Abstract
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disorder associated with repetitive traumatic brain injury. Multiple system atrophy (MSA) is a Parkinsonian disorder that can result in repetitive falls with associated head trauma. We hypothesized that patients with neurodegenerative disorders like MSA could develop CTE pathology. Therefore, we assessed CTE pathology in 139 MSA cases in our brain bank. Sections from convexity cerebral cortices were screened by immunohistochemistry with anti-phospho-tau antibody. For cases with suggestive CTE pathology, further sections of basal forebrain and hippocampus were immunostained. Consensus criteria were used to make the diagnosis of CTE and aging-related tau astrogliopathy (ARTAG) was differentiated from CTE pathology. Pertinent clinical information was derived from the available records and online searches. Of the 139 MSA cases, 8 (6%) had CTE pathology and 10 (8%) had ARTAG pathology. All 8 cases with CTE were male and 4 of them had a documented history of contact sports. The median age at death in MSA with CTE was younger than in MSA without CTE or MSA with ARTAG (60, 67, and 74 years, respectively; p = 0.002). Even without a known history of contact sports or head trauma, a small subset of cases with MSA had CTE pathology.
Keywords: α-Synuclein, Aging-related tau astrogliopathy, Chronic traumatic encephalopathy, Head trauma, Multiple system atrophy, Tau.
INTRODUCTION
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disorder associated with repetitive traumatic brain injury (1–3). Individuals with CTE can develop a variety of clinical symptoms, such as headache, loss of attention and concentration, depression, memory loss, executive dysfunction, impulsivity, visuospatial abnormalities, and heightened suicidality (4). Pathologically, CTE is classified as atauopathy with phospho-tau-immunoreactive neurofibrillary tangles (NFTs) and thorn-shape astrocytes (TSAs) in perivascular foci, often at the depths of cerebral sulci of convexity frontal and temporal cortices (5, 6).
CTE was originally described as “punch drunk syndrome” and “dementia pugilistica” in boxers with repetitive brain trauma (7, 8). Subsequently, recognition that activities other than boxing, including participation in contact sports, can be associated with this condition has become widespread, with the disorder currently termed CTE (9, 10). Although the incidence and prevalence of CTE has yet to be elucidated fully, an autopsy case series has revealed that 80% (68/85) of individuals with a history of repetitive mild traumatic brain injuries, most commonly sustained through athletic activities, had CTE pathology (6). Furthermore, some of these cases had concurrent neurodegenerative pathologies, including motor neuron disease, Alzheimer disease, and Parkinson disease. Recently, published research from the Mayo Clinic brain bank has also demonstrated that a subset of individuals (32%, 21/66) with documented participation in contact sports (including both professional and amateur levels) had CTE pathology concomitant with other neurodegenerative processes but it was not detected in individuals without a known history of exposure to contact sports. (11). Similarly, Ling et al. (12) reported CTE pathology in a variety of neurodegenerative diseases in the University College London brain bank, including a single case with multiple system atrophy (MSA).
MSA is a sporadic, progressive neurodegenerative disorder classified as an α-synucleinopathy; glial cytoplasmic inclusions (GCIs) in oligodendrocytes are the pathognomic features of the disorder. GCIs are found throughout the brain, with a predilection for striatonigral and olivopontocerebellar systems (13). Clinically, patients with MSA present with a variable combination of autonomic failure, parkinsonism, cerebellar ataxia, and pyramidal symptoms (14–16). Individuals with MSA generally develop difficulty with gait, balance problem, and falls due to the parkinsonism, cerebellar ataxia, or orthostasis. The patient with MSA and CTE pathology reported by Ling et al (12) was a woman with a history of multiple falls and traumatic brain injuries; she did not participate in contact sports. Two additional MSA patients, both men with known participation in contact sports (1 high school football player reported by McKee et al (6) and 1 football player described by Bieniek et al (11)), did not have CTE pathology.
The aims of this study were to determine the frequency of CTE pathology in patients with MSA and to address the hypothesis that CTE pathology may develop in a subset of patients with MSA, a disease associated with repetitive falls, even without known participation in contact sports. To address these research questions, we assessed CTE pathology in 139 MSA cases in our brain bank.
MATERIALS AND METHODS
Case Selection
Between 2000 and 2015, 153 cases in the Mayo Clinic brain bank were given the neuropathologic diagnosis of MSA. Of the 153 MSA cases, 139 cases with available paraffin-embedded tissue and at least minimal medical documentation such as clinical diagnosis and the age at onset were included in this study cohort. All brain autopsies were performed after consent of the legal next-of-kin or individual with power-of-attorney. Studies of autopsy samples were considered exempt from human subject research by Mayo Clinic Institutional Review Board.
Clinical Assessment
Pertinent clinical information derived from the available records or brain bank questionnaires filled out by a close family member included gender, age at symptom onset, symptom duration, age at death, clinical diagnosis, clinical symptoms (falls, cognitive impairment, and depression), clinical subtype of MSA, family history of dementia or parkinsonism, contact sports participation, the level of sports participation, and documented head trauma associated with sports participation (11, 17). Exposure to non-sports-related head trauma, including falls with head injury, motor vehicle accidents, violent assaults, and domestic abuse was also recorded. Online searches of obituaries were conducted to validate and supplement clinical records using full name, date of birth and date of death. Patients were considered to have cognitive impairment if at least short-term memory loss, disorientation, or executive dysfunction were diagnosed by a physician, or there were recorded complaints of these symptoms by the patient or their family members. Patients were considered to have depression if the patient was given a diagnosis of depression and prescribed antidepressant medications. Based on the clinical information, each patient was assigned a clinical subtype of MSA: MSA with predominant parkinsonism or MSA with predominant cerebellar ataxia.
Neuropathologic Assessment
Most of the brains were received with the left hemibrain fixed in 10% formalin (Leica Biosystems, Richmond, IL); the right hemibrain had been frozen at −80 °C. Formalin-fixed brains underwent systematic and standardized sampling with neuropathologic evaluation by a single, experienced neuropathologist (D.W.D.). Regions sampled on all cases included 6 regions of neocortex, 2 levels of hippocampus, a basal forebrain section that includes amygdala, lentiform nucleus and hypothalamus, anterior corpus striatum, thalamus at the level of the subthalamic nucleus, midbrain, pons, medulla, and 2 sections of cerebellum, 1 including the deep nuclei. Paraffin-embedded 5-μm-thick sections mounted on glass slides were stained with hematoxylin and eosin (Thermo Fisher Scientific Inc., Waltham, MA) and thioflavin S (Sigma-Aldrich, St. Louis, MO) stains. Braak NFT stage and Thal amyloid phase were assigned using thioflavin S slides according to published criteria (18–20).
Immunohistochemistry for α-synuclein (NACP, 1:3000 rabbit polyclonal, Mayo Clinic antibody, Jacksonville, FL) was performed on sections of basal forebrain, striatum, midbrain, pons, medulla, and cerebellum to establish the neuropathological diagnosis of MSA (21). MSA cases were pathologically subclassified as MSA with predominant striatonigral involvement, MSA with predominant olivopontocerebellar involvement, and MSA with equally severe involvement of striatonigral and olivopontocerebellar systems (22).
CTE pathology was determined when the following distinctive features were present (23): (1) perivascular foci of NFTs, tau-positive astrocytes and neurites in the neocortex; (2) irregular distribution of tau-positive lesions at the depths of sulci; (3) NFTs in the cerebral cortex preferentially in the superficial layers; (4) supportive, non-diagnostic features: clusters of subpial astrocytic tau pathology (23). Cases with CTE pathologic distribution was assessed according to the McKee staging scheme (6).
Tau-immunoreactive astrogliopathy is increasingly recognized in elderly brains. This type of pathology also known as aging-related tau astrogliopathy (ARTAG) should be differentiated from CTE pathology (24). Based on the recent consensus paper on ARTAG, the diagnosis of ARTAG was defined as follows: TSA or granular/fuzzy astrocytes (GFA) in subependymal, subpial, perivascular, gray matter, and white matter regions (24).
To assess CTE pathology, 5-μm-thick sections from convexity cerebral cortices [middle frontal gyrus (Brodmann area 46), superior temporal gyrus (Brodmann area 41/42), and inferior parietal lobule (Brodmann area 39/40)] were processed for tau immunohistochemistry. Following deparaffinization in xylene and reagent alcohol, antigen retrieval was performed by steaming slides in distilled water for 30 min. All immunohistochemistry was conducted using a DAKO Autostainer and DAKO EnVision + reagents (Dako, Carpinteria, CA). The phospho-tau antibody used was CP13 (mouse monoclonal; 1:1000; a gift from Dr. Peter Davies, Feinstein Institute for Medical Research). Immunostained slides were counterstained with hematoxylin and coverslipped. Tau-immunostained tissue from the frontal, temporal, and parietal cortices of all 139 MSA cases were evaluated blinded to clinical features, including contact sports participation. For cases suspected of having CTE or ARTAG pathology based on screening of the 3 cortical sections, additional sections were sampled from orbital frontal gyrus (Brodmann area 10/11), inferior frontal gyrus (Brodmann area 44/45), dorsolateral frontal gyrus (Brodmann area 9), and middle temporal gyrus (Brodmann area 21). Sections from cingulate cortex with superior frontal gyrus, basal forebrain and hippocampus were also processed for tau immunohistochemistry.
Immunofluorescence
We performed immunofluorescence with the rabbit polyclonal antibody NACP and mouse monoclonal antibody CP13. The deparaffinized and rehydrated sections were pretreated with 98% formic acid (EMD Chemicals Inc., Gibbstown, NJ) for 30 minutes and then steamed in distilled water for 30 min. Next, sections were blocked with Protein Block plus Serum Free (Dako) for 1 h and incubated with both anti-CP13 (1:500) and anti-NACP (1:2000) primary antibodies diluted in with Antibody Diluent with Background-Reducing Components (Dako) overnight at 4 °C. Sections were washed 3 times with 1 X PBS (Sigma-Aldrich) at room temperature, and then incubated with secondary antibodies Alexa Fluor 568 (1:500, Thermo Fisher Scientific Inc.).and Alexa Fluor 488 (1:500, Thermo Fisher Scientific Inc.) diluted with Antibody Diluent with Background-Reducing Components (Dako) for 1.5 h at room temperature in a dark chamber. Sections were washed 3 times with 1×PBS at room temperature, incubated with 1% Sudan Black (SPI Supplies, West Chester, PA) for 2 min, washed with distilled water and mounted with Vectashield mounting media containing DAPI (Vector Laboratories, Burlingame, CA). Representative images were taken with an Olympus BX50 fluorescent microscope (Olympus Co. Ltd., Tokyo, Japan).
Statistical Analysis
All statistical analyses were performed using SigmaPlot 12.3 (Systat Software Inc., San Jose, CA). A Chi-square test was performed for group comparisons of categorical data. ANOVA on ranks, followed by Dunn’s post hoc test, or 1-way ANOVA, followed by post hoc Holm-Sidak test, were used for analyses of continuous variables as appropriate. P values < 0.05 were considered statistically significant.
RESULTS
Summary of the Cohort
One hundred thirty-nine subjects with autopsy-confirmed MSA were included in this cohort. The median age at death was 66 years. Eighty-four patients were men (60%); 5 of the men (6%) had a history of participation in a contact sport. Thirteen patients (9%) had a family history of dementia and 13 (9%) had a family history of parkinsonism. The clinical MSA phenotypes were MSA with predominant parkinsonism in 96 patients (77%) and MSA with predominant cerebellar ataxia in 29 patients (23%); it was not possible to make a designation in 14 cases due to lack of detailed clinical information about motor symptoms. Of 107 patients with available clinical records, 85 (79%) had descriptions of falls, 50 (47%) had depression, and 44 (41%) had cognitive impairment. Alzheimer-type pathology was minimal, with median Braak NFT stage of I and Thal amyloid phase of 0. Only 7 cases (5%) had a concurrent pathologic diagnosis of Alzheimer disease. Fifty cases were pathologically subclassified as MSA with predominant striatonigral involvement, 25 cases were MSA with predominant olivopontocerebellar involvement, 60 cases were MSA with equally severe involvement of striatonigral and olivopontocerebellar systems, and 4 cases could not be classified.
Neuropathologic Assessment of CTE
Tau immunohistochemistry was performed on sections from the frontal, parietal, and temporal lobules as well as basal forebrain and hippocampus. Of the 139 cases with MSA, 8 cases (6%) had tau pathology consistent with CTE. Pathologic features in 8 CTE cases are summarized in Table 1. Most frequently affected regions were middle frontal gyrus (5/8) and inferior parietal gyrus (5/8), followed by orbital frontal gyrus (4/8), inferior frontal gyrus (4/8), superior temporal gyrus (2/8), dorsolateral frontal gyrus (2/8), superior frontal gyrus (1/8), and middle temporal gyrus (1/8). These 8 cases were assigned to CTE stages proposed by McKee et al (6): 1 case was stage III, 5 cases were stage II, and 2 cases were stage I.
Table 1.
Pathologic Features of Chronic Traumatic Encephalopathy or Aging-Related Tau Astrogliopathy in Multiple Systems Atrophy Cases
| Case no. | CTE Stage | Sport | Age | Sex | Clinical Dx | Path Dx | Braak | Thal | MFG | STG | IPG | SFG/CG | OG | IFG | DFG | MTG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTE-1 | III | FB | 72 | M | MSA v PD | SND | III | 0 | CTE | CTE | CTE | – | CTE | CTE | – | CTE |
| CTE-2 | II | FB | 53 | M | MSA-C | OPCA | V | 0 | CTE | – | CTE | – | CTE | CTE | CTE | – |
| CTE-3 | II | – | 59 | M | PSP | SND | I | 3 | CTE | – | CTE | – | – | – | CTE | – |
| CTE-4 | II | – | 54 | M | MSA | Mix | I | 0 | – | – | CTE | – | – | CTE | – | – |
| CTE-5 | II | – | 58 | M | MSA | Mix | II | 3 | – | – | CTE | – | CTE | – | – | – |
| CTE-6 | II | BB | 53 | M | MSA | Mix | 0 | 0 | – | CTE | – | – | CTE | – | – | – |
| CTE-7 | I | – | 55 | M | MSA | OPCA | I | 1 | CTE | – | ATG | – | – | CTE | – | – |
| CTE-8 | I | FB | 76 | M | MSA | OPCA | I | 0 | CTE | – | – | CTE | – | – | – | – |
| ATG-1 | – | – | 85 | M | PD v MSA | SND | III | 0 | – | ATG | – | – | – | – | ATG | – |
| ATG-2 | – | – | 83 | M | PD v PDD | SND | IV | 2 | – | – | ATG | – | – | – | – | – |
| ATG-3 | – | – | 89 | F | PSP v MSA | SND | III | 0 | – | ATG | – | – | – | – | – | – |
| ATG-4 | – | FB | 73 | M | PSP | SND | I | 0 | ATG | – | – | – | – | – | – | – |
| ATG-5 | – | – | 74 | F | PSP v MSA | OPCA | II | 0 | – | ATG | – | – | – | – | – | – |
| ATG-6 | – | – | 70 | M | DLB | SND | II | 1 | ATG | – | ATG | – | ATG | ATG | ATG | – |
| ATG-7 | – | – | 79 | M | MSA-C | OPCA | IV | 0 | – | ATG | ATG | – | – | – | – | – |
| ATG-8 | – | – | 65 | M | MSA | SND | II | 0 | ATG | – | – | – | – | – | – | – |
| ATG-9 | – | – | 70 | F | MSA-P | SND | I | 3 | – | ATG | – | – | – | – | – | – |
| ATG-10 | – | – | 56 | M | PSP | Mix | 0 | 0 | ATG | – | – | – | – | – | – | – |
FB, football; BB, basketball; Age, age at death; Dx, diagnosis; Braak, Braak neurofibrillary tangle stage; Thal, Thal amyloid phase; MFG, middle frontal gyrus; STG, superior temporal gyrus; IPG, inferior parietal gyrus; SFG, superior frontal gyrus; CG, cingulate gyrus; OG, orbital gyrus; IFG, inferior frontal gyrus; DFG, dorsolateral frontal gyrus; MTG, middle temporal gyrus; CTE, chronic traumatic encephalopathy; ATG, aging-related tau astrogliopathy; MSA, multiple system atrophy; MSA-C, multiple system atrophy with predominant cerebellar ataxia; MSA-P, multiple system atrophy with predominant parkinsonism; PD, Parkinson disease; PSP, progressive supranuclear palsy; DLB, dementia with Lewy bodies; SND, MSA with predominant striatonigral involvement; OPCA, MSA with predominant olivopontocerebellar involvement; Mix, MSA with equally severe involvement of striatonigral and olivopontocerebellar systems;.
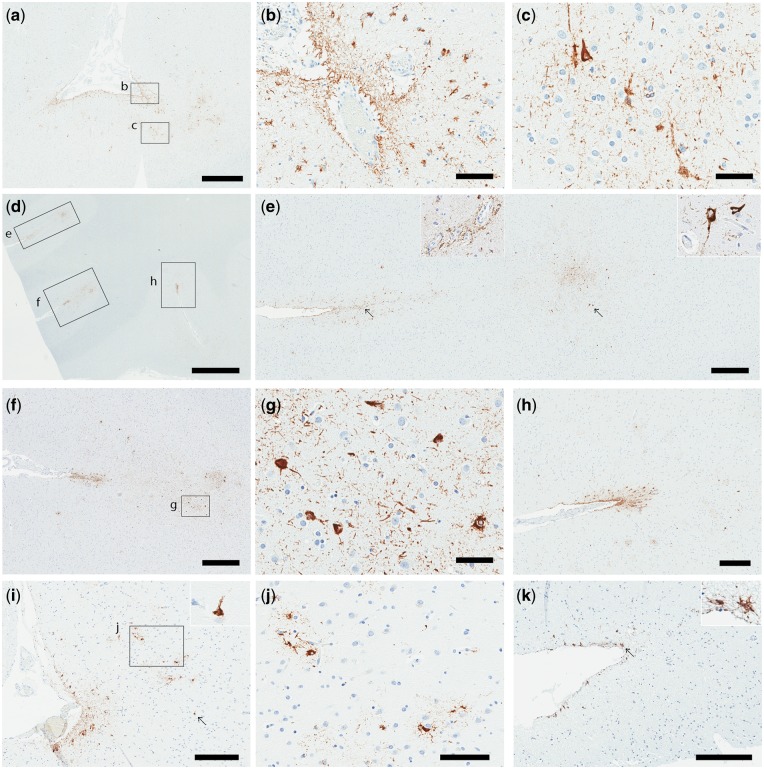
CTE pathology in 3 representative cases is illustrated in Figure 1. In CTE-1, numerous TSAs and NFTs were detected in the perivascular or subpial regions, and at the depth of sulci in superior temporal gyrus (Fig. 1a–c), as well as middle frontal gyrus, inferior parietal gyrus, orbital frontal gyrus, inferior frontal gyrus, and middle temporal gyrus. Given the distribution of CTE pathology, this case was considered CTE stage III. In the patient CTE-2, there were 3 epicenters in a section containing orbital frontal gyrus, consisting of perivascular TSAs and NFTs at the depth of sulci (Fig. 1d–h). As shown in Table 2, numerous TSAs surrounding the sulci with abundant NFTs were noted at the depths of sulci in the middle frontal gyrus, inferior frontal gyrus, dorsolateral frontal gyrus, and inferior parietal gyrus. Given the distribution of CTE pathology throughout the frontal lobe as well as inferior parietal lobe, this case was considered CTE stage II. Other stage II cases included CTE-3, 4, 5, and 6. CTE-3 through 5 had CTE pathology in inferior parietal gyrus and frontal gyrus, while CTE-6 had CTE pathology in superior temporal gyrus and orbital gyrus, but not in the parietal cortex. In contrast, the patient CTE-8 had mild CTE pathology. Sparse subpial TSAs and NFTs were detected at the depth of sulci in the middle frontal and superior frontal gyrus (Fig. 1i–k). Given the fact that this case was Braak NFT stage I, where tau pathology is restricted to medial temporal lobe, these NFTs were considered to be associated with CTE pathology rather than age-related Alzheimer pathology. The distribution of sparse CTE pathology restricted to frontal lobe was consistent with CTE stage I. CTE-7 also had sparse CTE pathology limited to frontal gyrus.
FIGURE 1.
Tau immunohistochemistry on tissue from 3 cases with CTE; CTE-1 (a–c), CTE-2 (d–h), and CTE-8 (i–k). (a-c) In case CTE-1, numerous thorn-shaped astrocytes (TSAs) surround the sulcus of superior temporal gyrus as well as perivascular regions (a, b). Abundant NFTs and pretangles were found at the depth of the sulcus (a, c). (d–h) In case CTE-2 there were 3 CTE epicenters in the orbital gyrus (d). Perivascular astrocytes (e, left inset) and NFTs were noted at the depth of the sulcus (e, right inset). Numerous TSAs surround the sulcus with perivascular astrocytes and NFTs at the depth of the sulcus. (i–k) In case CTE-8, relatively mild astrocytic pathology and sparse NFTs were seen in superior frontal gyrus (i, j) and middle frontal gyrus (k). Arrows indicate an inset in each image (e, i, k). Scale bars: a = 1 mm; b, c, g = 50 µm; d = 2 mm; e, f = 500 µm; h, i, k = 300 µm; j = 100 µm.
Table 2.
Demographic, and Clinical, Pathologic Findings
| MSAa | MSA + CTE | MSA + ARTAG | ||
|---|---|---|---|---|
| Features | (n = 121) | (n = 8) | (n = 10) | p Value |
| Demographic | ||||
| Sex, % male | 69 (57%) | 8 (100%) | 7 (70%) | 0.184 |
| History of contact sports | 0 (0%) | 4 (50%) | 1 (10%) | <0.001 |
| Age at death | 67 ± 8 | 60 ± 9 | 74 ± 10 | 0.002 |
| Family history of dementia | 13 (11%) | 1 (13%) | 1 (10%) | 0.985 |
| Family history of parkinsonism | 13 (11%) | 1 (13%) | 2 (20%) | 0.675 |
| Clinical characteristics | ||||
| Age at onset | 58 ± 10 | 50 ± 11 | 65 ± 12 | 0.032 |
| Disease duration | 7 (5, 10) | 11 (9, 13) | 6 (3, 12) | 0.058 |
| MSA-P/MSA-C ratio | 84/23 (79%) | 5/3 (63%) | 7/3 (70%) | 0.852 |
| Falls | 72/93 (77%) | 6/7 (86%) | 7/7 (100%) | 0.331 |
| Cognitive impairment | 36/93 (39%) | 3/7 (43%) | 5/7 (71%) | 0.577 |
| Depression | 43/93 (46%) | 3/7 (43%) | 4/7 (57%) | 0.986 |
| Pathologic features | ||||
| Brain weight (g) | 1210 ± 160 | 1180 ± 100 | 1250 ± 170 | 0.668 |
| Braak NFT stage | I (0, II) | I (I, III) | II (0, III) | 0.535 |
| Thal amyloid phase | 0 (0, 1) | 0 (0, 2) | 0 (0, 2) | 0.893 |
Data are displayed as mean ± SD or median (25th, 75th range) as appropriate.
aThis group includes MSA without CTE and ARTAG.
MSA, multiple system atrophy; MSA-C, multiple system atrophy with predominant cerebellar ataxia; MSA-P, multiple system atrophy with predominant parkinsonism; CTE, chronic traumatic encephalopathy; ARTAG, aging-related tau astrogliopathy; NFT, neurofibrillary tangle.
Of the 8 CTE cases, 4 cases had a history of contact sports: 1 with CTE stage III, 2 with stage II, and 1 with stage I. No association was observed regarding the severity of CTE pathology and a history of contact sports. Furthermore, CTE distribution and severity did not correlate with MSA α-synuclein pathology (data not shown).
Immunofluorescence
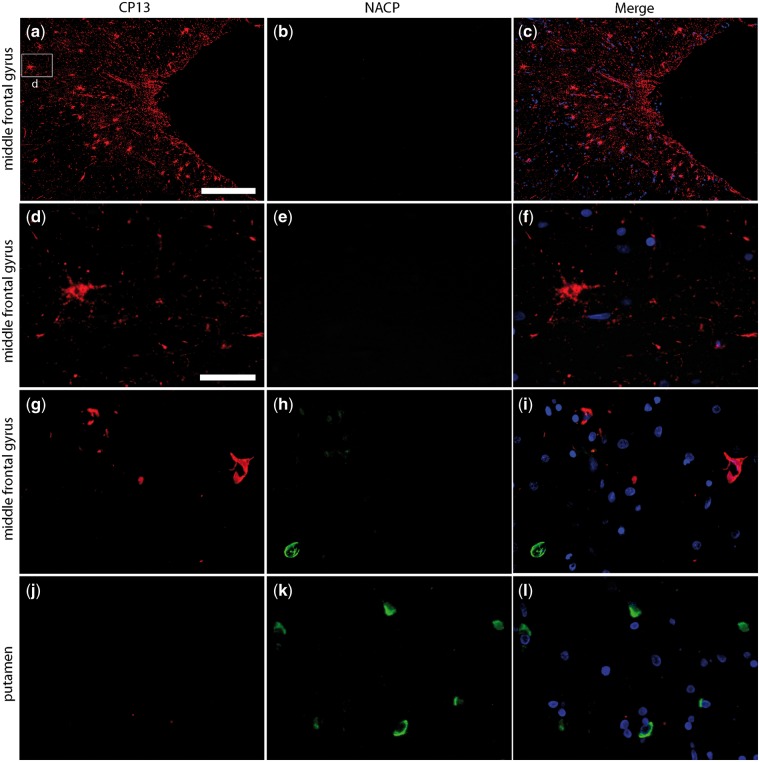
To assess the colocalization of tau and α-synuclein, we performed immunofluorescence staining for phospho-tau (CP13) and α-synuclein (NACP). Representative images from CTE-2 are shown in Figure 2. Phospho-tau-positive astrocytes and NFTs were detected in the cortex at the depths of a sulcus in the middle frontal gyrus, but α-synuclein positive inclusions in neurons and glial cells were not detected in this region (Fig. 2a–f). There were GCIs in the white matter of middle frontal gyrus, but not preferentially at the depths of sulci (Fig. 2g–i). There were abundant GCIs in putamen, characteristics of MSA, but tau pathology was not detected (Fig. 2j–l).
FIGURE 2.
Representative immunofluorescence images of CTE pathology and GCIs in a case with MSA and CTE (CTE-2). Tau-positive astrocytes and NFTs were detected in the middle frontal gyrus (a, d), but proximal NACP-positive neurons or glial cells were not observed (b, e). An NACP-positive GCI was detected the white matter of the middle frontal gyrus but it was not colocalized with CP13-positive lesions (g–i). (k) There were numerous GCIs in the putamen (k). Scale bars: a–c = 200 µm; d–l = 40 µm.
Neuropathologic Assessment of ARTAG
As shown in Table 1, 10 cases had tau pathology consistent with ARTAG, but not with CTE: TSAs and GFAs in subependymal, subpial, perivascular, gray matter, and white matter regions. Supplementary Figure 1 shows representative images of ARTAG pathology in case ARTAG-6. Mild subpial astrocytic pathology and a few TSAs were observed surrounding the sulcus in the middle frontal gyrus, but pretangles, NFTs, or perivascular astrocytic pathology were not detected (Supple-mentary Fig. S1a–c). Moderate numbers of GFAs were observed in hippocampal CA4 (Supplementary Fig. S1d). Numerous TSAs were detected around the lenticulostriate arteries and subpial mediobasal region in a section of basal forebrain (Supplementary Fig. S1e–f). ARTAG pathologies were distinguished from CTE pathology by the absence of pretangles and NFTs at the depth of sulci in the cortices (24). ARTAG pathology was detected in basal forebrain (8/10), mammillary body (6/8), superior temporal gyrus (5/10), middle frontal gyrus (4/10), hippocampus (4/10), inferior parietal gyrus (2/10), dorsal lateral frontal gyrus (2/10), orbital frontal gyrus (1/10), and inferior frontal gyrus (1/10), as shown in Supplementary Table S1.
Macroscopic Findings of CTE
Atrophy of the medial temporal lobe, thalamus, hypothalamus, and mammillary body are the most common macroscopic brain changes of CTE cases (6). We assessed these macroscopic findings in the 8 MSA cases with CTE pathology. Mild cortical atrophy was noted in 2 cases (CTE-2 and CTE-6), but the medial temporal lobe did not have atrophy. No cases had atrophy of thalamus, hypothalamus, mammillary body, or corpus callosum. A cavum septum pellucidum could not be accurately assessed as the autopsy removal process often artificially damaged the septum pellucidum because the brain was cut down the midline dividing it into 2 hemispheres. Atrophy and hemosiderosis of the lateral and posterior putamen as well as atrophy of middle cerebellar peduncle, pontine base and cerebellar white matter (typical macroscopic findings of MSA) were observed in all 8 cases. In summary, there was a paucity of macroscopic features associated with CTE in the 8 MSA brains with CTE pathology.
Demographic and Clinicopathologic Characteristics of CTE and ARTAG Cases
Demographic, clinical, and pathologic features are compared for MSA with CTE, MSA without CTE or ARTAG, and MSA with ARTAG cases in Table 2. All 8 CTE cases were male and 4 of them had a history of contact sports (Table 1). Case-1 was a 72-year-old Caucasian man who played high school football; CTE-2 was a 53-year-old African-American man who was a football player, playing throughout high school and college; CTE-6 was a 53-year-old Caucasian man who played basketball in high school; and CTE-8 was a 76-year-old Caucasian man who had a history of multiple football and skiing-related concussions, none of which reportedly were associated with a loss of consciousness. One ARTAG case (ARTAG-4) had a history of football and was described in our previous study (11). The age at death of CTE cases was younger than MSA (without CTE or ARTAG) cases or ARTAG cases (60 vs 67 vs 74 years, p = 0.002). Median age at onset tended to be younger in CTE than other 2 groups (50 vs 58 vs 65 years, p = 0.058), but only the post-hoc statistical difference between CTE and ARTAG reached significance (p = 0.027). The frequency of MSA with predominant parkinsonism clinical subtype (63% vs 79% vs 70%, p = 0.852), presence of falls (86% vs 77% vs 100%), cognitive impairment (43% vs 39% vs 71%, p = 0.577), and depression (43% vs. 46% vs. 57%, p = 0.986), were not different among the 3 groups. Brain weight (1180 vs 1210 vs 1250 g, p = 0.668), Braak NFT stage (I vs I vs II, p = 0.535), and Thal amyloid phase (0 vs 0 vs 0, p = 0.893) were not different amongst the 3 groups.
DISCUSSION
In this autopsy series of 139 neuropathologically confirmed MSA, 8 cases (6%) had concomitant CTE pathology. All MSA patients with CTE pathology were men. In individuals without a documented participation in contact sports, 3% (4/134) had CTE pathology. This suggests that a small, subset of cases with MSA can develop CTE pathology possibly related to repetitive falls. Although the retrospective clinical review has an obvious limitation in assessing frequency and severity of falls, our results support findings from Ling et al (12) that movement disorder patients prone to frequent falls may have higher frequency of CTE pathology than subjects without a known history of such exposure. Another possible explanation is that a history of contact sports was not documented in some cases. The patient reported by Ling et al (12) was the only one of 50 MSA patients with CTE pathology. That patient was a 62-year-old woman without exposure to contact sports or military whose traumatic brain injuries and multiple falls occurred later in life. Interestingly, however, the prevalence of histological evidence of CTE in that study was 13% in control subjects older than 60 years at death, which is higher than the reported frequency of CTE pathology in MSA (2%) (12). The differences between the present study and the Ling et al (12) study have several possible explanations. First, different pathologic criteria were used for diagnosis of CTE pathology. Second, it is not clear that the Ling et al study made an effort to differentiate ARTAG pathology from CTE pathology. In the current report, we used consensus criteria for CTE and made a concerted effort to distinguish ARTAG features from CTE (23). In contrast, Ling et al used preliminary CTE criteria, and it is unclear whether all the CTE pathology they described would meet stringent consensus criteria for CTE in accordance with newly defined NINDS guidelines (23). In addition, it is not possible to exclude that some of their CTE cases were actually ARTAG. In particular, the frequency of “CTE” amongst the neurodegenerative diseases and control subjects was quite high and seems to be influenced by age at death. In their study, the frequencies of “CTE” were 24% in PSP, 16% in Parkinson disease, and 13% in control subjects and the mean ages at death were 80, 84, and 92 years, respectively. On the other hand, the frequencies of “CTE” were 2% in MSA, 4% in FTLD, and 7% in CBD for which the mean ages were 62, 66, and 65 years, respectively. ARTAG is considered to be an aging process, hence the name; therefore, the frequency of ARTAG is inevitably higher in autopsy cohorts with an older age at death regardless of other concomitant pathologies (24).
The results of the current study found that age at death was younger in MSA with CTE than in MSA without CTE; 6 out of 8 patients died in the sixth decade of life. This earlier age at death in MSA with CTE may be explained by the younger age of onset in MSA with CTE, although it did not reach statistical significance. In terms of initial symptoms, 3 of 7 patients with adequate medical documentation had rapid eye movement sleep behavior disorder, 3 presented with autonomic dysfunction, and 1 had difficulty using in his left hand. These symptoms seem to be unrelated with CTE, so the association between CTE pathology and younger onset of disease is unclear. It could be speculated that CTE pathology affects regions vulnerable to α-synuclein pathology. Immunofluorescence studies did not support a spatial relationship between NFTs or TSAs in CTE and GCIs in MSA. Future studies are needed to clarify whether CTE pathology exacerbates the clinical or pathologic process of concomitant neurodegenerative diseases.
Regarding clinical symptoms suggestive of CTE, some MSA patients with CTE pathology developed memory loss, behavioral changes or chronic headaches, but the frequency of cognitive impairments and depression were not significantly different amongst the 3 groups. This could be explained by the relatively mild CTE pathology in this cohort; 1 with CTE stage III, 5 with stage II, and 2 with stage I. The fact that not all individuals with CTE were symptomatic is consistent with the literature, which has reported that 11% of individuals with CTE pathology were asymptomatic especially in early stages (6).
There are some limitations of this study. First, it is a retrospective analysis; hence, clinical information, especially as it relates to exposure to head trauma as well as the frequency and severity of falls, is limited. To supplement the medical records, we conducted online searches of obituaries. Although we obtained further information about the participation in contact sports, detailed information about falls is not typically mentioned in such inquiries. To assess the precise association between repetitive falls and CTE pathology, prospective studies with standardized questionnaires pertaining to falls and other sources of head trauma are needed. Second, some CTE pathology could be missed by the regional pathologic screening scheme; however, we selected all sections, including sulcal depths of the superior and middle frontal gyrus, superior and middle temporal gyrus, and inferior parietal gyrus, that are recommended as most valuable for detecting CTE (23). Third, there is a possibility that the 4 CTE patients without a known history of contact sports (CTE-3, 4, 5, and 7) actually participated in a contact sport(s), with lack of documentation in the medical records or online sources. Intriguingly, relevant synergistic effects of contact sports and repetitive falls are another possibility to explain the association between MSA and CTE pathology. However, we did not find the correlation between the burden of GCI and the severity of CTE pathology in eight cases with MSA and CTE, although it is uncertain whether the burden of GCI most correctly reflects the severity of MSA pathology as opposed to neuronal loss or other pathologic features.
A notable strength of this study is the number of patients with MSA screened for CTE pathology and diagnostic method for CTE. Another advantage of this study is MSA as our targeted neurodegenerative disorder. As MSA is an α-synucleinopathy, the diagnosis of CTE is not complicated by additional comorbid tau pathology, which complicates assessment of CTE pathology in AD, PSP and other primary tauopathies. Furthermore, 7 of 8 CTE cases had low Braak NFT stage (≤III), so age-related tau pathology would not be expected to mask CTE pathology. Although the greatest risk factor for developing CTE pathology in this study was participation in contact sports, a small subset of individuals with CTE pathology had no history of exposure to contact sports. Results in this study indicate that the threshold that triggers mild CTE pathology might be lower than previously appreciated.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Dr. Peter Davies (Feinstein Institute for Medical Research, LIJ-North Shore Health System, NY) for the monoclonal anti-tau antibodies CP13, Linda Rousseau (Mayo Clinic, Jacksonville, FL) and Virginia Phillips (Mayo Clinic) for histologic support, and Monica Castanedes-Casey (Mayo Clinic) for immunohistochemistry support.
Funding: Dr. Dickson receives support from the NIH (P50-AG016574; P50-NS072187; P01-AG003949) and CurePSP: Foundation for PSP|CBD and Related Disorders.
Supplementary Data can be found at http://www.jnen.oxfordjournals.org.
REFERENCES
- 1.DeKosky ST, Blennow K, Ikonomovic MD, et al. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol 2013;9:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavett BE, Stern RA, Cantu RC, et al. Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res Ther 2010;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30:179–88, xi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geddes JF, Vowles GH, Nicoll JA, et al. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol 1999;98:171–8 [DOI] [PubMed] [Google Scholar]
- 6.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martland HS. Punch Drunk. Jama 1928;91:1103–7 [Google Scholar]
- 8.Millspaugh JA. Dementia pugilistica. US Naval Med Bull 1937;35:297–303 [Google Scholar]
- 9.Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J 1957;1:357–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Critchley M. Punch-drunk syndromes: The chronic traumatic encephalopathy of boxers. In: Verbiest H, ed. Hommage a Clovis Vincent. Maloine, Paris: Maloin; 1949; 131 [Google Scholar]
- 11.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling H, Holton JL, Shaw K, et al. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015;130:891–3 [DOI] [PubMed] [Google Scholar]
- 13.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 1989;94:79–100 [DOI] [PubMed] [Google Scholar]
- 14.Wenning GK, Tison F, Ben Shlomo Y, et al. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 1997;12:133–47 [DOI] [PubMed] [Google Scholar]
- 15.Geser F, Wenning GK, Seppi K, et al. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG). Mov Disord 2006;21:179–86 [DOI] [PubMed] [Google Scholar]
- 16.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015;372:249–63 [DOI] [PubMed] [Google Scholar]
- 17.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 2015;85:404–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 20.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 2007;33:615–20 [DOI] [PubMed] [Google Scholar]
- 22.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 2004;127:2657–71 [DOI] [PubMed] [Google Scholar]
- 23.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016;131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.