Abstract
Purpose of review
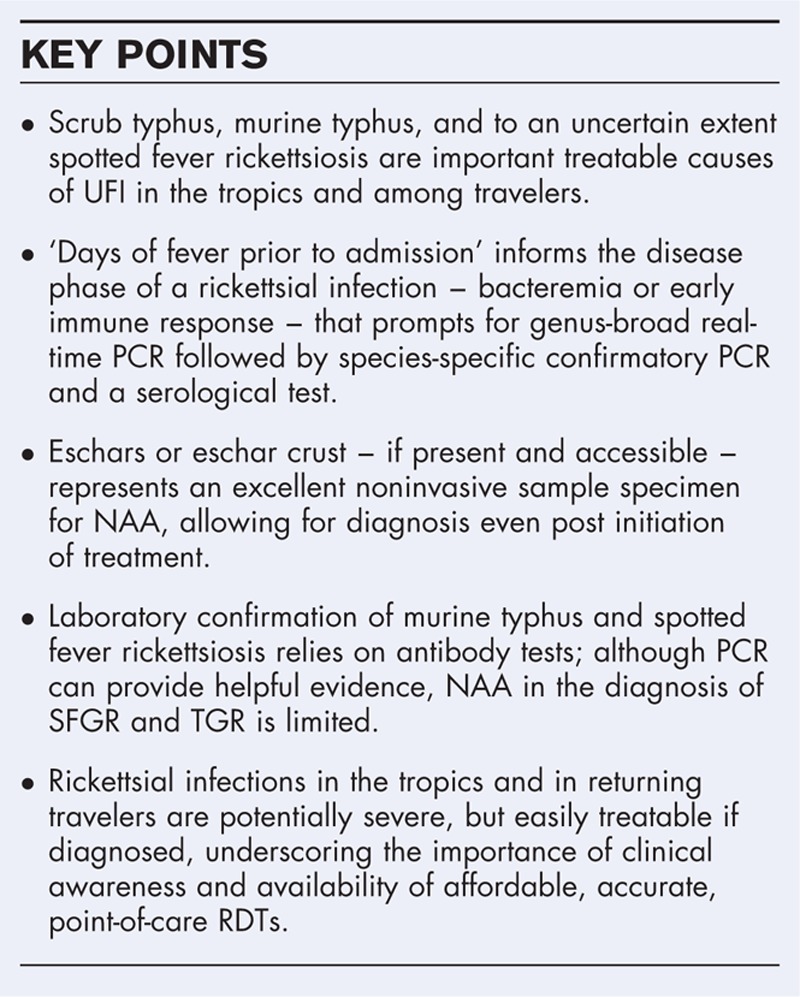
With improved malaria control, acute undifferentiated febrile illness studies in tropical regions reveal a startling proportion of rickettsial illnesses, especially scrub typhus, murine typhus, and spotted fever group rickettsioses. Laboratory diagnosis of these infections evolved little over the past 40 years, but combinations of technologies like PCR and loop-mediated isothermal amplification, with refined rapid diagnostic tests and/or ELISA, are promising for guidance for early antirickettsial treatment.
Recent findings
The long-term reliance on serological tests – useful only late in rickettsial infections – has led to underdiagnosis, inappropriate therapies, and undocumented morbidity and mortality. Recent approaches integrate nucleic acid amplification and recombinant protein-based serological tests for diagnosing scrub typhus. Optimized using Bayesian latent class analyses, this strategy increases diagnostic confidence and enables early accurate diagnosis and treatment – a model to follow for lagging progress in murine typhus and spotted fever.
Summary
A laboratory diagnostic paradigm shift in rickettsial infections is evolving, with replacement of indirect immunofluorescence assay by the more objective ELISA coupled with nucleic acid amplification assays to expand the diagnostic window toward early infection intervals. This approach supports targeted antirickettsial therapy, reduces morbidity and mortality, and provides a robust evidence base for further development of diagnostics and vaccines.
Keywords: ELISA, indirect immunofluorescent assay, murine typhus, Orientia tsutsugamushi, PCR, rickettsia, scrub typhus, spotted fever
INTRODUCTION
The global decline of malaria revealed an array of acute undifferentiated febrile illnesses (UFI) that extract a high toll on human health [1]. Recent large-scale studies of UFI in (sub)tropical regions reveal that rickettsial diseases, predominantly scrub typhus and murine typhus, are among the leading causes of treatable UFI [2–4,5▪▪,6–12]. Rickettsial illnesses are often misdiagnosed as malaria, dengue, or typhoid, and are important preventable causes of morbidity and mortality [2–4,5▪▪,6,13,14]. Rickettsial infections affect the vasculature to present with nonspecific signs and symptoms rendering early clinical diagnosis difficult [15]. The dissemination dynamics of Rickettsia and Orientia with their early limited bacteremic phase and subsequent appearance of antibodies have hindered the development of effective diagnostic tools for targeted early antirickettsial therapy. Especially in disease endemic areas, the occurrence of high background antibody titers poses an additional challenge to the already difficult serodiagnosis [16,17▪▪,18,19]. More hurdles involve translating promising technologies with high analytical sensitivity and specificity into clinically useful tests [2,20▪]. Here, we describe recent advances and major knowledge gaps in diagnosing rickettsial diseases, focusing on blood specimen-based tests conducted at the time of acute illness to inform targeted treatment.
Box 1.
no caption available
SCRUB TYPHUS AND RICKETTSIOSES OVERVIEW
Scrub typhus is arguably the world's most important rickettsial illness in terms of disease burden and is a leading cause of treatable UFI in Asia and Pacific regions where it accounts for up to 20% of febrile hospital admissions in endemic regions [2,4,5▪▪,6,13]. Recent evidence of Orientia spp. found in Africa, Europe, and South America indicates a wide genetic diversity and geographic distribution [2]. Scrub typhus, caused by Orientia tsutsugamushi and transmitted by Leptotrombidium mites, presents with ‘flu-like’ symptoms, and often with an eschar and/or a macular/maculopapular rash. Although effectively treated with tetracyclines, macrolides, and chloramphenicol, delayed treatment responses and severe disease with case fatality rates reaching 12–13% in northern Thailand and southern India are documented. Scrub typhus remains severely underrecognized, mainly because of diagnostic difficulties and lack of awareness.
The genus Rickettsia is divided based on antigenic and genomic distinctions and has an enlarging taxonomy [21]. The major pathogens are globally distributed and classified primarily within spotted fever and typhus group clusters [21,22]. Among tropical and travel-related infections, murine (fleaborne) typhus, caused by Rickettsia typhi in the typhus group rickettsia (TGR), is a common cause of UFI in tropical areas and travelers, especially in southeast Asia [3,21,23]. The expanding spotted fever group rickettsiae (SFGR) are less well studied globally. The GeoSentinel Network describes the highest rate in travelers returning from sub-Saharan Africa [7], but seroepidemiologic data and clinical studies show high prevalences of SFGR in the Americas, Mediterranean basin, north and south of Africa, Australia, and increasingly in Asia [13,21,24–26]. Major diagnostic challenges for SFGR are the close genetic relatedness and serological crossreactivity [27]. These rickettsioses also present with ‘flu-like’ symptoms, sometimes with an eschar and/or a macular/maculopapular rash, further complicating diagnosis [28].
DIAGNOSTIC ASPECTS FOR SCRUB TYPHUS, MURINE TYPHUS, AND SPOTTED FEVER RICKETTSIOSES
Major modalities for diagnosing rickettsial illnesses include culture, nucleic acid amplification (NAA), and serology; the latter includes rapid diagnostic tests (RDTs), indirect immunofluorescence assays (IFAs), and ELISA. Antigen detection in skin, eschar, or tissue biopsies can be advantageous during the acute phase of infection, and culture is useful for definitive identification and characterization, but either invasive sampling or long incubation times and biosafety aspects render these suboptimal for acute setting diagnosis.
SCRUB TYPHUS NUCLEIC ACID AMPLIFICATION TESTS
In 1990, PCR was first shown useful for detecting O. tsutsugamushi in clinical specimens. Nucleic acid detection is accurate in the early phase of infection up to 10 days of fever, after which serology becomes better at diagnosing scrub typhus. Common target genes include the htrA (47-kDa periplasmic serine protease), 56-kDa type-specific antigen, rrs (16S rRNA), and groEL (heat shock protein Hsp60). The 56-kDa type-specific antigen gene is specific to Orientia spp. only and PCR positivity and/or product sequencing provides strong evidence for the presence of pathogen DNA [29–32].
Real-time PCR prevails for diagnosis of scrub typhus, but high costs and training limit its use in rural areas. PCR assays are only as good as the samples used and depend on bacterial load and time point of disease. Common samples are whole blood, buffy coat, and eschars. Samples from eschar biopsies or noninvasive eschar crust are excellent for PCR, but only in areas of high eschar rates (e.g., Korea or China report >95%). Loop-mediated isothermal amplification (LAMP) assays are easy to use, need no thermocycler, provide a simple readable endpoint, and have comparable diagnostic accuracies to PCR, but are not widely used. Combined algorithms incorporating a NAA assay with an antibody-based test should be used as they expand the interval for successful laboratory diagnosis in the acute setting [33,34].
Scrub typhus rapid diagnostic tests
The availability of affordable and accurate point-of-care RDTs has improved directed treatment, and their widespread use enhances the awareness of scrub typhus. Comparisons of RDTs demonstrate improved diagnostic accuracy when using IgM over total antibody. Anti-O. tsutsugamushi IgG can persist leading to high RDT false-positive rates in endemic areas, for which assay adjustments might be required. Currently available RDTs are immunochromatographic or semiquantitative dot-blot assays, increasingly incorporating recombinant antigens, allowing greater standardization, and simple readout for point-of-care testing in resource-constrained settings [16,35–38].
Scrub typhus serology by indirect immunofluorescence assays
The IFA has been the mainstay in scrub typhus diagnostics for decades. However, the lack of standardization, variable cutoff titers for endemic regions, requirement for paired sera, high cost, and subjective endpoints are causes for concern [39]. The rigorous use of at least four-fold antibody titer rise in paired sera improves confidence, but confounding factors such as preexisting antibodies and crossreactivity remain. A combination of diagnostic modalities were incorporated into the scrub typhus infection criteria (STIC), as a composite endpoint for diagnostic comparisons; STIC was considered positive if either O. tsutsugamushi was isolated, at least two of three PCR assays were positive, and an admission IFA IgM titer was at least 12 800, or at least four-fold rise in convalescent IFA IgM titer was present [33]. STIC became the standard, but evaluations of new tests against a flawed gold standard inexorably lead to suboptimal biased results. Bayesian latent class modeling overcomes these difficulties, as it estimates accuracies of diagnostic tests using the true disease status of each patient (infected or noninfected), does not require a gold standard, does not assume that any diagnostic test or combination is perfect, and provides unbiased sensitivity and specificity estimates [17▪▪]. Using this analytical tool, the initial STIC recommendations improved to using a single admission IgM IFA titer of at least 3200 and/or a four-fold rise to at least 3200 in paired samples. This corrected for false positive rates associated with low-rising IFA titers and significantly increased sensitivity and specificity of the modified STIC [18].
Scrub typhus serology by ELISAs
Improved anti-Orientia IgM and IgG-based RDTs and ELISAs are replacing subjective IFAs. Increasingly, new assays use O. tsutsugamushi recombinant proteins to detect specific antibodies and have become less expensive, with improved sensitivity, specificity, and reproducibility [35,36,38,40]. ELISA offers advantages over IFA in simplicity, standardization, objectivity, and throughput. However, establishing a validated diagnostic cutoff is often overlooked, especially in endemic areas. A recent evaluation of ELISA found a strong association between optical density (OD) values and IFA titers. This enabled the determination of an ELISA optical density positive cutoff corresponding to a single IFA titer of at least 1600 with 93% sensitivity and 91% specificity [35], and is congruent with the improved composite indicators of STIC.
MURINE TYPHUS AND SPOTTED FEVER GROUP NUCLEIC ACID AMPLIFICATION TESTS
The major NAA methods for SFGR and TGR include LAMP and PCR; for PCR, a large array of gene targets are published, but none is substantially more effective than others [41]. Frequently used genes include 16S rRNA (rrs), citrate synthase (gltA), 17-kDa lipoprotein, and other conserved genes [41,42]. ‘Diagnosis-to-treat’ approaches incorporate Rickettsia genus-specific real-time PCRs [24,43▪,44–46]. However, unique gene regions can be targeted for species and subspecies-level identification [21,41], or broad-range PCR amplicons can be sequenced [47], as real-time PCR target sequences of 75–150 nucleotides provide only limited taxonomic information. Although simple and field applicable, SFGR and TGR LAMP assays are not well studied [48,49]; for murine typhus, low diagnostic LAMP accuracy is attributed to low R. typhi bacterial loads [48].
Frequent sample types include whole blood and buffy coat, and as with scrub typhus, skin or eschar biopsies/crusts or swabs are excellent for PCR, if available [21,50]. Although, real-time PCR provides reduced contamination, quantitation, and multiplex potential for species identification, or high-throughput analyses for epidemiologic investigations, conventional PCR methods, especially nested PCRs, are often used because of good diagnostic sensitivity and the potential for amplicon sequencing. In general, analytical sensitivity ranges from low for conventional PCR, to moderate/good (1000–10 000 genome equivalents/ml blood DNA) for nested PCR, and to good (<100–5000 genome equivalents/ml of blood DNA) for real-time PCR. Unfortunately, bacterial loads less than 100/ml blood (equivalent to 0.1 genome copies/μl reaction mixture) and poor DNA yield severely challenge analytical sensitivity limits and hinder NAA applicability [45,46,51,52].
High-quality clinical PCR evaluations are limited by small patient numbers, lack of prospective design, poorly controlled specificity, and a wide variety of techniques, targets, analytical approaches, and uncertain gold standards. Among published PCR methods since 2013 with more than 10 samples compared with serological standards [43▪,44,53,54], clinical sensitivity varied from good (>75%) to very poor (<5%), with a median of 18% (interquartile range 4–30) (Table 1). For pan-Rickettsia PCRs, blood DNA median sensitivity was 18% (6–27%), for SFGR PCR was 42% (6–69%), and for TGR was 3% (1–18%). Despite real-time PCR's appeal, there are insufficient clinical data to conclude that these assays are superior to nested or conventional PCR for diagnosis of human rickettsioses. Although data are limited, the use of skin biopsy or eschar samples improves sensitivity for pan-Rickettsia (43 vs. 18%) and SFGR assays (67 vs. 42%), but not for TGR (6 vs. 3%). Real-time PCR modestly enhances clinical sensitivity vs. nested PCR among a cohort of 223 human blood and tissue samples examined for rickettsial infection (18 vs. 16%) [46]. Additional support for use of skin and real-time PCR comes from guinea pig SFGR model studies: median sensitivity of skin vs. blood detection was 31 vs. 3%; 44% for real-time PCR, 7% for nested PCR, and 3% for conventional PCR [55▪].
Table 1.
Median clinical sensitivity of PCR methods for detection of spotted fever group and typhus group rickettsia in blood and skin/eschar biopsy samplesa
| Percentage clinical sensitivity | |||||
| Sample | Rickettsia | Method | Number of assays | Median (IQR) | References |
| All | PanRick | All | 145 | 23 (15–34) | [43,▪, 44,54] |
| SFGR | 331 | 48 (34–65) | [53, 54] | ||
| TGR | 257 | 5 (3–7) | [43▪,44] | ||
| Skin | All | All | 233 | 43 (7–55) | [43▪,54] |
| SFGR | 101 | 67 (55–79) | |||
| TGR | 88 | 6 (5–6) | |||
| Blood | All | All | 331 | 18 (4–30) | [43▪,44] |
| PanRick | 101 | 18 (12–23) | |||
| SFGR | 230 | 42 (24–56) | |||
| TGR | 169 | 3 (2–10) | |||
| All | PanRick | Real-time PCR | 525 | 7 (4–23) | [43▪,44] |
| SFGR | Real-time PCR | 123 | 23 (14–33) | ||
| TGR | Real-time PCR | 257 | 5 (3–7) | ||
| SFGR | Nested PCR | 29 | 31 (31–31) | [53] | |
| SFGR | Conventional PCR | 179 | 69 (61–80) | [53, 54] | |
aDerived from studies for which serologic and PCR results on more than 10 patients were reported since 2013 identified using search terms ‘rickettsia’, ‘spotted fever’, ‘typhus’ and ‘PCR’, ‘real-time PCR’, ‘nested-PCR’, ‘qPCR’, ‘quantitative PCR’. PanRick – assays that target the genus Rickettsia; SFGR – assays that target spotted fever group rickettsiae; TGR – assays that target typhus group rickettsiae. Number of assays column includes total assays reported, including some on the same samples but different approaches or targets. IQR, interquartile range.
Current methods for SFGR and TGR diagnosis are limited when using whole blood. This could be addressed by using skin rash or eschar biopsies, which are not always present in all patients. Diagnostic improvements could include bacterial enrichment, high blood volume use, or multicopy gene targets to overcome low rickettsial loads. Thus, the PCR target itself is not the major limiting factor for increasing clinical sensitivity.
SERODIAGNOSIS OF TYPHUS AND SPOTTED FEVER GROUP RICKETTSIOSES
Serodiagnosis remains the gold standard for SFGR and TGR infections using seroconversion and four-fold antibody titer increases [20▪,41]. Specificity, where examined, tends to be good to excellent, with the potential exception of IgM assays [20▪,56]. Major disadvantages include poor sensitivity during acute infection (antibodies are often not detectable within the first 10–14 days), the indirect nature of diagnostic evidence (detection of host responses), and crossreactions with other Rickettsia[13,20▪,57]. Unlike with scrub typhus, development of RDTs lags for SFGR and TGR. The preferred method remains IFA despite requirement of experience for accuracy and precision [20▪,58]. ELISA and related protein immunoblot and immunochromatographic methods are described. Although some manufacturers provide data documenting IFA comparisons, there is a paucity of studies that evaluate diagnostic methods on well characterized patient samples. The use of insufficiently validated ELISAs is associated with reduced studies of confirmed SFG rickettsioses in the United States [59].
Sensitivity and specificity of serological assays for SFGR and TGR are shown in Table 2[16,35,36,67,68,70–73]. Important limitations include the use of single samples for diagnosis and assumptions about cause based on serologic results. The latter is particularly relevant as all SFGR crossreact to some extent, as they do also with TGR, and as titers to individual species can vary considerably among poorly standardized methods such as IFA [13,20▪,57]. Most assays utilize antigens derived from Rickettsia rickettsii or Rickettsia conorii, but a positive result simply indicates a likely Rickettsia infection, and to a lesser extent a SFG Rickettsia infection. If several distinct SFGR antigens are used and titers differ by more than four-fold, the higher titer does not identify the etiologic agent. Although this could be in part resolved by crossabsorption studies, this is not a feasible approach for most clinical laboratories, is not rapid, and often does not resolve the specific cause [57,74]. For diagnostic purposes, the results are unlikely to be useful during the acute stage even if positive as the background seropositive rate in many tropical regions is either high or undefined [18,19,75]. Thus, reliance on single samples is discouraged and further confounded by a lack of specificity for IgM testing.
Table 2.
Sensitivity and specificity of serological tests for confirmation of scrub typhus, spotted fever rickettsiosis, and murine typhus
| Disease | Serological assay | Sensitivity (%) | Specificity (%) | References |
| Scrub typhus | IFA IgG | 91 | 96 | [60] |
| IFA IgM | 70–87 | 84–100 | [16,17▪▪,60] | |
| ELISA IgG | 80–97 | 89–98 | [60,61,62,63] | |
| ELISA IgM | 84–100 | 73–99 | [60, 64] | |
| ImmChrom IgG RDT | 86–95 | 96–100 | [38,60,65] | |
| ImmChrom IgM RDT | 82–94 | 86–100 | [35,38,40,60,65] | |
| Dot EIA | 60–100 | 94–99 | [36,60,66] | |
| Spotted fever rickettsiosis | IFA IgG | 85–100 | 99–100 | [67,68,69] |
| IFA IgM | 83–85 | 100 | [68, 69] | |
| ELISA IgG | 83 | 87 | [68, 70] | |
| ELISA IgM | 98 | 94a | [68] | |
| Murine typhus | IFA IgG | ≥83 | ≥93 | [67] |
| IFA IgM | 53–85 | 99 |
CONCLUSION
Rickettsial infections require early diagnosis and treatment to prevent severe outcomes, but this is rarely achieved using serology. For scrub typhus, combining NAA and IgM RDTs or ELISAs improves diagnostic accuracy and allows earlier detection. For SFGR and TGR infections, limited comparative studies, restricted RDT availability, and poor evidence for IgM-based testing make NAA tests attractive. To reliably guide clinical decisions, NAA tests for Rickettsia require considerable improvement to resolve challenges of genus-wide detection and methods improvement to overcome low-level rickettsemia. Prospective clinical studies in endemic areas are a critical test for the next generation of highly sensitive diagnostics for rickettsioses.
Acknowledgements
The opinions expressed herein are those of the author(s) and are not necessarily representative of those of the Department of Defense (DOD), Uniformed Services University of the Health Sciences (USUHS) or the US Army, Navy, or Air Force.
Financial support and sponsorship
This work was supported by funding from the Wellcome Trust, NIH/NIAID, Li Ka Shing Foundation and MDRIP to D.H.P., and by grants from the National Institutes of Allergy and Infectious Diseases, Bill and Melinda Gates Foundation, Fisher Discovery Center (Johns Hopkins University), Department of Pathology, University of Maryland School of Medicine, and PAT-74–3977 from the Uniformed Services University of the Health Sciences to J.S.D.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Walker DH. After malaria is controlled, what's next? Am J Trop Med Hyg 2014; 91:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris DH, Shelite TR, Day NP, et al. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg 2013; 89:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson CN, Blacksell SD, Paris DH, et al. Undifferentiated febrile illness in Kathmandu, Nepal. Am J Trop Med Hyg 2015; 92:875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu M, Nicholson WL, Roche AJ, et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis 2011; 53:e8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪▪.Mayxay M, Castonguay-Vanier J, Chansamouth V, et al. Causes of nonmalarial fever in Laos: a prospective study. Lancet Glob Health 2013; 1:e46–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive ‘causes of febrile illness study,’ which contributed significantly to the discovery of the importance of rickettsial illnesses, and introduced the aspect of targeting ‘doxycycline-treatable illnesses,’ which cover rickettsioses and leptospirosis.
- 6.Acestor N, Cooksey R, Newton PN, et al. Mapping the aetiology of nonmalarial febrile illness in Southeast Asia through a systematic review: terra incognita impairing treatment policies. PLoS One 2012; 7:e44269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006; 354:119–130. [DOI] [PubMed] [Google Scholar]
- 8.Jensenius M, Davis X, von Sonnenburg F, et al. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers. Emerg Infect Dis 2009; 15:1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensenius M, Han PV, Schlagenhauf P, et al. Acute and potentially life-threatening tropical diseases in western travellers: a GeoSentinel multicenter study. Am J Trop Med Hyg 2013; 88:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi NH, Taylor WR, Murphy GS, et al. Etiology of acute, nonmalaria, febrile illnesses in Jayapura, northeastern Papua, Indonesia. Am J Trop Med Hyg 2012; 86:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasper MR, Blair PJ, Touch S, et al. Infectious etiologies of acute febrile illness among patients seeking healthcare in south-central Cambodia. Am J Trop Med Hyg 2012; 86:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leelarasamee A, Chupaprawan C, Chenchittikul M, et al. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai 2004; 87:464–472. [PubMed] [Google Scholar]
- 13.Reller ME, Bodinayake C, Nagahawatte A, et al. Unsuspected rickettsioses among patients with acute febrile illness, Sri Lanka, 2007. Emerg Infect Dis 2012; 18:825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chheng K, Carter MJ, Emary K, et al. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS One 2013; 8:e60634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valbuena G, Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb Haemost 2009; 102:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blacksell SD, Lim C, Tanganuchitcharnchai A, et al. Optimal cut-off and accuracy of an IgM ELISA for diagnosis of acute scrub typhus in northern Thailand: an alternative reference method to the IgM IFA. J Clin Microbiol 2016; 54:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Lim C, Paris DH, Blacksell SD, et al. How to determine the accuracy of an alternative diagnostic test when it is actually better than the reference tests: a re-evaluation of diagnostic tests for scrub typhus using Bayesian LCMs. PLoS One 2015; 10:e0114930. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive study on the use of Bayesian latent class modeling methods to determine the diagnostic accuracy of a new test when the actual gold standard is flawed or inaccurate. This method uses a minimum of three different diagnostic methods to estimate the disease prevalence (true disease status of each patient), to provide unbiased sensitivity and specificity estimates, and does not assume that any diagnostic test or combination is perfect.
- 18.Lim C, Blacksell SD, Laongnualpanich A, et al. Optimal cutoff titers for indirect immunofluorescence assay for diagnosis of scrub typhus. J Clin Microbiol 2015; 53:3663–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall GS, Stout GG, Jacobs RF, et al. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch Pediatr Adolesc Med 2003; 157:443–448. [DOI] [PubMed] [Google Scholar]
- 20▪.Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis: United States. MMWR Recomm Rep 2016; 65:1–44. [DOI] [PubMed] [Google Scholar]; Comprehensive review of rickettsial infections that impact the United States, whether domestic or imported. The laboratory diagnostic approaches are useful and extensively referenced.
- 21.Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker DH. Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis 2007; 45 Suppl 1:S39–S44. [DOI] [PubMed] [Google Scholar]
- 23.Delord M, Socolovschi C, Parola P. Rickettsioses and Q fever in travelers. Travel Med Infect Dis 2014; 12:443–458. [DOI] [PubMed] [Google Scholar]
- 24.Prakash JA, Sohan Lal T, Rosemol V, et al. Molecular detection and analysis of spotted fever group Rickettsia in patients with fever and rash at a tertiary care centre in Tamil Nadu, India. Pathog Glob Health 2012; 106:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang LQ, Liu K, Li XL, et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis 2015; 15:1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhengsri S, Baggett HC, Edouard S, et al. Sennetsu neorickettsiosis, spotted fever group, and typhus group rickettsioses in three provinces in Thailand. Am J Trop Med Hyg 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merhej V, Angelakis E, Socolovschi C, et al. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect Genet Evol 2014; 25:122–137. [DOI] [PubMed] [Google Scholar]
- 28.Parola P, Raoult D. Tropical rickettsioses. Clin Dermatol 2006; 24:191–200. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Chan TC, Temenak JJ, et al. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg 2004; 70:351–356. [PubMed] [Google Scholar]
- 30.Horinouchi H, Murai K, Okayama A, et al. Genotypic identification of Rickettsia tsutsugamushi by restriction fragment length polymorphism analysis of DNA amplified by the polymerase chain reaction. Am J Trop Med Hyg 1996; 54:647–651. [DOI] [PubMed] [Google Scholar]
- 31.Paris DH, Aukkanit N, Jenjaroen K, et al. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect 2009; 15:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonthayanon P, Chierakul W, Wuthiekanun V, et al. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol 2009; 47:430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paris DH, Blacksell SD, Nawtaisong P, et al. Diagnostic accuracy of a loop-mediated isothermal PCR assay for detection of Orientia tsutsugamushi during acute scrub typhus infection. PLoS Negl Trop Dis 2011; 5:e1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blacksell SD, Paris DH, Chierakul W, et al. Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin Vaccine Immunol 2012; 19:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blacksell SD, Tanganuchitcharnchai A, Nawtaisong P, et al. Diagnostic accuracy of the InBios scrub typhus detect enzyme-linked immunoassay for the detection of IgM antibodies in Northern Thailand. Clin Vaccine Immunol 2015; 23:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodkvamtook W, Zhang Z, Chao C-C, et al. Dot-ELISA rapid test using recombinant 56-kDa protein antigens for serodiagnosis of scrub typhus. Am J Trop Med Hyg 2015; 92:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash JA, Abraham OC, Mathai E. Evaluation of tests for serological diagnosis of scrub typhus. Trop Doct 2006; 36:212–213. [DOI] [PubMed] [Google Scholar]
- 38.Silpasakorn S, Srisamut N, Ekpo P, et al. Development of new, broadly reactive, rapid IgG and IgM lateral flow assays for diagnosis of scrub typhus. Am J Trop Med Hyg 2012; 87:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blacksell SD, Bryant NJ, Paris DH, et al. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis 2007; 44:391–401. [DOI] [PubMed] [Google Scholar]
- 40.Kingston HW, Blacksell SD, Tanganuchitcharnchai A, et al. Comparative accuracy of the InBios scrub typhus detect IgM rapid test for the detection of IgM antibodies by using conventional serology. Clin Vaccine Immunol 2015; 22:1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luce-Fedrow A, Mullins K, Kostik AP, et al. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol 2015; 10:537–564. [DOI] [PubMed] [Google Scholar]
- 42.Renvoise A, Rolain JM, Socolovschi C, et al. Widespread use of real-time PCR for rickettsial diagnosis. FEMS Immunol Med Microbiol 2012; 64:126–129. [DOI] [PubMed] [Google Scholar]
- 43▪.Znazen A, Sellami H, Elleuch E, et al. Comparison of two quantitative real time PCR assays for Rickettsia detection in patients from Tunisia. PLoS Negl Trop Dis 2015; 9:e0003487. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of real-time PCR assays to detect Rickettsia genus, R. typhi, and spotted fever group rickettsia infections compared with serologic diagnosis, including improved detection in skin samples, illustrating limitations in clinical sensitivity despite high analytical sensitivity.
- 44.Watthanaworawit W, Turner P, Turner C, et al. A prospective evaluation of real-time PCR assays for the detection of Orientia tsutsugamushi and Rickettsia spp. for early diagnosis of rickettsial infections during the acute phase of undifferentiated febrile illness. Am J Trop Med Hyg 2013; 89:308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakash JAJ, Reller ME, Barat N, et al. Assessment of a quantitative multiplex 5’ nuclease real-time PCR for spotted fever and typhus group rickettsioses and Orientia tsutsugamushi. Clin Microbiol Infect 2009; 15:292–293. [DOI] [PubMed] [Google Scholar]
- 46.Kato CY, Chung IH, Robinson LK, et al. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 2013; 51:314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffith M, Peter JV, Karthik G, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med 2014; 18:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dittrich S, Castonguay-Vanier J, Moore CE, et al. Loop-mediated isothermal amplification for Rickettsia typhi (the causal agent of murine typhus): problems with diagnosis at the limit of detection. J Clin Microbiol 2014; 52:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan L, Zhang L, Wang G, et al. Rapid, simple, and sensitive detection of the ompB gene of spotted fever group rickettsiae by loop-mediated isothermal amplification. BMC Infect Dis 2012; 12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JM, Hudson BJ, Watts MR, et al. Diagnosis of Queensland tick typhus and African tick bite fever by PCR of lesion swabs. Emerg Infect Dis 2009; 15:963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paris DH, Blacksell SD, Stenos J, et al. Real-time multiplex PCR assay for detection and differentiation of rickettsiae and orientiae. Trans R Soc Trop Med Hyg 2008; 102:186–193. [DOI] [PubMed] [Google Scholar]
- 52.Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am J Trop Med Hyg 2005; 73:1083–1085. [PubMed] [Google Scholar]
- 53.Kondo M, Akachi S, Kawano M, et al. Improvement in early diagnosis of Japanese spotted fever by using a novel Rick PCR system. J Dermatol 2015; 42:1066–1071. [DOI] [PubMed] [Google Scholar]
- 54.Kurokawa I, Kondo M, Akachi S. Early diagnosis of Japan spotted fever by PCR using skin samples. J Infect Chemother 2013; 19:628–632. [DOI] [PubMed] [Google Scholar]
- 55▪.Zemtsova GE, Montgomery M, Levin ML. Relative sensitivity of conventional and real-time PCR assays for detection of SFG Rickettsia in blood and tissue samples from laboratory animals. PLoS One 2015; 10:e0116658. [DOI] [PMC free article] [PubMed] [Google Scholar]; In-vivo demonstration of comparative sensitivity of real-time vs. nested vs. conventional PCR, and the use of skin material vs. blood for identification of spotted fever group rickettsia infection in a guinea pig model.
- 56.McQuiston JH, Wiedeman C, Singleton J, et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg 2014; 91:767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaughn MF, Delisle J, Johnson J, et al. Seroepidemiologic study of human infections with spotted fever group rickettsiae in North Carolina. J Clin Microbiol 2014; 52:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phetsouvanh R, Thojaikong T, Phoumin P, et al. Inter- and intra-operator variability in the reading of indirect immunofluorescence assays for the serological diagnosis of scrub typhus and murine typhus. Am J Trop Med Hyg 2013; 88:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010; 83:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coleman RE, Sangkasuwan V, Suwanabun N, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg 2002; 67:497–503. [DOI] [PubMed] [Google Scholar]
- 61.Kim YJ, Yeo SJ, Park SJ, et al. Improvement of the diagnostic sensitivity of scrub typhus using a mixture of recombinant antigens derived from Orientia tsutsugamushi serotypes. J Korean Med Sci 2013; 28:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Land MV, Ching WM, Dasch GA, et al. Evaluation of a commercially available recombinant-protein enzyme-linked immunosorbent assay for detection of antibodies produced in scrub typhus rickettsial infections. J Clin Microbiol 2000; 38:2701–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lijuan Z, Si H, Yuming J, et al. A rapid, sensitive and reliable diagnostic test for scrub typhus in China. Indian J Med Microbiol 2011; 29:368–371. [DOI] [PubMed] [Google Scholar]
- 64.Jang WJ, Huh MS, Park KH, et al. Evaluation of an immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Orientia tsutsugamushi infection. Clin Diagn Lab Immunol 2003; 10:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao M, Guo H, Tang T, et al. Preparation of recombinant antigen of O. tsutsugamushi Ptan strain and development of rapid diagnostic reagent for scrub typhus. Am J Trop Med Hyg 2007; 76:553–558. [PubMed] [Google Scholar]
- 66.Kim YJ, Yeo SJ, Park SJ, et al. Improvement of the diagnostic sensitivity of scrub typhus using a mixture of recombinant antigens derived from Orientia tsutsugamushi serotypes. J Korean Med Sci 2013; 28:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newhouse VF, Shepard CC, Redus MD, et al. A comparison of the complement fixation, indirect fluorescent antibody, and microagglutination tests for the serological diagnosis of rickettsial diseases. Am J Trop Med Hyg 1979; 28:387–395. [DOI] [PubMed] [Google Scholar]
- 68.Clements ML, Dumler JS, Fiset P, et al. Serodiagnosis of Rocky Mountain spotted fever: comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J Infect Dis 1983; 148:876–880. [DOI] [PubMed] [Google Scholar]
- 69.Philip RN, Casper EA, MacCormack JN, et al. A comparison of serologic methods for diagnosis of Rocky Mountain spotted fever. Am J Epidemiol 1977; 105:56–67. [DOI] [PubMed] [Google Scholar]
- 70.Keysary A, Strenger C. Use of enzyme-linked immunosorbent assay techniques with cross-reacting human sera in diagnosis of murine typhus and spotted fever. J Clin Microbiol 1997; 35:1034–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Philip RN, Casper EA, Ormsbee RA, et al. Microimmunofluorescence test for the serological study of Rocky Mountain spotted fever and typhus. J Clin Microbiol 1976; 3:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hechemy KE, Stevens RW, Sasowski S, et al. Discrepancies in Weil-Felix and microimmunofluorescence test results for Rocky Mountain spotted fever. J Clin Microbiol 1979; 9:292–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L, Si H, Yuming J, et al. A rapid, sensitive and reliable diagnostic test for scrub typhus in China. Indian J Med Microbiol 2011; 29:368–371. [DOI] [PubMed] [Google Scholar]
- 74.Delisle J, Mendell NL, Stull-Lane A, et al. Human infections by multiple spotted fever group rickettsiae in Tennessee. Am J Trop Med Hyg 2016; 94:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Premaratna R, Weerasinghe S, Ranaweera A, et al. Clinically helpful rickettsial disease diagnostic IgG titers in relation to duration of illness in an endemic setting in Sri Lanka. BMC Res Notes 2012; 5:662. [DOI] [PMC free article] [PubMed] [Google Scholar]


