phosphorylated) AKT (p-AKT S473) in U87 (F) and JX6 (G) cells. UT, untreated.
PKC Inhibition Recapitulates Effects of OHT on EGFR
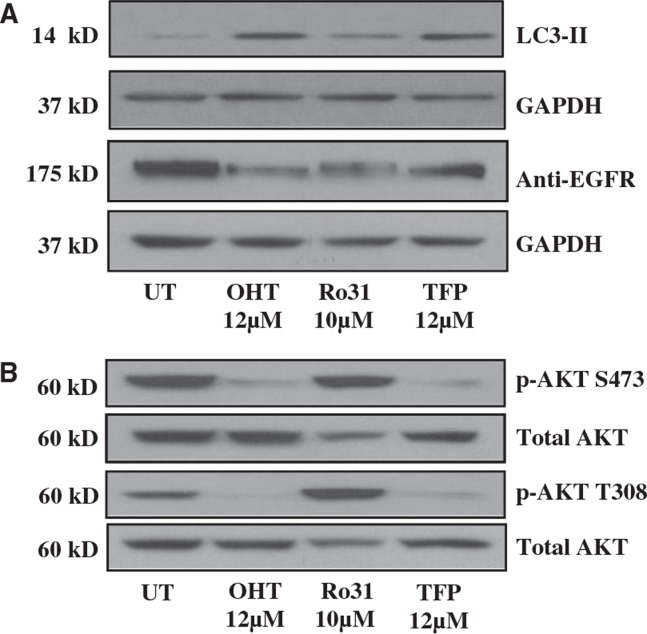
Inhibition of Ca2+ flux through endoplasmic reticulum Ca2+ release channels triggers rapid autophagy (47, 48) and active PKC has been shown to inhibit autophagy (49). Because OHT is known to inhibit Ca2+ signaling in GBM (50–52), which exerts a negative regulatory effect on autophagy (49), it is possible that Ca2+ signaling modulation mediates the observed effects of OHT on autophagy. Previous studies from our laboratory and others have demonstrated that inhibition of signaling through PKC can recapitulate various effects of OHT (15, 16). These investigations were based on observations made in the 1990s that TMX inhibits PKC activity (53, 54). We hypothesized that the effects of OHT on EGFR were dependent, at least in part, on Ca2+ signaling inhibition. To test this hypothesis, we collected total protein following treatment of U87 cells with OHT or 2 known Ca2+ signaling modulators that antagonize separate arms of Ca2+ signaling: Ro-31-8220 (Ro-31) inhibits PKC while TFP inhibits calmodulin. As expected, reduction in EGFR was observed in all drug treatments (Fig. 4A). We next queried levels of pAKT at both phosphorylation sites (S473 and T308) and observed that, as with OHT, activation of AKT was markedly inhibited by TFP while Ro-31 had no effect (Fig. 4B). These data demonstrate that Ca2+ modulation phenocopies the observed effects of OHT on EGFR and downstream signaling, suggesting that the effects of OHT on GBM cells are at least in part a function of Ca2+ signaling modulation.
FIGURE 4.
Inhibition of protein kinase C (PKC) recapitulates the effects of OHT on EGFR. (A) PKC inhibitors Ro31 and trifluoperazine (TFP) increase LC3-II accumulation and reduce EGFR protein levels after 24 hours as determined by Western blot. (B) Phosphorylation of AKT at both S473 and T308 is markedly reduced by OHT and TFP but not Ro31 in U87 cells after 24 hours of treatment.
DISCUSSION
GBM are the most common and deadly form of human primary malignant brain tumor (8). Patients receiving radiotherapy plus the most efficacious chemotherapeutic option, TMZ, exhibit only a slight increase in median survival from 12.2 to 14.6 months compared with radiotherapy alone (55). Compounding this dismal prognosis is the fact that roughly 50% of GBMs express the DNA repair enzyme MGMT and are consequently resistant to TMZ (10–12). Contributing significantly to GBM’s resistance to other chemotherapeutic approaches is its ability to evade apoptosis. GBM cells exhibit marked overexpression of anti-apoptotic proteins such as BCL-2, BCL-XL, and MCL-1 (5, 56), in addition to inactivation of p53, a critical proapoptotic protein (7). Considered together, additional chemotherapeutic options for GBM are desperately needed and therapies that do not rely on induction of apoptosis are a logical avenue of exploration.
The goal of this study was to explore the effects of OHT on GBM cells in vitro in an effort to provide a rationale for further investigations of OHT in GBM chemotherapy in vivo. In support of our hypothesis that OHT can exert cytotoxic effects on GBM cells, we demonstrate a concentration-dependent increase in GBM cell death following OHT treatment. Although OHT is typically utilized as an ER antagonist in the treatment of ER-positive breast cancer, several studies have shown that ER-negative malignancies also respond to OHT (57, 58). Further, OHT variably induces caspase 3-like activity in GBM cells and OHT-induced cell death is largely unaffected by broad-spectrum caspase inhibition, suggesting a caspase-independent cell death mechanism. Because many GBM cells are resistant to apoptotic induction (5–7), the lack of strong caspase 3-like activity after OHT exposure is not surprising. These data support the conclusion that OHT might prove useful in the treatment of patients whose tumors are refractory to apoptosis-targeting therapies.
Because OHT is known to induce autophagy in some cell types (59), we queried protein levels of LC3-II, a surrogate marker of AVs. We observed a concentration-dependent increase in LC3-II accumulation upon treatment with OHT that was further increased upon inhibition of lysosomal function with Baf A1. These data confirmed that OHT-induced autophagy in human GBM cells. Further, genetic inhibition of autophagy via partial ATG5 knockdown in GBM cells imparted a significant protection from OHT-induced cell death. Taken together, our data support the conclusion that OHT induces human GBM cell death that at least in part results from cytotoxic autophagy.
Enhanced EGFR signaling, either through overexpression of WT EGFR or activating mutations, occurs commonly in GBM (60, 61); thus, in addition to the WT receptor, we also examined the effects of OHT on the most common EGFR mutant variant, EGFRvIII (44, 45). In contrast to the WT receptor, EGFRvIII was not only refractory to OHT-induced degradation, but it also conferred a survival advantage to U87 cells when challenged with OHT. Because EGFRvIII protein is stable in the presence of OHT and promotes a survival advantage, we speculate that accelerated degradation of WT EGFR plays a role in OHT-induced human GBM cytotoxicity. Studies from The Cancer Genome Atlas have defined 4 relatively distinct molecular subtypes of GBM: classical, mesenchymal, neural, and proneural (62). Given that classical GBM is characterized by aberrations in EGFR expression (63), this subtype might prove more responsive to OHT treatment than its mesenchymal, neural, or proneural counterparts. Our data provide a potential explanation for observations made in various clinical trials of TMX in GBM suggesting a 20%–40% response rate (64, 65). Considering that these trials were performed only on the basis of histologically defined GBM, patient stratification based on molecular subtype and EGFR mutation status has the potential to improve response rates.
Finally, given the literature connecting OHT and autophagy with Ca2+ modulation, we compared OHT with 2 specific Ca2+ signaling inhibitors and observed an induction of autophagy concurrent with decreased protein levels of WT EGFR and inhibition of downstream signaling (AKT) with most drugs tested. These data support previous work suggesting a role for Ca2+ signaling modulation in the effects of OHT on human GBM cell viability (64) and suggest a novel role for OHT and PKC inhibitors in modulation of steady state EGFR protein levels.
In summary, this study elucidated a novel mechanism of action for OHT in human GBM cells in which cytotoxicity is at least in part autophagy-dependent and occurs concurrently with accelerated degradation of EGFR. Although autophagy is typically a survival-promoting process, its cell death promoting function is recognized and characterized histologically by the large scale sequestration of portions of the cytoplasm in AVs, giving the cell a characteristic vacuolated appearance (66). We utilized established techniques to assess autophagy as well as flux through the pathway (67) and have demonstrated that partial inhibition of autophagy protects against the cytotoxic effects of OHT in human GBM cells. Additional studies are needed to determine if the effects of OHT on EGFR might prove useful in the context of other malignancies that are driven by this oncogenic kinase, such as nonsmall cell lung carcinoma (68). TMX’s FDA-approved status (69) provides a basis for expeditious repurposing of this drug to non-ER driven tumor types.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Shawn Williams of the University of Alabama at Birmingham (UAB) High Resolution Imaging Facility (supported by the Cancer Center Support Grant CA013148 and the Rheumatic Disease Core Center Grant AR048311) for his expertise and guidance with confocal imaging, Terry Lewis and Marissa Menard of the University of Alabama at Birmingham (UAB) Molecular Detection Core (supported by grant NS0474666) for assistance with immunocytochemistry, and Enid Keyser of the University of Alabama at Birmingham (UAB) Flow Cytometry Core (supported by grants AR048311 and AI027767) for support with flow cytometry analysis.
This work was supported by National Cancer Institute grant CA134773 (K.A.R.) and Department of Defense grants W81XWH-14-1-0073 (K.A.R.), W81XWH-09-1-0086 (S.L.C.), and W81XWH-15-1-0193 (S.L.C.). Funding sources for this study had no involvement in study design, collection/analysis/interpretation of data, writing of the report, or decision to publish.
Conflict of interest: The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at http://www.jnen.oxfordjournals.org.
REFERENCES
- 1.Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol 2015;22:e273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldape K, Zadeh G, Mansouri S, et al. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol 2015;129:829–48 [DOI] [PubMed] [Google Scholar]
- 3.Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012;338:1080–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70 [DOI] [PubMed] [Google Scholar]
- 5.Tagscherer KE, Fassl A, Campos B, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 2008;27:6646–56 [DOI] [PubMed] [Google Scholar]
- 6.Krakstad C, Chekenya M. Survival signaling and apoptosis resistance in glioblastomas: Opportunities for targeted therapeutics. Mol Cancer 2010;9:135–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455:1061–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013;310:1842–50 [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 10.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 2000;343:1350–4 [DOI] [PubMed] [Google Scholar]
- 11.Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of 0-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 2004;10:1871–4 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Watanabe T, Yonekawa Y, et al. Promoter methylation of the DNA repair gene MGMT in astrocytomas is frequently associated with G:C → A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis 2001;22:1715–9 [DOI] [PubMed] [Google Scholar]
- 13.Lim YC, Desta Z, Flockhart DA, et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 2005;55:471–8 [DOI] [PubMed] [Google Scholar]
- 14.Clarke R, Leonessa F, Welch JN, et al. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev 2001;53:25–71 [PubMed] [Google Scholar]
- 15.Byer SJ, Eckert JM, Brossier NM, et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor-independent manner. Neuro Oncol 2011;13:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli L, Kaza N, Coric T, et al. 4-Hydroxytamoxifen induces autophagic cell death through K-Ras degradation. Cancer Res 2013;73:4395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol 2008;266:207–47 [DOI] [PubMed] [Google Scholar]
- 18.Kundu M, Thompson CB. Autophagy: Basic principles and relevance to disease. Annu Rev Pathol 2008;3:427–55 [DOI] [PubMed] [Google Scholar]
- 19.Eskelinen EL, Saftig P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochem Biophys Acta 2009;1793:664–73 [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto Y, Shimizu S. Another way to die: Autophagic programmed cell death. Cell Death Differ 2005;12(Suppl 2):1528–34 [DOI] [PubMed] [Google Scholar]
- 21.Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007;8:741–52 [DOI] [PubMed] [Google Scholar]
- 22.Samara C, Syntichaki P, Tavernarakis N. Autophagy is required for necrotic cell death in Caenorhabditis elegans. Cell Death Differ 2008;15:105–12 [DOI] [PubMed] [Google Scholar]
- 23.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell 2007;131:1137–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koike M, Shibata M, Tadakoshi M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 2008;172:454–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn SJ, Yoon MS, Hyuk S, et al. Phospholipase C-protein kinase C mediated phospholipase D activation pathway is involved in tamoxifen induced apoptosis. J Cell Biochem 2003;89:520–8 [DOI] [PubMed] [Google Scholar]
- 26.Baltuch GH, Couldwell WT, Villemure JG, et al. Protein kinase C inhibitors suppress cell growth in established and low-passage glioma cell lines. A comparison between staurosporine and tamoxifen. Neurosurgery 1993;33:495–501 [DOI] [PubMed] [Google Scholar]
- 27.Couldwell WT, Antel JP, Apuzzo ML, et al. Inhibition of growth of established human glioma cell lines by modulators of the protein kinase-C system. J Neurosurg 1990;73:594–600 [DOI] [PubMed] [Google Scholar]
- 28.Horgan K, Cooke E, Hallett MB, et al. Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance of antitumor activity. Biochem Pharmacol 1986;35:4463–5 [DOI] [PubMed] [Google Scholar]
- 29.Mishima K, Johns TG, Luwor RB, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res 2001;61:5349–54 [PubMed] [Google Scholar]
- 30.Geng Y, Kohli L, Klocke BJ, et al. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol 2010;12:473–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls KC, Ghosh AP, Franklin AV, et al. Lysosome dysfunction triggers Atg7-dependent neural apoptosis. J Biol Chem 2010;285:10497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol 2008;445:77–88 [DOI] [PubMed] [Google Scholar]
- 33.Kaza N, Kohli L, Roth KA. Autophagy in brain tumors: A new target for therapeutic intervention. Brain Pathol 2012;22:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N, Yamamato A, Hatano M, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 2001;152:657–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N, Noda T, Yoshimori T, et al. A protein conjugation system essential for autophagy. Nature 1998;395:395–8 [DOI] [PubMed] [Google Scholar]
- 36.Woodhoo A, Sommer L. Development of the Schwann cell lineage: From the neural crest to the myelinated nerve. Glia 2008;56:1481–90 [DOI] [PubMed] [Google Scholar]
- 37.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 2005;6:671–82 [DOI] [PubMed] [Google Scholar]
- 38.Lu A, Tebar F, Alvarez-Moya B, et al. A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J Cell Biol 2009;184:863–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsatsanis C, Spandidos DA. The role of oncogenic kinases in human cancer. Int J Mol Med 2000;5:583–90 [DOI] [PubMed] [Google Scholar]
- 40.Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): Where the wild things are altered. FEBS J 2013;280:5350–70 [DOI] [PubMed] [Google Scholar]
- 41.Huang HS, Nagane M, Klingbeil CK, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 1997;272:2927–35 [DOI] [PubMed] [Google Scholar]
- 42.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumors of glial origin. Nature 1985;313:144–7 [DOI] [PubMed] [Google Scholar]
- 43.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 1992;89:2965–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000;60:1383–7 [PubMed] [Google Scholar]
- 45.Sugawa N, Ekstrand AJ, James CD, et al. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA 1990;87:8602–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorkin A, Duex JE. Quantitative analysis of endocytosis and turnover of epidermal growth factor (EGF) and EGF receptor. Curr Protoc Cell Biol 2010;Chapter:Unit-15.14 [DOI] [PMC free article] [PubMed]
- 47.Criollo A, Maiuri MC, Tasdemir E, et al. Regulation of autophagy by the inositol triphosphate receptor. Cell Death Differ 2007;14:1029–39 [DOI] [PubMed] [Google Scholar]
- 48.Khan MT, Joseph SK. Role of inositol triphosphate receptors in autophagy in DT40 cells. J Biol Chem 2010;285:16912–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang H, Cheng D, Liu W, et al. Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem Biophys Res Commun 2010;395:471–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brian CA, Ioannides CG, Ward NE, et al. Inhibition of protein kinase C and calmodulin by the geometric isomers cis- and trans-tamoxifen. Biopolymers 1990;29:97–104 [DOI] [PubMed] [Google Scholar]
- 51.Gulino A, Barrera G, Vacca A, et al. Calmodulin antagonism and growth-inhibiting activity of triphenylethylene antiestrogens in MCF-7 human breast cancer cells. Cancer Res 1986;46:6274–8 [PubMed] [Google Scholar]
- 52.O’Brian CA, Housey GM, Weinstein IB. Specific and direct binding of protein kinase C to an immobilized tamoxifen analogue. Cancer Res 1988;48:3626–9 [PubMed] [Google Scholar]
- 53.Baltuch GH, Dooley NP, Villemure JG, et al. Protein kinase C and growth regulation of malignant gliomas. Can J Neurol Sci 1995;22:264–71 [DOI] [PubMed] [Google Scholar]
- 54.Gelmann EP. Tamoxifen for the treatment of malignancies other than breast and endometrial carcinoma. Sem Oncol 1997;24(1 Suppl 1):S1-65–70 [PubMed] [Google Scholar]
- 55.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: Mechanisms of action, repair and resistance. Curr Mol Pharmacol 2012;5:102–14 [DOI] [PubMed] [Google Scholar]
- 56.Krajewski S, Krajewska M, Ehrmann J, et al. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1 and Bax in tumors of central and peripheral nervous system origin. Am J Pathol 1997;150:805–14 [PMC free article] [PubMed] [Google Scholar]
- 57.Gelmann EP. Tamoxifen induction of apoptosis in estrogen receptor-negative cancers: New tricks for an old dog? J Natl Cancer Inst 1996;88:224–6 [DOI] [PubMed] [Google Scholar]
- 58.Ferlini C, Scambia G, Marone M, et al. Tamoxifen induces oxidative stress and apoptosis in oestrogen receptor-negative human cancer cell lines. Br J Cancer 1999;79:257–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho KS, Yoon YH, Choi JA, et al. Induction of autophagy and cell death by tamoxifen in cultured retinal pigment epithelial and photoreceptor cells. Invest Ophthalmol Vis Sci 2012;53:5344–53 [DOI] [PubMed] [Google Scholar]
- 60.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev 2007;21:2683–710 [DOI] [PubMed] [Google Scholar]
- 61.Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: Molecular basis to overcome resistance. Curr Cancer Drug Targets 2012;12:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Couldwell WT, Weiss MH, DeGiorgio CM, et al. Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen. Neurosurgery 1993;32:485–9 [DOI] [PubMed] [Google Scholar]
- 65.Vertosick FT, Jr, Selker RG, Pollack IF, et al. The treatment of intracranial malignant gliomas using orally administered tamoxifen therapy: Preliminary results in a series of “failed” experiments. Neurosurgery 1992;30:897–902 [DOI] [PubMed] [Google Scholar]
- 66.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009;16:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140:313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pal SK, Figlin RA, Reckamp K. Targeted therapies for non-small cell lung cancer: An evolving landscape. Mol Cancer Ther 2010;9:1931–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown PH, Lippman SM. Chemoprevention of breast cancer. Breast Cancer Res Treat 2000;62:1–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.