Abstract
Background
Age‐related macular degeneration (AMD) is a progressive, late‐onset disorder of the macula affecting central vision. It is the leading cause of blindness in people over 65 years in industrialized countries. Recent epidemiologic, genetic, and pathological evidence has shown that AMD shares a number of risk factors with atherosclerosis, leading to the hypothesis that statins may exert protective effects in AMD.
Objectives
The objective of this review was to examine the effectiveness of statins compared with other treatments, no treatment, or placebo in delaying the onset and progression of AMD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 3), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2016), EMBASE (January 1980 to March 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2016), PubMed (January 1946 to March 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched 5 June 2014), ClinicalTrials.gov (www.clinicaltrials.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 31 March 2016.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐randomized trials that compared statins with other treatments, no treatment, or placebo in people who were diagnosed as having the early stages of AMD.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently evaluated the search results against the selection criteria, abstracted data, and assessed risk of bias. We did not perform meta‐analysis due to heterogeneity in the interventions and outcomes between the included studies.
Main results
Two RCTs with a total of 144 participants met the selection criteria. Both trials compared simvastatin versus placebo in older people (older than 50 or 60 years) with high risk of developing AMD (drusen present on examination). Overall, we judged the quality of the evidence to be low, as we downgraded all outcomes due to limitations in the designs of the trials and insufficient outcome reporting. The larger trial, with 114 participants, was conducted in Australia and used a higher dose (40 mg daily) of simvastatin for three years. Participants and study personnel in this trial were adequately masked, however data were missing for 30% of participants at three years' follow‐up. The smaller trial, with 30 participants, was conducted in Italy and used a lower dose (20 mg) of simvastatin for three months. This trial reported insufficient details to assess the risk of bias.
Neither trial reported data for change in visual acuity. Low‐quality evidence from the smaller trial, with 30 participants, did not show a statistically significant difference between the simvastatin and placebo groups in visual acuity values at three months of treatment (decimal visual acuity 0.21 ± 0.56 in simvastatin group and 0.19 ± 0.40 in placebo group) or 45 days after the completion of treatment (decimal visual acuity 0.20 ± 0.50 in simvastatin group and 0.19 ± 0.48 in placebo group). The lack of a difference in visual acuity was not explained by lens or retina status, which remained unchanged during and after the treatment period for both groups.
Preliminary analyses of 42 participants who had completed 12 months' follow‐up in the larger trial did not show a statistically significant difference between simvastatin and the placebo groups for visual acuity, drusen score, or visual function (effect estimates and confidence intervals were not available). Complete data for these outcomes at three years' follow‐up were not reported. At three years, low‐quality evidence showed an effect of simvastatin in slowing progression of AMD compared with placebo to be uncertain (odds ratio 0.51, 95% confidence interval 0.23 to 1.09).
One trial did not report adverse outcomes. The second trial reported no difference between groups in terms of adverse events such as death, muscle aches, and acute hepatitis.
Authors' conclusions
Evidence from currently available RCTs is insufficient to conclude that statins have a role in preventing or delaying the onset or progression of AMD.
Keywords: Female, Humans, Male, Middle Aged, Hydroxymethylglutaryl‐CoA Reductase Inhibitors, Hydroxymethylglutaryl‐CoA Reductase Inhibitors/adverse effects, Hydroxymethylglutaryl‐CoA Reductase Inhibitors/therapeutic use, Macular Degeneration, Macular Degeneration/prevention & control, Randomized Controlled Trials as Topic, Simvastatin, Simvastatin/adverse effects, Simvastatin/therapeutic use, Visual Acuity
Plain language summary
Statins for delaying the onset and progression of age‐related macular degeneration
Review question What are the effects of statins on delaying the onset and progression of age‐related macular degeneration (AMD)?
Background AMD is a progressive disease of the macula (area in the back of the eye affecting central vision). AMD is the leading cause of blindness in people over 65 years in industrialized countries. Studies have shown that some of the factors that may lead to heart disease and strokes are the same as those that may lead to AMD. Statins are a type of drug that aims to lower blood cholesterol levels. As statins are very effective in preventing strokes, it is possible that they are also protective for AMD.
Study characteristics We included two trials (144 total participants) in this review. Participants included men and women, most of whom were older than 50 years, who had good visual acuity. Participants were either susceptible to or had been diagnosed with an early stage of AMD. Both trials compared simvastatin with placebo. The larger trial, with 114 participants and conducted in Australia, used a higher dose of 40 mg per day and had a treatment period of three years. The smaller trial, with 30 participants and conducted in Italy, used a lower dose of 20 mg per day and had a treatment period of three months. The evidence provided in this review was up‐to‐date as of March 2016.
Key results Neither trial provided sufficient evidence to determine whether statins are effective in delaying the onset or progression of AMD. Information was lacking for outcomes related to vision, quality of life, and adverse events.
Quality of the evidence The overall quality of the evidence was low. In the smaller trial, the number of participants enrolled and the short treatment period may not have been sufficient for detecting the effect of statins on AMD, which develops over time. In the larger trial, 30% of participants did not attend the three‐year follow‐up visit, and the amount of missing data hindered our ability to draw any reliable conclusions for this trial.
Summary of findings
Summary of findings for the main comparison. Statins compared with placebo for age‐related macular degeneration.
| Statins compared with placebo for age‐related macular degeneration | ||||||
|
Patient or population: People aged 50 years or older with age‐related macular degeneration Settings: Australia and Italy Intervention: statins (simvastatin) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with comparator* | Risk with intervention | |||||
| Change in visual acuity | See comment | ‐ | ‐ | 30 (1) | ⊕⊕⊝⊝ low1,2 | Authors of Guymer 2013 did not report visual‐acuity data. Only authors of Martini 1991 reported this outcome, showing the decimal visual acuity 0.21 ± 0.56 in simvastatin group and 0.19 ± 0.40 in placebo group at 3 months |
| Onset and progression of AMD | See comment | ‐ | ‐ | 114 (1) | ⊕⊕⊝⊝ low1,2 | Authors of Martini 1991 did not report data for the onset and progression of AMD. Only authors of Guymer 2013 reported this outcome, albeit using the last‐observation‐carried‐forward method to account for AMD assessment data for 34 of 144 participants (24%) who missed the 3‐year follow‐up visit. Participants assigned to simvastatin had a lower, but not statistically significant, odds of having AMD progression at 3 years compared with participants in the placebo group (OR 0.51, 95% CI 0.23 to 1.09) |
| Adverse outcomes | See comment | ‐ | ‐ | 114 (1) | ⊕⊕⊝⊝ low1,2 | Authors of Martini 1991 did not report adverse events data. Only authors of Guymer 2013 reported adverse events, which included death, muscle aches, and acute hepatitis. Guymer 2013 reported that adverse events occurred in 25/57 participants (44%) in the simvastatin group and 39/57 participants (68%) in the placebo group |
| *Risk was estimated from the comparator group in the included studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMD: age‐related macular degeneration; CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for limitations in the design of available studies suggesting high likelihood of bias (‐1). 2Downgraded for poor reporting of outcomes (‐1).
Background
Description of the condition
Age‐related macular degeneration (AMD) is a progressive, late‐onset disorder of the macula that affects central vision. Although the leading cause of blindness in people over 65 years in industrialized countries (Congdon 2003), its pathogenesis is not clearly understood. It is believed that both genetic and environmental factors play a significant role in the development of the disease.
Epidemiology
The prevalence, incidence, and rate of progression of AMD increase with age. The prevalence of any AMD (referred to as age‐related maculopathy) in the Beaver Dam Eye Study was less than 10% in people aged 43 to 54 years, but more than tripled for people aged 75 to 85 years (AAO 2014; Klein 1992; Klein 2001). Joint data from the United States, Netherlands, and Australia indicate the prevalence for late AMD is 0.2% in people 55 to 64 years old, rising sharply to 13% in people over 85 years old (Mitchell 1995; Smith 2001). The 10‐year incidence of early AMD and late AMD was 12.1% and 2.1%, respectively in the Beaver Dam Eye Study (Klein 2002). Individuals 75 years of age or older at baseline had significantly higher 10‐year incidences of both early and advanced AMD (Klein 2002). The main risk factors for the development and progression of AMD include increasing age, smoking, and ethnicity. Other reported risk factors include low levels of antioxidants, dietary fat, heart disease, hypertension, genetic influences, alcohol consumption, and sunlight (AAO 2014). Most of the epidemiologic studies have based their conclusions on few incident cases of advanced AMD. Also, the long intervals between follow‐up examinations or population surveys have meant that some incident cases were likely to have been missed due to deaths, so that the incidence and progression of AMD could not be assessed reliably.
Clinical presentation and diagnosis
The early manifestations of AMD are the presence of yellowish deposits known as drusen or retinal pigment epithelium abnormalities such as hypopigmentation or hyperpigmentation observed during retinal examination of eyes. In general, these early clinical signs are not associated with significant vision loss. However, a proportion of people with drusen and pigmentary changes will progress to advanced AMD with geographic atrophy (large area of atrophy centered in the macula) or choroidal revascularization, both of which can have severe effects on central visual function (AAO 2014; AREDS 2001; CAPT 2004; Smeeth 2005).
Description of the intervention
The treatments currently used for neovascular AMD include agents that interfere with vascular endothelial growth factor (VEGF), such as ranibizumab (Lucentis), bevacizumab (Avastin), and aflibercept (Eylea). Administration of these drugs, however, requires multiple intravitreous injections. Although the neovascularization may no longer appear to be 'active', visual recovery or preservation is neither complete nor universal. The risks and benefits of long‐term use are unknown (Solomon 2014). Photocoagulation and photodynamic therapy were shown to have some benefit in preventing severe visual loss in a small proportion of people with neovascular AMD, and only for a limited time after treatment (MPS 1991; MPS 1994; Virgili 2007; Wormald 2007). The evidence as to the benefits and harms of surgical injection or implantation of steroids with antiangiogenic properties for treating neovascular AMD is weak (Geltzer 2013). Antioxidant vitamin and mineral supplements were revealed in the Age‐Related Eye Disease Study (AREDS) to reduce progression to advanced AMD, both the neovascular ('wet') form and the geographic atrophy ('dry') form, in people with intermediate AMD (AREDS 2001). However, aside from the AREDS formulation, there is no proven medical intervention for preventing the onset and progression of this disease (Evans 2012a; Evans 2012b).
How the intervention might work
The burden of disease would be greatly diminished if a treatment could prevent or delay the onset of early AMD or the progression of early AMD to advanced AMD. Epidemiologic, genetic, and pathological evidence has shown that a number of risk factors are shared by AMD and atherosclerosis, leading to suggestions that statins, which are known to be beneficial in people with atherosclerotic disease and hyperlipidemia, may exert protective effects in AMD.
Possible pharmacological mechanisms of statins in preventing AMD include the following (Guymer 2005).
Serum lipid‐lowering effects: statins may alter the deposition or resorption characteristics, or both, of lipids in Bruch's membrane (a thin, semi‐permeable cellular structure that acts as the basement membrane for the retinal pigment epithelium and effectively mediates metabolic exchange between the retina and the choroid).
Preserving vascular supply: statins may preserve vascular supply to the outer retina through a protective effect against atherosclerosis (Friedman 2004).
The anti‐inflammatory actions of statins: inflammation may be important in AMD pathogenesis (Penfold 2001). The anti‐inflammatory properties of statins may provide additional protective effects. Statins down‐regulate the activation of transcription factors NF‐B, AP‐1, and hypoxia‐inducible factor‐1. They therefore have potentially anti‐inflammatory and antiproliferative effects that are relevant in the treatment of atherosclerotic diseases (Dichtl 2003). Elevated intraocular levels of vascular endothelial growth factor (VEGF) have an important role in the development of choroidal neovascularization (CNV) in AMD. Prior work indicates that statins reduce plasma levels of VEGF and down‐regulate transcription factors involved in VEGF expression. It is therefore conceivable that systemic statin use may reduce the incidence and progression of CNV via such cellular and molecular effects (Dichtl 2003).
The antioxidant effect of statins: oxidized lipids and low‐density lipoproteins (LDL) may be the initial stimulus leading to inflammation in AMD (Gurne 1991; Spaide 1999). Statins may protect the outer retina, Bruch's membrane, and choroid from oxidative damage.
Inhibition of metalloproteinases: statins may also inhibit secretion of matrix metalloproteinases, which may be involved in fissuring and rupture of plaques and development of neovascularization (Guymer 2005).
Why it is important to do this review
The high prevalence of AMD, the anticipated increase in the aged population, and the limited role of available effective treatments highlight the need to search for new treatment strategies for preventing or delaying onset or progression of AMD. A number of observational studies have examined the relationship between AMD and the use of statins; the results have been contradictory. In the Rotterdam Study, those using statins for more than 12 months had a similar incidence of AMD to those not using these drugs (adjusted hazard ratio 1.1, 95% confidence interval (CI) 0.7 to 1.9) (van Leeuwen 2003). The Beaver Dam Eye Study found the five‐year incidence of neovascular AMD not to be associated with statin use (odds ratio (OR) 0.9, 95% CI 0.46 to 1.78) (Klein 2001). A more recent analysis by Klein et al found that a history of statin use was not associated with the five‐year incidence of early AMD (OR 1.16, 95% CI 0.71 to 1.91), progression of AMD (OR 1.16, 95% CI 0.75 to 1.78), or incidence of late AMD (OR 1.27, 95% CI 0.60 to 2.69) (Klein 2007b). The Women's Health Initiative Sight Examination (WHISE), an ancillary study to the Women's Health Initiative's clinical trial of hormone replacement therapy, reported similar negative results (Klein 2007a). One study suggests increased risk with statin use (McGwin 2006). By contrast, animal experiments showed that pitavastatin (so‐called vascular statin) suppressed the formation and development of CNV in rats (Sagara 2007). A strong inverse association between statin use and AMD was reported by Hall et al in a cross‐sectional study with 392 participants (OR 0.14, 95% CI 0.02 to 0.83) and by McGwin et al in a study that involved 550 cases of AMD and 5500 controls (OR 0.3, 95% CI 0.21 to 0.45) (Hall 2001; McGwin 2003). Tan et al found that statin use was protective for indistinct soft drusen (hazard ratio 0.33, 95% CI 0.13 to 0.84), a key late AMD precursor lesion, based on their analysis of data from the Blue Mountains Eye Study (Tan 2007). In a population‐based cohort study, Smeeth et al assessed the effect of statins on a range of health outcomes. A sample of 129,288 people who initiated treatment with a statin were compared with a matched sample of 600,241 people who did not initiate treatment. The hazard ratio of AMD among non‐exposed to exposed study participants was 1.17 (99% CI 1.00 to 1.38), providing no evidence to support a beneficial effect of statin treatment with respect to AMD (Smeeth 2009). Observational and animal studies have methodological limitations and may be subject to bias and confounding. A systematic collection and summary of currently available data from high‐quality clinical trials provide the best evidence regarding this issue.
Objectives
The objective of this review was to examine the effectiveness of statins compared with other treatments, no treatment, or placebo in delaying the onset and progression of AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐randomized trials in this review. We considered studies that did not use randomization to allocate participants but utilized techniques intended to allocate participants in an unbiased fashion to be quasi‐randomized trials (for example allocation based on day of the week, year of birth, or hospital admission number of consecutive patients).
Types of participants
We included trials that enrolled participants who were diagnosed as having early stages of AMD with no signs of choroidal neovascularization as determined by their study criteria.
Types of interventions
We included trials comparing statins, which inhibit the enzyme 3‐hydroxy 3‐methylglutaryl CoA reductase, with other treatments, no treatment, or placebo. We planned to include trials that compared different types of statin therapy, as well as trials in which statins in combination with another treatment were compared with the other treatment alone.
Types of outcome measures
Primary outcomes
The primary outcome for comparison of interventions was the change in visual acuity, categorized by loss of three or more lines, no change (within three lines from baseline), and improvement in three or more lines. When continuous LogMAR data were available we analyzed the visual acuity and degree of change as continuous data. The primary time of outcome assessment was at three years' follow‐up, with different follow‐up times analyzed as reported.
Secondary outcomes
The secondary outcomes for comparison of interventions were onset and progression of AMD, defined as:
incidence of early signs of AMD using definitions specified in the included studies;
incidence of progression from early AMD to intermediate or late stages of AMD using definitions specified in the included studies.
Adverse outcomes
We tabulated all systemic and ocular adverse effects related either to statins or other treatments as reported in the included studies. Specific adverse effects of interest were:
ocular adverse effects;
systemic adverse effects.
Economic data
We planned to document cost‐benefit analyses and other data on economic outcomes in the reported studies.
Quality‐of‐life data
We planned to assess quality‐of‐life data when validated measures were reported.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 3), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2016), EMBASE (January 1980 to March 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2016), PubMed (January 1946 to March 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched 5 June 2014), ClinicalTrials.gov (www.clinicaltrials.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 31 March 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), PubMed (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and ICTRP (Appendix 8).
Searching other resources
We searched the reference lists of the trials included in the review for additional trials. We used the Science Citation Index to find studies that had cited the identified trials.
Data collection and analysis
Selection of studies
Two review authors independently evaluated the titles and abstracts resulting from the electronic and manual searches to identify potentially relevant studies for inclusion. We obtained full copies of all potentially or definitely relevant articles. Two review authors worked independently to determine which studies met the selection criteria. We resolved discrepancies by discussion. We documented the excluded studies and reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors independently abstracted data from the included study published in English (Guymer 2013). Cochrane Eyes and Vision's Italian‐speaking editor (Dr Gianni Virgili) extracted data from the included study published in Italian (Martini 1991). This information was verified by a second Italian‐speaking colleague (Dr Fabrizio Giansanti). Study characteristics extracted included: study design, participant characteristics, interventions, and outcomes assessed. One review author entered all data into Review Manager (RevMan 2014), and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors or colleagues (GV and FG) assessed the risk of bias of the included studies according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following parameters:
sequence generation;
allocation concealment;
masking (blinding) of participants, personnel, and outcome assessors;
incomplete outcome data;
selective outcome reporting;
other sources of bias.
We judged each included study to be at low risk of bias, high risk of bias, or unclear risk of bias for each parameter.
Measures of treatment effect
We followed guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).Martini 1991 reported the decimal visual acuity (for example 20/20 = 1.0; 20/25 = 0.8, ... 20/200 = 0.10, etc.) and standard deviation by trial group. However, the authors did not report the mean difference between the two treatment arms (P value was mentioned). We could calculate the mean difference in visual acuity based on available data; however, we could not calculate the 95% confidence interval because decimal visual acuity generally does not follow a normal or near‐normal distribution.
Dealing with missing data
We contacted authors of RCT reports in an effort to obtain unreported outcome information. We did not impute data for the purposes of this review; however, we used imputed data when reported in the included study reports and documented how missing data were handled. We will update the review when additional data become available.
Data synthesis
We did not perform a meta‐analysis as the review included only two trials and each trial reported different outcomes. We will consider meta‐analysis when additional data become available.
Sensitivity analysis
We did not perform sensitivity analyses to determine the impact of exclusion of studies with lower methodological quality, exclusion of unpublished studies, or exclusion of industry‐funded studies because no meta‐analysis was conducted. We will perform a sensitivity analysis when data become available from additional clinical trials.
'Summary of findings' table
We prepared a 'Summary of findings' table for the following outcomes: mean change in visual acuity, onset and progression of AMD, and adverse outcomes. We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group approach to assess the overall quality of the evidence for each outcome (Guyatt 2011). We assessed the five major domains of risk of bias, imprecision, inconsistency, publication bias, and indirectness.
Results
Description of studies
Results of the search
The electronic searches from 30 April 2009 retrieved 97 titles and abstracts, of which five appeared to be relevant (Gehlbach 2009). After examining the full text, we excluded three reports of two studies (Della Valle 2000; Sen 2002), included one study (Martini 1991), and identified one ongoing study (Guymer 2013).
Updated electronic searches on 16 September 2011 retrieved 35 additional titles and abstracts (Gehlbach 2012), of which three studies were assessed in full and two studies excluded as they were not RCTs (Drobek‐Slowik 2008; Maguire 2009). We included another report from the ongoing study first published as an abstract in 2005 (Guymer 2013).
Revised and updated electronic searches on 5 June 2014 yielded 187 new records. We assessed four potentially relevant reports in full, excluding two studies, Berendschot 2009 and Mao 2009, and including two reports from the completed Guymer 2013 study. Overall, we excluded six studies and included two distinct trials in this review (Gehlbach 2015).
As of 31 March 2016, the most recent updated electronic searches yielded 39 new records (Figure 1). None of the new records met our inclusion criteria and all were excluded.
1.
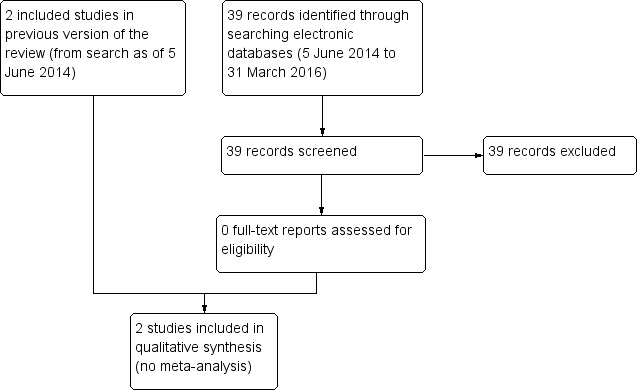
Study flow diagram.
Included studies
We included two RCTs with a total of 144 participants. The trials included men and women with good baseline visual acuity who were at risk for AMD (presence of drusen). Guymer 2013 included participants with normal cholesterol levels, whereas Martini 1991 included participants with high cholesterol levels (above 260 mg/dL). Guymer 2013, the larger trial, enrolled 114 participants and was conducted in Australia. Martini 1991, the smaller trial, enrolled 30 participants and was conducted in Italy. Both trials compared simvastatin with placebo: Guymer 2013 used a higher dose (40 mg/day) long term (three years), and Martini 1991 used a lower dose (20 mg/day) short term (three months). Although both trials assessed vision‐related outcomes, they used different outcome definitions and time points, precluding pooling of data. Only Guymer 2013 reported AMD‐related outcomes and adverse events. The primary outcome of Martini 1991 was serum cholesterol level, which was not an outcome assessed in this review.
Excluded studies
We excluded six studies because they did not meet our inclusion criteria: four were not RCTs of statin use (Berendschot 2009; Della Valle 2000; Drobek‐Slowik 2008; Maguire 2009), one enrolled participants with diabetic retinopathy (Sen 2002), and one did not include outcomes relevant to this review (Mao 2009).
See: Characteristics of excluded studies table.
Risk of bias in included studies
We have summarized 'Risk of bias' assessments in Figure 2. We judged Martini 1991 to be at unknown ('unclear') risk of bias on all domains assessed (see Characteristics of included studies table) because insufficient methodological details were reported.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of selection bias was low for Guymer 2013 and unclear for Martini 1991. Guymer 2013 employed a robust method of generating the random sequence by using permuted blocks of randomly varying size and storing the allocation list remotely.
Masking (performance bias and detection bias)
We assessed the methods used for masking as adequate for Guymer 2013 and unclear for Martini 1991. Guymer 2013 implemented proper masking of appropriate personnel. Each intervention, simvastatin and placebo, was identically packaged and properly masked limiting performance and detection bias.
Incomplete outcome data
Outcome data were missing for more than 25% of participants in Guymer 2013, resulting in a judgement of high risk of attrition bias. The authors of Guymer 2013 disclosed that 34 out of 114 participants (30%), 20 out of 57 participants (35%) in the simvastatin group, and 14 out of 57 participants (25%) in the placebo group missed the three‐year follow‐up examination. As a result, the authors carried forward the AMD assessment from the last follow‐up visit in their analysis of outcomes. However, use of the last‐observation‐carried‐forward method is not a robust analytic practice in most situations and may not be appropriate when measuring a progressive disease such as AMD (Li 2013).
Selective reporting
Guymer 2013 published results for their primary outcome as stated in their 1998 protocol, and we thus assessed the trial to be at low risk of reporting bias. No protocol for Martini 1991 was available, and we assessed the trial to be at unclear risk of selective outcome reporting.
Other potential sources of bias
Guymer 2013 reported imbalances between the statin and placebo groups with respect to participants with unilateral advanced AMD and smoking at baseline, with the statin group at higher risk of progression to advanced AMD.
Effects of interventions
See: Table 1
Due to heterogeneity in outcome measures and time points between trials, we did not combine the trial results in a meta‐analysis. The overall quality of the evidence was low for all review outcomes (Table 1); we downgraded for limitations in the designs of the available studies suggesting high likelihood of bias (‐1) and lack of reported outcomes suggesting high probability of publication bias (‐1).
Change in visual acuity
Neither trial reported change in visual acuity at three years' follow‐up, the primary outcome and time point for this review. Although visual‐acuity data were not reported at three years in Guymer 2013, possibly because progression of AMD was their primary outcome and not visual acuity, data were reported in preliminary 12‐month results. Analyses of 42 participants (19 in the simvastatin arm and 23 in the placebo arm) who completed 12 months' follow‐up showed no statistically significant difference between the two groups in visual acuity, drusen score, or visual function (effect estimates and confidence intervals (CIs) were not available). We contacted the trial investigators and will update the review as data become available.
In Martini 1991, analysis of 30 participants after three months of treatment showed similar results between the simvastatin and placebo groups in terms of visual acuity (decimal visual acuity 0.21 ± 0.56 in the simvastatin group and 0.19 ± 0.40 in the placebo group). The authors did not report values for mean changes in visual acuity. Visual acuity results were also similar at 45 days after the completion of the treatment period (decimal visual acuity 0.20 ± 0.50 in the simvastatin group and 0.19 ± 0.48 in the placebo group).
Onset and progression of AMD
Guymer 2013 used the last‐observation‐carried‐forward method to account for AMD assessment data for 34 of 144 participants (24%) who missed the three‐year follow‐up visit. Participants assigned to simvastatin had lower, but not statistically significant, odds of having AMD progression at three years compared with participants in the placebo group (odds ratio (OR) 0.51, 95% CI 0.23 to 1.09). Martini 1991 did not report onset or progression of AMD but reported that the lens and retina status were unchanged during and after the treatment period for both groups.
Adverse outcomes
Guymer 2013 reported that adverse events occurred in 25 out of 57 participants (44%) in the simvastatin group and 39 out of 57 participants (68%) in the placebo group. Reported adverse events included death, muscle aches, and acute hepatitis. Participants did not have abnormal liver function. Martini 1991 did not report adverse events.
Economic and quality‐of‐life outcomes
Neither study reported economic or quality‐of‐life outcomes.
Discussion
Summary of main results
We identified two RCTs, with a total of 144 participants, comparing simvastatin with placebo. We did not perform meta‐analysis due to the heterogeneity between the trials: Guymer 2013 used a higher dose (40 mg/day) of simvastatin over a longer time period (three years), and Martini 1991 used a lower dose (20 mg/day) of simvastatin over a shorter time period (three months). This review did not find sufficient evidence to determine the effectiveness and safety of statins to prevent or delay the progression of AMD.
Overall completeness and applicability of evidence
One trial, Martini 1991, had a small sample size of 30 participants at an early stage of disease. The other trial, Guymer 2013, had a larger sample size of 114 participants, but 30% of participants missed the three‐year follow‐up. The evidence appears to be incomplete given that slightly less than one‐third of the participants did not complete the last follow‐up visit in the largest trial. The two included trials were limited to simvastatin. It is unknown whether other statins have similar or different effects. Since the studies included participants with early AMD, this evidence is applicable to people at an earlier stage of the disease; the applicability of these findings to those with advanced AMD is unknown. The primary outcomes differed between the two studies, and neither trial reported change in visual acuity at three years' follow‐up as an outcome. The trials were conducted in Italy and Australia, and the applicability of this evidence to other settings, such as the US and low‐ to ‐middle‐income countries, is unknown. Limited information was available on whether findings were different across age and gender categories.
Quality of the evidence
Martini 1991 assessed visual acuity at the completion of treatment (three months from baseline) and 45 days afterwards. Due to the slow, progressive nature of the disease, the short duration of treatment and follow‐up may not provide useful information about the effectiveness of treatment because it may be too short to show an effect. In addition, we judged the trial as unclear for all risk of bias domains because of insufficient reporting of methodological details. Although no ophthalmological adverse effects were reported during the study period, there was still a degree of uncertainty about the accuracy of this finding because of the short follow‐up period.
Guymer 2013 assessed progression and onset of AMD at three‐year follow‐up. However, the level of missing data was about 30% and the trial investigators used the last‐observation‐carried‐forward method to account for the missing data. This method of dealing with missing data is not robust and may not be appropriate in assessing outcomes for progressive diseases, thus making the conclusions unreliable.
Potential biases in the review process
We followed standard methodological procedures recommended by Cochrane in order to minimize potential biases in the review process.
The absence of evidence on harms in the Martini 1991 trial may relate to the short duration of follow‐up and inadequate power of the trial to detect meaningful differences in important but infrequent events between treatment groups. The long‐term treatment and follow‐up of the participants in the larger Guymer 2013 trial provide more information about the effectiveness of treatment and the potential harms; however, caution is needed in interpreting these results as data were missing for a substantial proportion of participants.
Agreements and disagreements with other studies or reviews
The American Academy of Ophthalmology does not list statins as a treatment option for AMD, which is consistent with our finding of inadequate evidence to support the use of statins to slow progression of early AMD. We also found little or no evidence that statins improve visual‐acuity outcomes in AMD.
Authors' conclusions
Implications for practice.
There is insufficient evidence from RCTs to justify the use of statins to delay the onset and progression of AMD or to improve visual acuity. The American Academy of Ophthalmology does not list statins as a treatment option for AMD, which is consistent with the results of the review.
Implications for research.
Challenges exist in answering the question of whether statins have beneficial effect in the prevention and treatment of AMD. Due to the slow progressive nature of the disease, trials with a short duration of treatment or follow‐up, or with a small sample size, provide limited information about the effectiveness of statins in the prevention and treatment of AMD. Given that direct treatments for AMD are available (for example anti‐VEGF therapy), and a large proportion of older adults in Western populations are already on statins, it is unlikely that a new RCT would be designed to answer this question specifically. We envision that subsequent data addressing the effectiveness of statins for AMD would come from RCTs evaluating a separate clinical question in which AMD participants are included as a subgroup or AMD is analyzed as a secondary outcome. Future updates of this systematic review may provide conclusive findings that answer this question.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2016 | New search has been performed | Issue 8, 2016: No new studies and citations were added. |
| 31 March 2016 | New citation required but conclusions have not changed | Issue 8, 2016: Electronic searches were updated. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 5 February 2012 | New search has been performed | Issue 3, 2012: Electronic searches were updated. |
| 5 February 2012 | New citation required but conclusions have not changed | Issue 3, 2012: No new studies were identified for inclusion. |
| 18 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Iris Gordon and Lori Rosman at Cochrane Eyes and Vision for devising and conducting the electronic search for this review. We thank peer reviewers (Barbara Hawkins and other masked reviewers) and the editorial team, especially Jennifer Evans, for their advice and assistance during the preparation of this review. We thank Gianni Virgili and Fabrizio Giansanti, who abstracted data for the Italian trial included in this review, and Kristina Lindsley and Andrew Law for assisting with the update. Many thanks to: David Brandon, Harold Burton, Bill Crowther, Peter Dyson, Esme Green, Brenda Rogers, David Yule of the Cochrane Eyes and Vision AMD Consumer Panel for comments on the plain language summary.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Retinal Degeneration] explode all trees #2 MeSH descriptor: [Macular Degeneration] explode all trees #3 MeSH descriptor: [Retinal Neovascularization] explode all trees #4 MeSH descriptor: [Choroidal Neovascularization] explode all trees #5 MeSH descriptor: [Macula Lutea] explode all trees #6 ((macul* or retina* or choroid*) near/4 degener*) #7 ((macul* or retina* or choroid*) near/4 neovasc*) #8 maculopath* #9 (macul* near/2 lutea*) #10 (macul* near/3 dystroph*) #11 (macul* near/2 syndrome) #12 ((macul* or geographic) near/2 atroph*) #13 ((macul* or retina*) near/2 edema*) #14 (AMD or ARMD or CNV) #15 {or #1‐#14} #16 MeSH descriptor: [Hydroxymethylglutaryl‐CoA Reductase Inhibitors] explode all trees #17 (Statin* or vastatin*) #18 ("hmg coenzyme" or "hmg co‐enzyme") #19 ((HMG near/3 COA*):ti,ab or (HMG near/3 "Co A")) #20 3‐hydroxy‐3‐methylglutaryl #21 hydroxymethylglutaryl* #22 (hydroxymethyl near/3 glutaryl) #23 Atorvastatin* or liptonorm or Atorlip or atovarol or ci981 or "CI 981" or glustar or lipibec or lipitor or lowlipen or sortis or storvas or tahor or torvast or "ym 548" or ym548 or zarator or ezetimibe* or liptruzet or Ramipril* or "atocor R" #24 Cerivastatin or Kazak or rivastatin or Certa or "Bay w 6228" or "bay w6228" or Baycol or Lipobay #25 Bervastatin or "ls 2904" or ls2904 #26 Crilvastatin or "pmd 387" or pmd387 #27 dalvastatin or rg12561 or "rg 12561" #28 Fluvastatin or fluindostatin or Lescol or "XU 62‐320" or "XU 62320" or xu62320 or canef or cranoc or "fractal lp" or leucol or lochol or locol or "sri 62320" or sri62320 or vastin #29 Glenvastatin or "hr 780" or hr780 #30 MeSH descriptor: [Lovastatin] explode all trees #31 Lovastatin* or Mevinolin or "Monacolin K" or "6 Methylcompactin" or "MK 803" or MK803 or mk0803 or Mevacor or altocor or altoprev or artein or belvas or birotin or cholestra or cysin or ellanco or elstatin or "l 654969" or lipdip or lipivas or lofacol or lomar or lostatin or lovacel or lovacol or lovalip or lovalord or lovastan or lovasterol or lovastin or lovatadin or lowachol or lozutin or medostatin or meverstin or mevinacor or monakolin k or "msd 803" or neolipid or nergadan or ovasta or rodatin or rovacor or taucor or advicor #32 MeSH descriptor: [Meglutol] explode all trees #33 Meglutol or "3 Hydroxy 3 methylpentanedioic Acid" or "beta Hydroxy beta Methylglutarate" or "3 Hydroxy 3 methylglutaric Acid" #34 Mevastatin or compactin or mevastin or 6‐demethylmevinolin or "CS 500" or CS500 or "ML 236B" or ML236B #35 mevinolin* or monacolin* #36 "Phosphoadenosine diphosphoribose" or "phospho‐ADP ribose" #37 Pitavastatin or nisvastatin or itavastatin or alipza or itavastatin or livalo or livazo or pitava or ribar or vezepra or "P 872441" or "NK 104" or "nk104" or "nks 104" or nks104 #38 MeSH descriptor: [Pravastatin] explode all trees #39 Pravastatin* or Eptastatin or Vasten or "CS 514" or CS514 or Lipemol or Liplat or Nu‐Pravastatin or Prareduct or Mevalotin or Pravachol or Elisor or Selektine or Pravacol or Pravasin or Lipostat or "RMS 431" or RMS431 or "SQ 31000" or SQ31000 or "SQ 31,000" or SQ31,000 or Bristacol or astin or bristacol or cholespar or epatostantin or eptastatine or kenstatin or lipidal or liprevil or novales or prascolend or prastan or prava or pravaselect or pravasine or pravator or pravyl or sanaprav or selipran or stanidine or vasopran or xipral or pravafenix #40 Rosuvastatin* or ZD4522 or "ZD 4522" or Crestor or rosuvas or "s 4522" or s4522 or certriad #41 MeSH descriptor: [Simvastatin] explode all trees #42 Simvastatin* or Synvinolin or "MK 733" or MK733 or Zocor or avastinee or cholestat or clinfar or colastatina or colestricon or covastin or denan or epistatin or esvat or ethicol or eucor or ifistatin or kavelor or klonastin or kolestevan or "l 644128" or l644128 or lipecor or lipex or lipinorm or liponorm or lipovas or lodales or medipo or mersivas or nor‐vastina or normofat or orovas or rechol or simbado or simcard or simchol or simovil or simtin or simvacor or simvahex or simvalord or simvastar or simvata or simvatin or simvor or simvotin or sinvacor or sinvastatin or sinvinolin or sivastin or starzoco or torio or valemia or vasilip or vasotenal or vazim or vidastat or zimmex or zocord or zovast or inegy or vytorin or zetsim or zintrepid or cholib or fenofibrate* or niacin‐simvastatin or simcor or rosiglitazone‐simvastatin or avandastat or sitagliptin‐simvastatin or sitagliptin phosphate* or juvisync #43 tenivastatin #44 {or #16‐#43} #45 #15 and #44
Appendix 2. MEDLINE (Ovid) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Macular Degeneration/ 13. exp Retinal Degeneration/ 14. exp Retinal Neovascularization/ 15. exp Choroidal Neovascularization/ 16. exp Macula Lutea/ 17. ((macul* or retina* or choroid*) adj4 degener*).tw. 18. ((macul* or retina* or choroid*) adj4 neovasc*).tw. 19. Maculopath*.tw. 20. (macul* adj2 lutea*).tw. 21. (macul* adj3 dystroph*).tw. 22. (macul* adj2 syndrome).tw. 23. ((macul* or geographic) adj2 atroph*).tw. 24. ((macul* or retina*) adj2 edema*).tw. 25. (AMD or ARMD or CNV).tw. 26. or/12‐25 27. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 28. (Statin* or vastatin*).tw. 29. ((HMG adj3 COA*) or (HMG adj3 Co A)).tw. 30. (hmg coenzyme or hmg co‐enzyme).tw. 31. 3‐hydroxy‐3‐methylglutaryl*.tw. 32. hydroxymethylglutaryl*.tw. 33. (hydroxymethyl adj3 glutaryl).tw. 34. (Atorvastatin* or liptonorm or Atorlip or atovarol or ci981 or "CI 981" or glustar or lipibec or lipitor or lowlipen or sortis or storvas or tahor or torvast or "ym 548" or ym548 or zarator or ezetimibe* or liptruzet or Ramipril* or "atocor R").tw. 35. (Bervastatin or "ls 2904" or ls2904).tw. 36. (Cerivastatin or Kazak or rivastatin or Certa or "Bay w 6228" or "bay w6228" or Baycol or Lipobay).tw. 37. (Crilvastatin or "pmd 387" or pmd387).tw. 38. (dalvastatin or rg12561 or "rg 12561").tw. 39. (Fluvastatin or fluindostatin or Lescol or "XU 62‐320" or "XU 62320" or xu62320 or canef or cranoc or "fractal lp" or leucol or lochol or locol or "sri 62320" or sri62320 or vastin).tw. 40. (Glenvastatin or "hr 780" or hr780).tw. 41. exp Lovastatin/ 42. (Lovastatin* or Mevinolin or "Monacolin K" or "6 Methylcompactin" or "MK 803" or MK803 or mk0803 or Mevacor or altocor or altoprev or artein or belvas or birotin or cholestra or cysin or ellanco or elstatin or "l 654969" or lipdip or lipivas or lofacol or lomar or lostatin or lovacel or lovacol or lovalip or lovalord or lovastan or lovasterol or lovastin or lovatadin or lowachol or lozutin or medostatin or meverstin or mevinacor or monakolin k or "msd 803" or neolipid or nergadan or ovasta or rodatin or rovacor or taucor or advicor).tw. 43. exp Meglutol/ 44. (Meglutol or "3 Hydroxy 3 methylpentanedioic Acid" or "beta Hydroxy beta Methylglutarate" or "3 Hydroxy 3 methylglutaric Acid").tw. 45. (Mevastatin or compactin or mevastin or 6‐demethylmevinolin or "CS 500" or CS500 or "ML 236B" or ML236B).tw. 46. (mevinolin* or monacolin*).tw. 47. ("Phosphoadenosine diphosphoribose" or "phospho‐ADP ribose").tw. 48. (Pitavastatin or nisvastatin or itavastatin or alipza or itavastatin or livalo or livazo or pitava or ribar or vezepra or "P 872441" or "NK 104" or "nk104" or "nks 104" or nks104).tw. 49. exp Pravastatin/ 50. (Pravastatin* or Eptastatin or Vasten or "CS 514" or CS514 or Lipemol or Liplat or Nu‐Pravastatin or Prareduct or Mevalotin or Pravachol or Elisor or Selektine or Pravacol or Pravasin or Lipostat or "RMS 431" or RMS431 or "SQ 31000" or SQ31000 or "SQ 31,000" or SQ31,000 or Bristacol or astin or bristacol or cholespar or epatostantin or eptastatine or kenstatin or lipidal or liprevil or novales or prascolend or prastan or prava or pravaselect or pravasine or pravator or pravyl or sanaprav or selipran or stanidine or vasopran or xipral or pravafenix).tw. 51. (Rosuvastatin* or ZD4522 or "ZD 4522" or Crestor or rosuvas or "s 4522" or s4522 or certriad).tw. 52. exp Simvastatin/ 53. (Simvastatin* or Synvinolin or "MK 733" or MK733 or Zocor or avastinee or cholestat or clinfar or colastatina or colestricon or covastin or denan or epistatin or esvat or ethicol or eucor or ifistatin or kavelor or klonastin or kolestevan or "l 644128" or l644128 or lipecor or lipex or lipinorm or liponorm or lipovas or lodales or medipo or mersivas or nor‐vastina or normofat or orovas or rechol or simbado or simcard or simchol or simovil or simtin or simvacor or simvahex or simvalord or simvastar or simvata or simvatin or simvor or simvotin or sinvacor or sinvastatin or sinvinolin or sivastin or starzoco or torio or valemia or vasilip or vasotenal or vazim or vidastat or zimmex or zocord or zovast or inegy or vytorin or zetsim or zintrepid or cholib or fenofibrate* or niacin‐simvastatin or simcor or rosiglitazone‐simvastatin or avandastat or sitagliptin‐simvastatin or sitagliptin phosphate* or juvisync).tw. 54. tenivastatin.tw. 55. or/27‐54 56. 11 and 26 and 55
The search filter for trials at the beginning of the MEDLINE strategy was from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'retina maculopathy'/exp #34 'retina degeneration'/exp #35 'retina macula degeneration'/exp #36 'retina neovascularization'/exp #37 'subretinal neovascularization'/exp #38 'retina macula lutea'/exp #39 ((macul* OR retina* OR choroid*) NEAR/4 degener*):ab,ti #40 ((macul* OR retina* OR choroid*) NEAR/4 neovasc*):ab,ti #41 maculopath*:ab,ti #42 (macul* NEAR/2 lutea*):ab,ti #43 (macul* NEAR/3 dystroph*):ab,ti #44 (macul* NEAR/2 syndrome):ab,ti #45 ((macul* OR geographic) NEAR/2 atroph*):ab,ti #46 ((macul* OR retina*) NEAR/2 edema*):ab,ti #47 amd:ab,ti OR armd:ab,ti OR cnv:ab,ti #48 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 #49 'hydroxymethylglutaryl coenzyme a reductase inhibitor'/exp #50 statin*:ab,ti OR vastatin*:ab,ti #51 'hmg coenzyme':ab,ti OR 'hmg co‐enzyme':ab,ti #52 (hmg NEAR/3 coa*):ab,ti OR (hmg NEAR/3 'co a'):ab,ti #53 '3 hydroxy 3 methylglutaryl':ab,ti #54 hydroxymethylglutaryl*:ab,ti #55 (hydroxymethyl NEAR/3 glutaryl):ab,ti #56 atorvastatin*:ab,ti OR liptonorm:ab,ti OR atorlip:ab,ti OR atovarol:ab,ti OR ci981:ab,ti OR 'ci 981':ab,ti OR glustar:ab,ti OR lipibec:ab,ti OR lipitor:ab,ti OR lowlipen:ab,ti OR sortis:ab,ti OR storvas:ab,ti OR tahor:ab,ti OR torvast:ab,ti OR 'ym 548':ab,ti OR ym548:ab,ti OR zarator:ab,ti OR ezetimibe*:ab,ti OR liptruzet:ab,ti OR ramipril*:ab,ti OR 'atocor r':ab,ti #57 cerivastatin:ab,ti OR kazak:ab,ti OR rivastatin:ab,ti OR certa:ab,ti OR 'bay w 6228':ab,ti OR 'bay w6228':ab,ti OR baycol:ab,ti OR lipobay:ab,ti #58 bervastatin:ab,ti OR 'ls 2904':ab,ti OR ls2904:ab,ti #59 crilvastatin:ab,ti OR 'pmd 387':ab,ti OR pmd387:ab,ti #60 dalvastatin:ab,ti OR rg12561:ab,ti OR 'rg 12561':ab,ti #61 fluvastatin:ab,ti OR fluindostatin:ab,ti OR lescol:ab,ti OR 'xu 62‐320':ab,ti OR 'xu 62320':ab,ti OR xu62320:ab,ti OR canef:ab,ti OR cranoc:ab,ti OR 'fractal lp':ab,ti OR leucol:ab,ti OR lochol:ab,ti OR locol:ab,ti OR 'sri 62320':ab,ti OR sri62320:ab,ti OR vastin:ab,ti #62 glenvastatin:ab,ti OR 'hr 780':ab,ti OR hr780:ab,ti #63 lovastatin*:ab,ti OR mevinolin:ab,ti OR 'monacolin k':ab,ti OR '6 methylcompactin':ab,ti OR 'mk 803':ab,ti OR mk803:ab,ti OR mk0803:ab,ti OR mevacor:ab,ti OR altocor:ab,ti OR altoprev:ab,ti OR artein:ab,ti OR belvas:ab,ti OR birotin:ab,ti OR cholestra:ab,ti OR cysin:ab,ti OR ellanco:ab,ti OR elstatin:ab,ti OR 'l 654969':ab,ti OR lipdip:ab,ti OR lipivas:ab,ti OR lofacol:ab,ti OR lomar:ab,ti OR lostatin:ab,ti OR lovacel:ab,ti OR lovacol:ab,ti OR lovalip:ab,ti OR lovalord:ab,ti OR lovastan:ab,ti OR lovasterol:ab,ti OR lovastin:ab,ti OR lovatadin:ab,ti OR lowachol:ab,ti OR lozutin:ab,ti OR medostatin:ab,ti OR meverstin:ab,ti OR mevinacor:ab,ti OR 'monakolin k':ab,ti OR 'msd 803':ab,ti OR neolipid:ab,ti OR nergadan:ab,ti OR ovasta:ab,ti OR rodatin:ab,ti OR rovacor:ab,ti OR taucor:ab,ti OR advicor:ab,ti #64 meglutol:ab,ti OR '3 hydroxy 3 methylpentanedioic acid':ab,ti OR 'beta hydroxy beta methylglutarate':ab,ti OR '3 hydroxy 3 methylglutaric acid':ab,ti #65 mevastatin:ab,ti OR compactin:ab,ti OR mevastin:ab,ti OR '6 demethylmevinolin':ab,ti OR 'cs 500':ab,ti OR cs500:ab,ti OR 'ml 236b':ab,ti OR ml236b:ab,ti #66 mevinolin*:ab,ti OR monacolin*:ab,ti #67 'phosphoadenosine diphosphoribose':ab,ti OR 'phospho‐adp ribose':ab,ti #68 pitavastatin:ab,ti OR nisvastatin:ab,ti OR alipza:ab,ti OR itavastatin:ab,ti OR livalo:ab,ti OR livazo:ab,ti OR pitava:ab,ti OR ribar:ab,ti OR vezepra:ab,ti OR 'p 872441':ab,ti OR 'nk 104':ab,ti OR 'nk104':ab,ti OR 'nks 104':ab,ti OR nks104:ab,ti #69 pravastatin*:ab,ti OR eptastatin:ab,ti OR vasten:ab,ti OR 'cs 514':ab,ti OR cs514:ab,ti OR lipemol:ab,ti OR liplat:ab,ti OR 'nu pravastatin':ab,ti OR prareduct:ab,ti OR mevalotin:ab,ti OR pravachol:ab,ti OR elisor:ab,ti OR selektine:ab,ti OR pravacol:ab,ti OR pravasin:ab,ti OR lipostat:ab,ti OR 'rms 431':ab,ti OR rms431:ab,ti OR 'sq 31000':ab,ti OR sq31000:ab,ti OR 'sq 31,000':ab,ti OR sq31,000:ab,ti OR astin:ab,ti OR bristacol:ab,ti OR cholespar:ab,ti OR epatostantin:ab,ti OR eptastatine:ab,ti OR kenstatin:ab,ti OR lipidal:ab,ti OR liprevil:ab,ti OR novales:ab,ti OR prascolend:ab,ti OR prastan:ab,ti OR prava:ab,ti OR pravaselect:ab,ti OR pravasine:ab,ti OR pravator:ab,ti OR pravyl:ab,ti OR sanaprav:ab,ti OR selipran:ab,ti OR stanidine:ab,ti OR vasopran:ab,ti OR xipral:ab,ti OR pravafenix:ab,ti #70 rosuvastatin*:ab,ti OR zd4522:ab,ti OR 'zd 4522':ab,ti OR crestor:ab,ti OR rosuvas:ab,ti OR 's 4522':ab,ti OR s4522:ab,ti OR certriad:ab,ti #71 simvastatin*:ab,ti OR synvinolin:ab,ti OR 'mk 733':ab,ti OR mk733:ab,ti OR zocor:ab,ti OR avastinee:ab,ti OR cholestat:ab,ti OR clinfar:ab,ti OR colastatina:ab,ti OR colestricon:ab,ti OR covastin:ab,ti OR denan:ab,ti OR epistatin:ab,ti OR esvat:ab,ti OR ethicol:ab,ti OR eucor:ab,ti OR ifistatin:ab,ti OR kavelor:ab,ti OR klonastin:ab,ti OR kolestevan:ab,ti OR 'l 644128':ab,ti OR l644128:ab,ti OR lipecor:ab,ti OR lipex:ab,ti OR lipinorm:ab,ti OR liponorm:ab,ti OR lipovas:ab,ti OR lodales:ab,ti OR medipo:ab,ti OR mersivas:ab,ti OR 'nor vastina':ab,ti OR normofat:ab,ti OR orovas:ab,ti OR rechol:ab,ti OR simbado:ab,ti OR simcard:ab,ti OR simchol:ab,ti OR simovil:ab,ti OR simtin:ab,ti OR simvacor:ab,ti OR simvahex:ab,ti OR simvalord:ab,ti OR simvastar:ab,ti OR simvata:ab,ti OR simvatin:ab,ti OR simvor:ab,ti OR simvotin:ab,ti OR sinvacor:ab,ti OR sinvastatin:ab,ti OR sinvinolin:ab,ti OR sivastin:ab,ti OR starzoco:ab,ti OR torio:ab,ti OR valemia:ab,ti OR vasilip:ab,ti OR vasotenal:ab,ti OR vazim:ab,ti OR vidastat:ab,ti OR zimmex:ab,ti OR zocord:ab,ti OR zovast:ab,ti OR inegy:ab,ti OR vytorin:ab,ti OR zetsim:ab,ti OR zintrepid:ab,ti OR cholib:ab,ti OR fenofibrate*:ab,ti OR 'niacin simvastatin':ab,ti OR simcor:ab,ti OR 'rosiglitazone simvastatin':ab,ti OR avandastat:ab,ti OR 'sitagliptin simvastatin':ab,ti OR sitagliptin:ab,ti AND phosphate*:ab,ti OR juvisync:ab,ti #72 tenivastatin:ab,ti #73 #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 #74 #48 AND #73 #75 #32 AND #74
Appendix 4. LILACS search strategy
((Macul$ OR Mácul$ OR Retina$ OR Retiniana OR Choroid$ OR Coroide) AND (Degenera$ OR Neovasculariza$) OR MH:C11.768.585$ OR MH:C11.768.585.439$ OR MH: C11.768.725$ OR MH:C23.550.589.500.725$ OR MH:C11.941.160.244$ OR MH:C23.550.589.500.145$ OR MH:A09.371.729.522$ OR maculopath$ OR AMD OR ARMD OR CNV) AND ("Inhibidores de Hidroximetilglutaril‐CoA Reductasas" OR "Inibidores de Hidroximetilglutaril‐CoA Redutases" OR MH:D27.505.519.186.071.202.370$ OR MH:D27.505.519.389.370$ OR MH:D27.505.954.557.500.202.370$ OR Statin$ OR vastatin$ OR "hmg coenzyme" OR "hmg co‐enzyme" OR hydroxymethylglutaryl$ OR Atorvastatin$ OR Cerivastatin OR Bervastatin OR Crilvastatin OR dalvastatin OR Fluvastatin OR Glenvastatin OR Lovastatin$ OR MH:D02.455.426.559.847.638.400$ OR MH:D04.615.638.400$ OR Meglutol OR MH:D02.241.081.337.351.550$ OR Mevastatin OR mevinolin$ OR " Phosphoadenosine diphosphoribose" OR Pitavastatin OR Pravastatin$ OR MH:D02.455.426.559.847.638.930$ OR MH:D04.615.638.930$ OR Rosuvastatin$ OR Simvastatin$ OR MH:D02.455.426.559.847.638.400.900$ OR MH:D04.615.638.400.900$ OR tenivastatin)
Appendix 5. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 ((macul*[tiab] OR retina*[tiab] OR choroid*[tiab]) AND degener*[tiab]) NOT Medline[sb] #3 ((macul*[tiab] OR retina*[tiab] OR choroid*[tiab]) AND neovasc*[tiab]) NOT Medline[sb] #4 Maculopath*[tiab] NOT Medline[sb] #5 (macul*[tiab] AND lutea*[tiab]) NOT Medline[sb] #6 (macul*[tiab] AND dystroph*[tiab]) NOT Medline[sb] #7 (macul*[tiab] AND syndrome[tiab]) NOT Medline[sb] #8 ((macul*[tiab] OR geographic[tiab]) AND atroph*[tiab]) NOT Medline[sb] #9 ((macul*[tiab] OR retina*[tiab]) AND edema*[tiab]) NOT Medline[sb] #10 (AMD[tiab] OR ARMD[tiab] OR CNV[tiab]) NOT Medline[sb] #11 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 (Statin*[tw] OR vastatin*[tw]) NOT Medline[sb] #13 ((HMG COA*[tw]) OR ("HMG Co A"[tw])) NOT Medline[sb] #14 ("hmg coenzyme"[tw] OR "hmg co‐enzyme"[tw]) NOT Medline[sb] #15 3‐hydroxy‐3‐methylglutaryl*[tw] NOT Medline[sb] #16 hydroxymethylglutaryl*[tw] NOT Medline[sb] #17 (hydroxymethyl glutaryl[tw]) NOT Medline[sb] #18 (Atorvastatin*[tw] OR liptonorm[tw] OR Atorlip[tw] OR atovarol[tw] OR ci981[tw] OR "CI 981"[tw] OR glustar[tw] OR lipibec[tw] OR lipitor[tw] OR lowlipen[tw] OR sortis[tw] OR storvas[tw] OR tahor[tw] OR torvast[tw] OR "ym 548"[tw] OR ym548[tw] OR zarator[tw] OR ezetimibe*[tw] OR liptruzet[tw] OR Ramipril*[tw] OR "atocor R"[tw]) NOT Medline[sb] #19 (Bervastatin[tw] OR "ls 2904"[tw] OR ls2904[tw]) NOT Medline[sb] #20 (Cerivastatin[tw] OR Kazak[tw] OR rivastatin[tw] OR Certa[tw] OR "Bay w 6228"[tw] OR "bay w6228"[tw] OR Baycol[tw] OR Lipobay[tw]) NOT Medline[sb] #21 (Crilvastatin[tw] OR "pmd 387"[tw] OR pmd387[tw]) NOT Medline[sb] #22 (dalvastatin[tw] OR rg12561[tw] OR "rg 12561"[tw]) NOT Medline[sb] #23 (Fluvastatin[tw] OR fluindostatin[tw] OR Lescol[tw] OR "XU 62‐320"[tw] OR "XU 62320"[tw] OR xu62320[tw] OR canef[tw] OR cranoc[tw] OR "fractal lp"[tw] OR leucol[tw] OR lochol[tw] OR locol[tw] OR "sri 62320"[tw] OR sri62320[tw] OR vastin[tw]) NOT Medline[sb] #24 (Glenvastatin[tw] OR "hr 780"[tw] OR hr780[tw]) NOT Medline[sb] #25 (Lovastatin*[tw] OR Mevinolin[tw] OR "Monacolin K"[tw] OR "6 Methylcompactin"[tw] OR "MK 803"[tw] OR MK803[tw] OR mk0803[tw] OR Mevacor[tw] OR altocor[tw] OR altoprev[tw] OR artein[tw] OR belvas[tw] OR birotin[tw] OR cholestra[tw] OR cysin[tw] OR ellanco[tw] OR elstatin[tw] OR "l 654969"[tw] OR lipdip[tw] OR lipivas[tw] OR lofacol[tw] OR lomar[tw] OR lostatin[tw] OR lovacel[tw] OR lovacol[tw] OR lovalip[tw] OR lovalord[tw] OR lovastan[tw] OR lovasterol[tw] OR lovastin[tw] OR lovatadin[tw] OR lowachol[tw] OR lozutin[tw] OR medostatin[tw] OR meverstin[tw] OR mevinacor[tw] OR "monakolin k" OR "msd 803"[tw] OR neolipid[tw] OR nergadan[tw] OR ovasta[tw] OR rodatin[tw] OR rovacor[tw] OR taucor[tw] OR advicor[tw]) NOT Medline[sb] #26 (Meglutol[tw] OR "3 Hydroxy 3 methylpentanedioic Acid"[tw] OR "beta Hydroxy beta Methylglutarate"[tw] OR "3 Hydroxy 3 methylglutaric Acid"[tw]) NOT Medline[sb] #27 (Mevastatin[tw] OR compactin[tw] OR mevastin[tw] OR 6‐demethylmevinolin[tw] OR "CS 500"[tw] OR CS500[tw] OR "ML 236B"[tw] OR ML236B[tw]) NOT Medline[sb] #28 (mevinolin*[tw] OR monacolin*[tw]) NOT Medline[sb] #29 ("Phosphoadenosine diphosphoribose"[tw] OR "phospho‐ADP ribose"[tw]) NOT Medline[sb] #30 (Pitavastatin[tw] OR nisvastatin[tw] OR itavastatin[tw] OR alipza[tw] OR itavastatin[tw] OR livalo[tw] OR livazo[tw] OR pitava[tw] OR ribar[tw] OR vezepra[tw] OR "P 872441"[tw] OR "NK 104"[tw] OR "nk104"[tw] OR "nks 104"[tw] OR nks104[tw]) NOT Medline[sb] #31 (Pravastatin*[tw] OR Eptastatin[tw] OR Vasten[tw] OR "CS 514"[tw] OR CS514[tw] OR Lipemol[tw] OR Liplat[tw] OR Nu‐Pravastatin[tw] OR Prareduct[tw] OR Mevalotin[tw] OR Pravachol[tw] OR Elisor[tw] OR Selektine[tw] OR Pravacol[tw] OR Pravasin[tw] OR Lipostat[tw] OR "RMS 431"[tw] OR RMS431[tw] OR "SQ 31000"[tw] OR SQ31000[tw] OR "SQ 31,000"[tw] OR SQ31,000[tw] OR Bristacol[tw] OR astin[tw] OR bristacol[tw] OR cholespar[tw] OR epatostantin[tw] OR eptastatine[tw] OR kenstatin[tw] OR lipidal[tw] OR liprevil[tw] OR novales[tw] OR prascolend[tw] OR prastan[tw] OR prava[tw] OR pravaselect[tw] OR pravasine[tw] OR pravator[tw] OR pravyl[tw] OR sanaprav[tw] OR selipran[tw] OR stanidine[tw] OR vasopran[tw] OR xipral[tw] OR pravafenix[tw]) NOT Medline[sb] #32 (Rosuvastatin*[tw] OR ZD4522[tw] OR "ZD 4522"[tw] OR Crestor[tw] OR rosuvas[tw] OR "s 4522"[tw] OR s4522[tw] OR certriad[tw]) NOT Medline[sb] #33 (Simvastatin*[tw] OR Synvinolin[tw] OR "MK 733"[tw] OR MK733[tw] OR Zocor[tw] OR avastinee[tw] OR cholestat[tw] OR clinfar[tw] OR colastatina[tw] OR colestricon[tw] OR covastin[tw] OR denan[tw] OR epistatin[tw] OR esvat[tw] OR ethicol[tw] OR eucor[tw] OR ifistatin[tw] OR kavelor[tw] OR klonastin[tw] OR kolestevan[tw] OR "l 644128"[tw] OR l644128[tw] OR lipecor[tw] OR lipex[tw] OR lipinorm[tw] OR liponorm[tw] OR lipovas[tw] OR lodales[tw] OR medipo[tw] OR mersivas[tw] OR nor‐vastina[tw] OR normofat[tw] OR orovas[tw] OR rechol[tw] OR simbado[tw] OR simcard[tw] OR simchol[tw] OR simovil[tw] OR simtin[tw] OR simvacor[tw] OR simvahex[tw] OR simvalord[tw] OR simvastar[tw] OR simvata[tw] OR simvatin[tw] OR simvor[tw] OR simvotin[tw] OR sinvacor[tw] OR sinvastatin[tw] OR sinvinolin[tw] OR sivastin[tw] OR starzoco[tw] OR torio[tw] OR valemia[tw] OR vasilip[tw] OR vasotenal[tw] OR vazim[tw] OR vidastat[tw] OR zimmex[tw] OR zocord[tw] OR zovast[tw] OR inegy[tw] OR vytorin[tw] OR zetsim[tw] OR zintrepid[tw] OR cholib[tw] OR fenofibrate*[tw] OR niacin‐simvastatin[tw] OR simcor[tw] OR rosiglitazone‐simvastatin[tw] OR avandastat[tw] OR sitagliptin‐simvastatin[tw] OR sitagliptin phosphate*[tw] OR juvisync[tw]) NOT Medline[sb] #34 tenivastatin[tw] NOT Medline[sb] #35 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 #36 #11 AND #35 #37 #1 AND #36
Appendix 6. metaRegister of Controlled Trials search strategy
(macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (statins OR Atorvastatin OR Cerivastatin OR Bervastatin OR Crilvastatin OR dalvastatin OR Fluvastatin OR Glenvastatin) (macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (Lovastatin OR mevinacor OR Meglutol OR Mevastatin OR mevinolin OR Phosphoadenosine diphosphoribose) (macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (Pitavastatin OR Pravastatin OR Rosuvastatin OR Simvastatin OR tenivastatin)
Appendix 7. ClinicalTrials.gov search strategy
(macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (statins OR Atorvastatin OR Cerivastatin OR Bervastatin OR Crilvastatin OR dalvastatin OR Fluvastatin OR Glenvastatin) (macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (Lovastatin OR mevinacor OR Meglutol OR Mevastatin OR mevinolin OR Phosphoadenosine diphosphoribose OR Pitavastatin) (macular degeneration OR maculopathy OR Retinal Degeneration OR Retinal Neovascularization OR Choroidal Neovascularization) AND (Pravastatin OR Rosuvastatin OR Simvastatin OR tenivastatin)
Appendix 8. ICTRP search strategy
macular degeneration AND statins OR macular degeneration AND Atorvastatin OR macular degeneration AND Cerivastatin OR macular degeneration AND Bervastatin OR macular degeneration AND Crilvastatin OR macular degeneration AND dalvastatin OR macular degeneration AND Fluvastatin OR macular degeneration AND Glenvastatin OR macular degeneration AND Lovastatin OR macular degeneration AND mevinacor OR macular degeneration AND Meglutol OR macular degeneration AND Mevastatin OR macular degeneration AND mevinolin OR macular degeneration AND Phosphoadenosine diphosphoribose OR macular degeneration AND Pitavastatin OR macular degeneration AND Pravastatin OR macular degeneration AND Rosuvastatin OR macular degeneration AND Simvastatin OR macular degeneration AND tenivastatin maculopathy AND statins OR maculopathy AND Atorvastatin OR maculopathy AND Cerivastatin OR maculopathy AND Bervastatin OR maculopathy AND Crilvastatin OR maculopathy AND dalvastatin OR maculopathy AND Fluvastatin OR maculopathy AND Glenvastatin OR maculopathy AND Lovastatin OR maculopathy AND mevinacor OR maculopathy AND Meglutol OR maculopathy AND Mevastatin OR maculopathy AND mevinolin OR maculopathy AND Phosphoadenosine diphosphoribose OR maculopathy AND Pitavastatin OR maculopathy AND Pravastatin OR maculopathy AND Rosuvastatin OR maculopathy AND Simvastatin OR maculopathy AND tenivastatin Retinal Degeneration AND statins OR Retinal Degeneration AND Atorvastatin OR Retinal Degeneration AND Cerivastatin OR Retinal Degeneration AND Bervastatin OR Retinal Degeneration AND Crilvastatin OR Retinal Degeneration AND dalvastatin OR Retinal Degeneration AND Fluvastatin OR Retinal Degeneration AND Glenvastatin OR Retinal Degeneration AND Lovastatin OR Retinal Degeneration AND mevinacor OR Retinal Degeneration AND Meglutol OR Retinal Degeneration AND Mevastatin OR Retinal Degeneration AND mevinolin OR Retinal Degeneration AND Phosphoadenosine diphosphoribose OR Retinal Degeneration AND Pitavastatin OR Retinal Degeneration AND Pravastatin OR Retinal Degeneration AND Rosuvastatin OR Retinal Degeneration AND Simvastatin OR Retinal Degeneration AND tenivastatin Retinal Neovascularization AND statins OR Retinal Neovascularization AND Atorvastatin OR Retinal Neovascularization AND Cerivastatin OR Retinal Neovascularization AND Bervastatin OR Retinal Neovascularization AND Crilvastatin OR Retinal Neovascularization AND dalvastatin OR Retinal Neovascularization AND Fluvastatin OR Retinal Neovascularization AND Glenvastatin OR Retinal Neovascularization AND Lovastatin OR Retinal Neovascularization AND mevinacor OR Retinal Neovascularization AND Meglutol OR Retinal Neovascularization AND Mevastatin OR Retinal Neovascularization AND mevinolin OR Retinal Neovascularization AND Phosphoadenosine diphosphoribose OR Retinal Neovascularization AND Pitavastatin OR Retinal Neovascularization AND Pravastatin OR Retinal Neovascularization AND Rosuvastatin OR Retinal Neovascularization AND Simvastatin OR Retinal Neovascularization AND tenivastatin Choroidal Neovascularization AND statins OR Choroidal Neovascularization AND Atorvastatin OR Choroidal Neovascularization AND Cerivastatin OR Choroidal Neovascularization AND Bervastatin OR Choroidal Neovascularization AND Crilvastatin OR Choroidal Neovascularization AND dalvastatin OR Choroidal Neovascularization AND Fluvastatin OR Choroidal Neovascularization AND Glenvastatin OR Choroidal Neovascularization AND Lovastatin OR Choroidal Neovascularization AND mevinacor OR Choroidal Neovascularization AND Meglutol OR Choroidal Neovascularization AND Mevastatin OR Choroidal Neovascularization AND mevinolin OR Choroidal Neovascularization AND Phosphoadenosine diphosphoribose OR Choroidal Neovascularization AND Pitavastatin OR Choroidal Neovascularization AND Pravastatin OR Choroidal Neovascularization AND Rosuvastatin OR Choroidal Neovascularization AND Simvastatin OR Choroidal Neovascularization AND tenivastatin
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Guymer 2013.
| Methods |
Study design: randomized controlled trial Number randomized: 114 total; 57 simvastatin; 57 placebo Exclusions after randomization: none Number analyzed: at 36 months: 114 total; 57 simvastatin; 57 placebo Unit of analysis: individuals Losses to follow‐up: 34 participants total; 20 simvastatin; 14 placebo How was missing data handled?: last‐observation‐carried‐forward method used for 34 participants; 11 participants with baseline data only and 23 participants who missed the 3‐year follow‐up visit Power calculation: 58 participants in each arm for power of 80% at alpha 0.05 to detect a 50% reduction in progression of disease |
|
| Participants |
Country: Australia Mean age: 74.6 years overall; 74.8 years for simvastatin group; 74.4 years for placebo group Gender: 77/114 (68%) women 37/114 (32%) men total 39/57 (68%) women 18/57 (32%) men in the simvastatin group 38/57 (67%) women 19/57 (33%) men in the placebo group Inclusion criteria: 1) males and females aged 50 years and older; 2) able to assess the macula in at least 1 eye; 3) visual acuity ≥ 20/60 in study eye; 4) high‐risk drusen in both eyes: 1 or more large soft drusen, > 10 intermediate drusen, or late AMD in 1 eye and any drusen or pigment change in study eye; 5) normal cholesterol levels; and 6) not currently on cholesterol‐lowering medications Exclusion criteria: 1) bilateral end‐stage AMD; 2) medical or ophthalmic conditions which could potentially affect visual function, such as cataract, diabetes, glaucoma; 3) use of medications that may affect visual function, such as hydroxychloroquine (Plaquenil), chloroquine, major tranquillizers; 4) currently on cholesterol‐lowering medication; 5) use of statins is contraindicated; 6) alanine aminotransferase (ALT) 2 times the upper limit of normal; and 7) previous severe adverse or allergic reactions to statins Equivalence of baseline characteristics: no; more participants in simvastatin group had unilateral advanced AMD as compared with placebo; fewer smokers in placebo group than in simvastatin group |
|
| Interventions |
Intervention 1: 2 tablets of simvastatin (40 mg daily) for 3 years Intervention 2: placebo with an identical appearance for 3 years Length of follow‐up: Planned: 3 years Actual: 3 years |
|
| Outcomes |
Primary outcome, as defined in study reports: "Primary outcome was progression of non‐advanced AMD to either advanced AMD or higher severity scores of non‐advanced AMD," evaluated every 6 months. "Advanced AMD was defined as presence of either CNV or geographic atrophy (GA). CNV was confirmed on angiography and GA was defined as an area of hypopigmentation 175 mm with a choroidal vessel in its base on colour photography." Secondary outcomes, as defined in study reports: 1) change in visual function over time; 2) genotype as an effect modifier of the association between statins and progression of AMD Adverse events reported: yes Intervals at which outcomes assessed: 1, 6, 12, 18, 24, 30, and 36 months |
|
| Notes |
Funding sources: Ian Potter Foundation, John Reid Charitable Trust and Royal Victorian Eye and Ear Hospital; National Health and Medical Research Council (NHMRC) supported the study through a Centre for Clinical Research Excellence award to CERA (#529923), a Practitioner Fellowship (#529905), and a Senior Research Fellowship (#1028444); Wagstaff Fellowship; Victorian Government Disclosures of interest: co‐author Paul Baird is a PLOS ONE Editorial Board member Study period: 3 years; 2003 to 2006 Reported subgroup analyses: yes Trial investigators provided information on loss to follow‐up by intervention at 3‐year follow‐up (email communication) Trial reported at ARVO (abstract); trial registration number: ACTRN12605000320651 (registered at WHO International Clinical Trials Registry Platform) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed by a biostatistician using permuted blocks of randomly varying size.” |
| Allocation concealment (selection bias) | Low risk | “The hospital pharmacist packed the medication into identical containers according to the randomization code. The sequentially numbered containers were allocated to the participants by the study coordinator in order of enrolment.” “The allocation list was stored at a remote site.” |
| Masking (performance bias and detection bias) | Low risk | “The study staff, the participants, and data analysts were masked to treatment allocation until the analysis was finalised.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data missing for 34/114 participants (30%) at 3 years' follow‐up: 20/57 (35%) in the simvastatin group and 14/57 (25%) in the placebo group. Reasons for missing the 3‐year visit were: personal, poor health, unable to contact, adverse reaction to study medication, reached late AMD, sick at 3‐year follow‐up, deceased, or developed macular hole. The study investigators imputed missing data using the last‐observation‐carried‐forward method |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the 2013 results paper matched the protocol published in 2008 |
| Other bias | Unclear risk | “Analysis was done ‘by person’ and used the data from the eye showing greatest progression. If one eye of a person worsened and the other eye showed improvement, the person was classified as having progressed,” but AMD progression by eye also was reported; at baseline, “the number of participants with unilateral advanced AMD was twice as large in the simvastatin group compared to the placebo group (x2 = 9.2, P = 0.002). Smoking was also less prevalent in the placebo group; the difference was marginally significant (x2 = 3.5, P = 0.06).” |
Martini 1991.
| Methods |
Study design: randomized controlled trial Number randomized: 30 participants total; 15 in each group Exclusions after randomization: none reported Number analyzed: 30 participants total; 15 in each group Unit of analysis: individuals Losses to follow‐up: none reported How was missing data handled?: not applicable Power calculation: none reported |
|
| Participants |
Country: Italy Age: greater than 60 years Gender: not reported Inclusion criteria: participants with drusen (no CNV), good visual acuity (mean 0.52 LogMAR), and serum cholesterol level > 260 mg/dL Exclusion criteria: participants' age less than 60 years Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: simvastatin (20 mg daily) for 3 months Intervention 2: placebo for 3 months Length of follow‐up: Planned: 4.5 months Actual: 4.5 months |
|
| Outcomes |
Primary outcome, as defined in study reports: serum cholesterol levels
Secondary outcomes, as defined in study reports: 1) visual acuity; 2) microscopic eye examination; 3) fluorescein angiography; 4) electroretinography; and 5) visual evoked potentials
Adverse events reported: no Intervals at which outcomes assessed: baseline, 3 months, and 4.5 months |
|
| Notes |
Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Attempted to contact trial investigators, but no reliable contact information found Article in Italian No trial registration identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description was found in the article |
| Allocation concealment (selection bias) | Unclear risk | No description was found in the article |
| Masking (performance bias and detection bias) | Unclear risk | No description was found in the article |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description was found in the article |
| Selective reporting (reporting bias) | Unclear risk | No description was found in the article |
| Other bias | Unclear risk | Inadequate information reported |
AMD: age‐related macular degeneration ARVO: Association for Research in Vision and Ophthalmology CNV: choroidal neovascularization mg/dL: milligrams per deciliter
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berendschot 2009 | Study participants were not randomized to statin use; randomized controlled trial of participants on statins assigned to 1 of 3 dietary groups (control margarine, plant sterol‐enriched margarine, or plant stanol‐enriched margarine) |
| Della Valle 2000 | Study participants were not randomized; control arm included participants who refused to use simvastatin for various reasons |
| Drobek‐Slowik 2008 | Study participants were not randomized; history of statin use for people with AMD compared with people without AMD |
| Maguire 2009 | Study participants were not randomized to statin use; retrospective study based on a cohort of trial participants |
| Mao 2009 | Not the outcome of interest; randomized controlled trial of participants with AMD assigned to statins or placebo; analyzed hemodynamic changes after 3 months (no vision‐related outcomes assessed) |
| Sen 2002 | The study was in people with diabetic retinopathy |
AMD: age‐related macular degeneration
Differences between protocol and review
This review adheres to the published protocol with the following exceptions (Gehlbach 2008).
We revised the Cochrane 'Risk of bias' table to the 2012 version.
We added the WHO International Clinical Trials Registry Platform (ICTRP) to the search strategy.
We did not implement specific methods pertaining to meta‐analysis due to insufficient data.
We assessed the quality of the evidence using the GRADE Working Group approach.
Contributions of authors
Conceiving the review: PG, TL Designing the review: PG, TL
Data collection for the review: Designing electronic search strategies: CEV Trials Search Co‐ordinator Undertaking electronic searches: CEV Trials Search Co‐ordinator Screening search results: PG, TL, EH, CEV US Project Organizing retrieval of papers: CEV US Project Screening retrieved papers against inclusion criteria: PG, TL, EH, CEV US Project Appraising quality of papers: PG, TL, EH, CEV US Project Extracting data from papers: PG, TL, EH, CEV US Project Writing to authors of papers for additional information: CEV US Project Providing additional data about papers: PG Obtaining and screening data on unpublished studies: CEV US Project
Data management for the review: Entering data into RevMan: CEV US Project Analysis of data: PG, TL, EH, CEV US Project
Interpretation of data: Providing a methodological perspective: PG, TL, EH Providing a clinical perspective: PG, EH Providing a policy perspective: PG, EH Providing a consumer perspective: PG, EH Writing the review: PG, TL, EH Providing general advice on the review: PG, EH Performing previous work that was the foundation of the current study: PG, TL, EH
Contributors to the update: PG, TL, EH, CEV US Project
Sources of support
Internal sources
Johns Hopkins Bloomberg School of Public Health, USA.
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research, UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
Peter Gehlbach: None known. Tianjing Li: None known. Elham Hatef: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Guymer 2013 {published data only}
- Guymer RH, Baird PN, Varsamidis M, Busija L, Dimitrov PN, Aung KZ, et al. Proof of concept, randomized, placebo‐controlled study of the effect of simvastatin on the course of age‐related macular degeneration. PLoS One 2013;8(12):e83759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymer RH, Chiu A, Robman L, O'Loughlin RK, Tikellis G, Dimitrov P, et al. Slowing progression of age‐related macular degeneration with statins: a randomised, controlled clinical trial. Investigative Ophthalmology and Visual Science 2003;44:ARVO E‐abstract 1807. [Google Scholar]
- Guymer RH, Dimitrov PN, Varsamidis M, Lim LL, Baird PN, Vingrys AJ, et al. Can HMG Co‐A reductase inhibitors ("statins") slow the progression of age‐related macular degeneration? The age‐related maculopathy statin study (ARMSS). Clinical Interventions in Aging 2008;3(3):581‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymer RH, Robman L, Varsamidis M, Dimitrov P, Hunt N, Lim L, et al. Can HMG Co‐A reductase inhibitors ("statins") slow the progression of AMD?. Investigative Ophthalmology and Visual Sciences 2005; Vol. 46:ARVO E‐abstract 2364.
Martini 1991 {published data only}
- Martini E, Scorolli L, Burgagni MS, Fessehaie S. Evaluation of the retinal effects of simvastatin in patients with age‐related macular degeneration [Valutazione degli effetti retinici della somministrazione di simvastatina in pazienti affetti da degenerazione maculare senile]. Annali Di Ottalmologia e Clinica Oculistica 1991;117(11):1121‐6. [Google Scholar]
References to studies excluded from this review
Berendschot 2009 {published data only}
- Berendschot TT, Plat J, Jong A, Mensink RP. Long‐term plant stanol and sterol ester‐enriched functional food consumption, serum lutein/zeaxanthin concentration and macular pigment optical density. British Journal of Nutrition 2009;101(11):1607‐10. [DOI] [PubMed] [Google Scholar]
Della Valle 2000 {published data only}
- Della Valle V, Scorolli L, Meduri R. Retinal effects of Simvastatin in SMD: Cases up‐date [Effetti retinici della Simvastatina nella DMS: Aggiornamento casistica]. Annali Di Ottalmologia e Clinica Oculistica 2000;126(11‐12):249‐55. [Google Scholar]
- Della Valle V, Scorolli L, Meduri R. Retinal effects of Simvastatin in patients affected by age related macular degeneration [Effetti retinici della Simvastatina in pazienti affetfi da degenerazione maculare senile correlata all'eta]. Annali Di Ottalmologia e Clinica Oculistica 2000;126(5‐6):89‐95. [Google Scholar]
Drobek‐Slowik 2008 {published data only}
- Drobek‐Slowik M, Karczewicz D, Safranow K, Jakubowska K, Chlubek D. Use of statins as a form of protection against age‐related macular degeneration (AMD) [Leczenie statynami jako forma ochrony przed zwyrodnieniem plamki zwiazanym z wiekiem (AMD)]. Klinika Oczna 2008;1‐3:50‐4. [PubMed] [Google Scholar]
Maguire 2009 {published data only}
- Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y, Complications of Age‐related Macular Degeneration Prevention Trial (CAPT) Research Group. Statin use and the incidence of advanced age‐related macular degeneration in the Complications of Age‐related Macular Degeneration Prevention Trial. Ophthalmology 2009;116(12):2381‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2009 {published data only}
- Mao YQ, Qi XY, Peng W, Sun JX, Wang AP. Effect of lovastatin on hemodynamic changes in early stage patients with age‐related macular degeneration. International Journal of Ophthalmology 2009;9(8):1521‐3. [Google Scholar]
Sen 2002 {published data only}
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Research and Clinical Practice 2002;56(1):1‐11. [DOI] [PubMed] [Google Scholar]
Additional references
AAO 2014
- American Academy of Ophthalmology. Preferred Practice Pattern Guidelines. Age‐Related Macular Degeneration. www.aao.org/ppp (accessed 7 November 2014).
AREDS 2001
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology 2001;119(10):1417‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
CAPT 2004
- Complications of Age‐Related Macular Degeneration Prevention Trial Study Group. The Complications of Age‐Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clinical Trials 2004;1(1):91‐107. [DOI] [PubMed] [Google Scholar]
Congdon 2003
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290(15):2057‐60. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dichtl 2003
- Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MPS, et al. HMG‐CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology 2003;23(1):58‐63. [DOI] [PubMed] [Google Scholar]
Evans 2012a
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000253.pub3] [DOI] [PubMed] [Google Scholar]
Evans 2012b
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000254.pub3] [DOI] [PubMed] [Google Scholar]
Friedman 2004
- Friedman E. Update of the vascular model of AMD. British Journal of Ophthalmology 2004;88(2):161‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geltzer 2013
- Geltzer A, Turalba A, Vedula SS. Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD005022.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gurne 1991
- Gurne DH, Tso MO, Edward DP, Ripp H. Antiretinal antibodies in serum of patients with age‐related macular degeneration. Ophthalmology 1991;98(5):602‐7. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Guymer 2005
- Guymer RH, Chiu AW, Lim L, Baird PN. HMG CoA reductase inhibitors (statins): do they have a role in age‐related macular degeneration?. Survey of Ophthalmology 2005;50(2):194‐206. [DOI] [PubMed] [Google Scholar]
Hall 2001
- Hall NF, Gale CR, Syddall H, Phillips DI, Martyn CN. Risk of macular degeneration in users of statins: cross sectional study. BMJ 2001;323(7309):375‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Klein 1992
- Klein R, Klein BE, Linton KL. Prevalence of age‐related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99(6):933‐43. [DOI] [PubMed] [Google Scholar]
Klein 2001
- Klein R, Klein BE, Jensen SC, Cruickshanks KJ, Lee KE, Danforth LG, et al. Medication use and the 5‐year incidence of early age‐related maculopathy: The Beaver Dam Eye Study. Archives of Ophthalmology 2001;119(9):1354‐9. [DOI] [PubMed] [Google Scholar]
Klein 2002
- Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten‐year incidence and progression of age‐related maculopathy: The Beaver Dam Eye Study. Ophthalmology 2002;109(10):1767‐79. [DOI] [PubMed] [Google Scholar]
Klein 2007a
- Klein R, Deng Y, Klein BE, Hyman L, Seddon J, Frank RN, et al. Cardiovascular disease, its risk factors and treatment, and age‐related macular degeneration: Women's Health Initiative Sight Exam ancillary study. Ophthalmology 2007;143(3):473‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2007b
- Klein R, Knudtson MD, Klein BE. Statin use and the five‐year incidence and progression of age‐related macular degeneration. American Journal of Ophthalmology 2007;144(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li T, Hutfless S, Scharfstein DO, Daniels MJ, Hogan JW, Little RJ, et al. Standards should be applied in the prevention and handling of missing data for patient‐centered outcomes research: a systematic review and expert consensus. Journal of Clinical Epidemiology 2013;67(1):15‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGwin 2003
- McGwin G Jr, Owsley C, Curcio CA, Crain RJ. The association between statin use and age related maculopathy. British Journal of Ophthalmology 2003;87(9):1121‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGwin 2006
- McGwin G, Modjarrad K, Hall TA, Xie A, Owsley C. 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors and the presence of age‐related macular degeneration in the Cardiovascular Health Study. Archives of Ophthalmology 2006;124(1):33‐7. [DOI] [PubMed] [Google Scholar]
Mitchell 1995
- Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age‐related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995;102(10):1450‐60. [DOI] [PubMed] [Google Scholar]
MPS 1991
- Anonymous. Argon laser photocoagulation for neovascular maculopathy. Five‐year results from randomized clinical trials. Macular Photocoagulation Study Group. Archives of Ophthalmology 1991;109(8):1109‐14. [PubMed] [Google Scholar]
MPS 1994
- Anonymous. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five‐year results from randomized clinical trials. Macular Photocoagulation Study Group. Archives of Ophthalmology 1994;112(4):500‐9. [PubMed] [Google Scholar]
Penfold 2001
- Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Progress in Retinal and Eye Research 2001;20(3):385‐414. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sagara 2007
- Sagara N, Kawaji T, Takano A, Inomata Y, Inatani M, Fukushima M, et al. Effect of pitavastatin on experimental choroidal neovascularization in rats. Experimental Eye Research 2007;84(6):1074‐80. [DOI] [PubMed] [Google Scholar]
Smeeth 2005
- Smeeth L, Cook C, Chakravarthy U, Hubbard R, Fletcher AE. A case control study of age related macular degeneration and use of statins. British Journal of Ophthalmology 2005;89(9):1171‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeeth 2009
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. British Journal of Clinical Pharmacology 2009;67(1):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2001
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, et al. Risk factors for age‐related macular degeneration: Pooled findings from three continents. Ophthalmology 2001;108(4):697‐704. [DOI] [PubMed] [Google Scholar]
Solomon 2014
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti‐vascular endothelial growth factor for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005139.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaide 1999
- Spaide RF, Ho‐Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch's membrane. Retina 1999;19(2):141‐7. [DOI] [PubMed] [Google Scholar]
Tan 2007
- Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long‐term risk of incident age‐related macular degeneration: The Blue Mountains Eye Study. American Journal of Ophthalmology 2007;143(4):685‐7. [DOI] [PubMed] [Google Scholar]
van Leeuwen 2003
- Leeuwen R, Vingerling JR, Hofman A, Jong PT, Stricker BH. Cholesterol lowering drugs and risk of age related maculopathy: prospective cohort study with cumulative exposure measurement. BMJ 2003;326(7383):255‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virgili 2007
- Virgili G, Bini A. Laser photocoagulation for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004763.pub2] [DOI] [PubMed] [Google Scholar]
Wormald 2007
- Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002030.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gehlbach 2008
- Gehlbach P, Li T, Hatef E. Statins for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006927] [DOI] [Google Scholar]
Gehlbach 2009
- Gehlbach P, Li T, Hatef E. Statins for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006927.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehlbach 2012
- Gehlbach P, Li T, Hatef E. Statins for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006927.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehlbach 2015
- Gehlbach P, Li T, Hatef E. Statins for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD006927.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


