Abstract
Metastasis is the main cause of death from cancer. To colonize distant organs, circulating cancer cells must overcome many obstacles through mechanisms that we are starting to understand. Infiltrating distant tissue, evading immune defences, adapting to supportive niches, surviving as latent tumour-initiating seeds, and eventually breaking out to replace the host tissue, are key steps for metastatic colonization. These obstacles make metastasis a highly inefficient process, but once metastases are established current treatments frequently fail to provide durable responses. A better understanding of the mechanistic determinants of metastatic colonization is needed to better prevent and treat metastatic cancer.
Keywords: Cancer, metastasis, organ specific metastasis, metastatic colonization
Malignant tumours start early on the road to metastasis. Cancer cells that are invasive and motile can enter the circulation long before the tumour is diagnosed. Most of these cells perish, but a small proportion manages to infiltrate and survive in distant organs as disseminated seeds for eventual relapse. Thus, at diagnosis, a primary tumour may already have seeded distant organs with thousands of cancer cells. These cells will continue to face many barriers before they can overtake the host organ and form clinically relevant lesions. Indeed, organ colonization is the most complex and rate-limiting phase of the metastatic process.
Until recently, research on metastatic colonization was hindered by the complexity of the biologic problem and a lack of adequate experimental models. However, the recent development of patient-derived and genetically engineered mouse models of metastasis, improved imaging technologies, advanced genomic sequencing, including the ability to analyse single cells, and an improved access to clinically annotated tissue samples, has brought new insights into the molecular mechanisms that allow circulating cancer cells to invade distant organs, settle in supportive niches, and eventually overtake the host tissue (Figure 1). This progress has allowed a better conceptualization of the metastatic process as a whole and provided a basis for better treatments. Though only a start, these advances show that elucidating metastatic colonization is a tractable problem with clinical benefits. The aspects of metastatic colonization that we explore in this article are individually rich areas of research, and we cite recent specialized reviews that cover each of these areas in depth. Here, we highlight current concepts and open key questions at the forefront of this field.
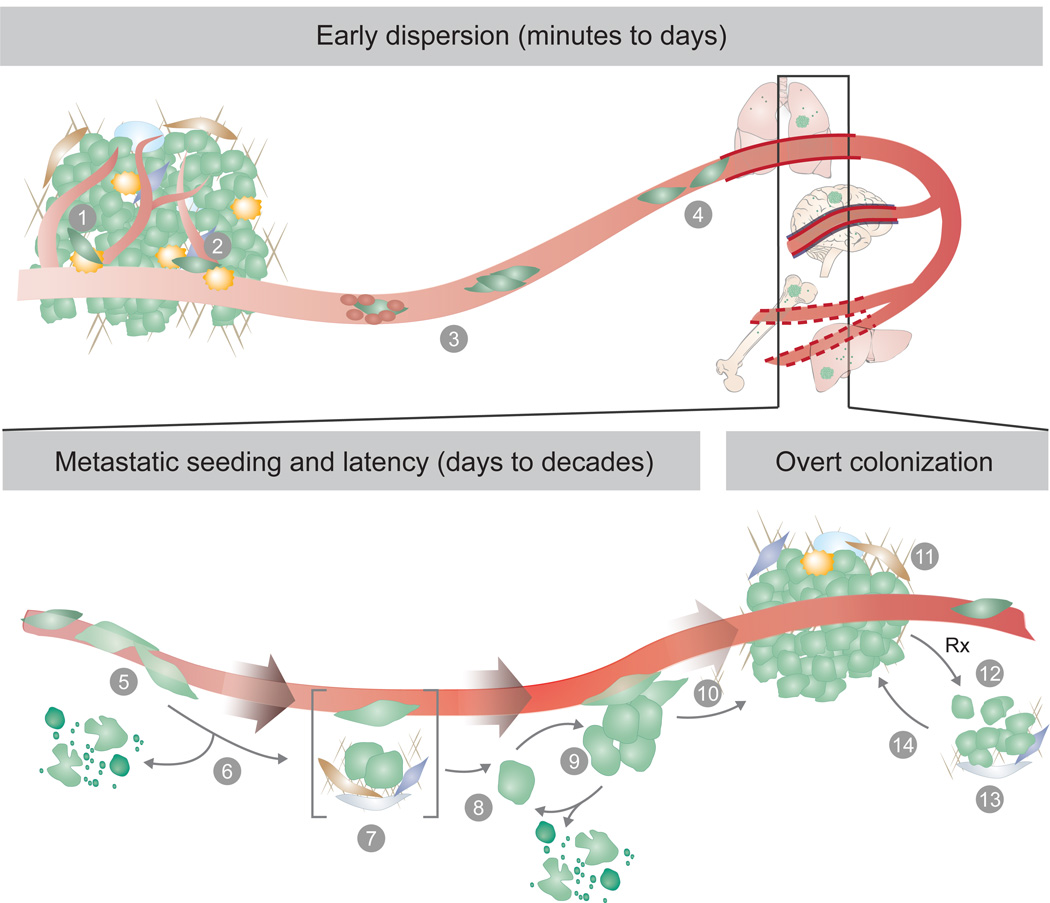
Figure 1. Metastatic colonization steps.
Metastasis proceeds through multiple steps and restrictive bottlenecks. Factors including the germline, tumour cell of origin, cancer cell plasticity, host tissue stroma, and response to therapy may influence the emergence of metastatic traits, and the probability that a cancer cell will complete all the steps towards overt metastasis. The pre-colonization phase of metastasis involves a series of events that cancer cells go through in a time scale of minutes to hours, including: (1) local invasion of cancer cells in the primary tumour, (2) intravasation into the tumour vasculature, (3) circulation of cancer cells as single cells or cell clusters, coated with platelets, (4) arrest in capillaries at the distant site and, (5) extravasation into the parenchyma of target organs for metastatic colonization. Colonization can be parsed into many steps that occur in a time scale of years. After extravasation, metastatic colonization comprises (6) resistance to immunity and other host tissue defences, (7) settlement in supportive niches for survival and retention of stem-like tumour-initiating capacity, (8) entry into latency as single cells, or (9) as indolent micrometastases. During the latency phase, which can last from months to decades, disseminated cancer cells must achieve long-term survival and may acquire traits for the eventually overtaking of the host tissue. The disseminated cancer cells may then break out of latency, reinitiating overt outgrowth (10), and overtaking the local tissue microenvironment (11). Once metastases become clinically manifest, therapeutic treatment may partially eliminate the tumour (12). However, under therapy-induced stress, cancer cells and non-neoplastic stromal cells mobilize survival signals (13) that nurse the residual disease until minority drug-resistant clones emerge and lead the outgrowth of a drug-resistant tumour (14). Different host tissue microenvironments select for cancer cells with distinct metastatic traits, giving rise to organ-specific metastatic cell populations.
The inefficiency of metastatic colonization
Even small tumours can release millions of cancer cells, yet many cancer patients never relapse or do so after a long period of latency without clinically manifesting disease. The number of cancer cells found in blood samples, called circulating tumour cells (CTCs), far exceeds the number of overt metastatic lesions that develop1. Cancer cells that survive after infiltrating distant organs, termed disseminated tumour cells (DTCs), can be present in the bone marrow of cancer patients for years, and yet only about half of these patients develop overt metastasis2. These clinical observations argue that metastatic colonization is a very inefficient process, in which most cancer cells die, and only a minority of the surviving cells forms macro-metastases.
Data from experimental mouse models is in line with the clinical evidence. Intravenously injected cancer cells that reach the lungs die massively within two days3, as do arterially injected cancer cells that lodge in the brain, liver, or bone marrow4. Even cell populations that are enriched for highly metastatic cells suffer extensive attrition after infiltration of distant organs5. The vast majority of melanoma cells injected in the portal vein failed to form micrometastases in the liver, and only 0.02% formed macrometastases6,7. Similarly, most cancer cells that infiltrate the brain die8–10. The inefficiency of metastasis cannot simply be attributed to a scarcity of cancer stem cells with metastasis-initiating potential. A majority of breast cancer stem cells that reach the lungs in mice undergo apoptosis11, and colorectal cancer stem cells are cleared quickly after infiltrating the liver parenchyma12. These observations in mouse models and in the clinic imply that factors influencing the survival and tumour-initiating activity of DTCs are key determinants of metastasis.
Early colonization steps
The early steps in the metastatic cascade, including cancer cell invasion, migration, and entry into the circulation, have been extensively studied13,14. Cytoskeletal rearrangements within the cancer cells15, combined with the action of adhesive interactions, secreted extracellular matrix metalloproteinases (MMPs) and cathepsins16,17 drive cancer cell invasion and migration through the stroma. Cancer cells may migrate as single cells boring a path through extracellular matrix18, move along collagen fibers19, or migrate collectively as ensembles that forge ahead from the tumour invasion front20.
In prostate cancer invasion along nerve fibers provides an additional route for dissemination21. In response to transforming growth factor-β (TGF-β) and other stromal signals, carcinoma cells may undergo an epithelial-to-mesenchymal transition (EMT), a reversible phenotypic change involving a loss of intercellular adhesion and epithelial polarization and a gain of motility and invasiveness22. EMT is key in gastrulation and other morphogenic events during development. In carcinoma cells, EMT can promote cell entry into the vasculature, called intravasation, and support a stem cell phenotype, whereas a reversal of this state after extravasation may facilitate organ colonization23. However, recent studies suggest that EMT is dispensable for the establishment of metastasis in models of breast and pancreatic cancer, although it contributes to the aggressiveness of cancer cells by increasing their chemoresistance24,25. Thus the contribution of EMT to metastasis may be more nuanced than previously thought.
Cancer cells may leave tumours as single cells or as cell clusters (Figure 1). Growing evidence indicates that distinct cancer cell clones can show cooperative behaviour, promoting mutual survival and metastatic ability26–29. Polyclonal metastatic seeding is, for example, documented in prostate cancer patients30, and in experimental models polyclonal CTC clusters establish metastases more efficiently than single cells31.
In the bloodstream cancer cells are exposed to significant shear forces, innate immunity, and oxidative stress. To protect themselves during transit, cancer cells associate with platelets32 and undergo reversible metabolic changes to increase their ability to withstand oxidative stress33. Melanoma cells show increased dependence on NADPH-generating enzymes in the folate pathway and inhibition of this pathway reduced overt metastasis34. In support of this finding, anti-oxidant supplementation increased lymph node metastases but had no effect on the growth of primary tumours33,34.
Mechanical entrapment of CTCs in capillaries is considered the main mechanism for cancer cell arrest prior to exit from blood into tissue. Circulation patterns dictate the first capillary bed that CTCs will encounter. In most organs, the venous circulation leads to the right cardiac ventricle and on to the lungs, whereas the mesenteric venous system from the gut first drains into the liver. The resulting retention of CTCs in lungs or liver, respectively, contributes to the high incidence of metastasis in these organs35. However, some CTCs bypass these first filters, perhaps through larger arterio-venous shunts, to reach all other organs through the arterial circulation.
CTCs lodged in the microvasculature may initiate intraluminal growth and form an embolus that eventually ruptures the vessel36 or extravasate by breaching vascular walls5,6. The composition of the vascular walls differs between organs and thus also influences where cancer cells extravasate (Figure 1). The capillaries in the liver and bone marrow, called sinusoids, are lined with fenestrated endothelial cells and a discontinuous basal lamina37, gaps that may facilitate the extravasation of CTCs and contribute to the high incidence of liver and bone metastasis13,38. In contrast, the endothelium of lung capillaries has tight junctions and a basement membrane, and brain capillary walls are additionally reinforced by pericytes and astrocyte processes, together constituting the blood-brain-barrier13,37. Diverse genes have been identified that mediate breast cancer CTC extravasation in the lungs in model systems and are associated with lung metastasis in the clinic. These mediators include Fascin-1 and other components of invading protrusions called invadopodia39, autocrine enhancers of cancer cell motility like epiregulin and WNT ligands39,40, mediators of endothelial disjunction and vascular permeability including angiopoietin-like 4 (ANGPTL4), vascular endothelial growth factor (VEGF), cyclooxygenase 2 (COX2), MMP1, and osteonectin41–44. CTC-associated platelets can stimulate extravasation by releasing TGF-β and triggering an EMT in the cancer cells32 or by secreting adenine nucleotides, which relax endothelial cell junctions45. Physical contacts with macrophages help pull CTCs across capillary walls in the lungs46. Many of these factors also enhance CTC extravasation in the brain, which additionally involves cancer-cell derived sialyltransferase ST6GalNac547, cathepsin S48, and microRNAs mir-105 and mir-181c49,50. These mediators individually provide a finite increase in the probability of metastatic seeding, and frequently act in parallel. In sum, a combination of priming signals from the tumour stroma, CTC cluster composition, circulation patterns, and cancer cell-autonomous functions determine metastatic infiltration of specific organs.
Tissue defences against infiltrating cancer cells
Cancer cells develop in primary tumours under a co-evolving microenvironment that suppresses immune surveillance17,51. However, this support is not immediately available to the cancer cells as they infiltrate distant organs, and most of these cells die5–7. The “seed and soil” hypothesis was based on the observation that different cancers show predilection for metastasis in different organs, and envisioned that certain organs are more hospitable to wandering cancer cells than others52. “Seed and soil” is an appealing metaphor, but it can be misleading. To disseminated cancer cells every distant soil is deadly, though some soils may be less deadly than others. In fact, the most welcoming of all soils for CTCs may be the primary tumour itself. The preferential re-seeding of CTCs back to a primary or metastatic tumour, over seeding tumour-free secondary sites, has been called tumour “self-seeding”53. Self-seeding can amplify the most aggressive clones released by a tumour53 and disperse drug resistant clones during treatment of metastatic melanoma with targeted therapy54.
While coping with a new and challenging microenvironment, newly disseminated cancer cells may be particularly vulnerable to immune surveillance (Figure 1). Major players in anti-metastatic immune surveillance include CD8 T-cells and natural killer (NK) cells55. Depletion of cytotoxic T-cells or NK cells increases metastasis56,57 and inhibition of the tyrosine kinase Mertk, a negative regulator of NK cells, suppresses metastasis58. Moreover, the specific immune cell composition of an organ may influence the susceptibility of that organ to overt metastasis. For example, the liver is particularly rich in NK cells, and neutralization of the pro-apoptotic NK-derived factor TRAIL, or genetic depletion of NK cells in mice, increases hepatic metastasis59,60. Recent advances in immunotherapy, most prominently using immune checkpoint inhibitors, have yielded striking results against metastatic melanoma and other tumours61,62. Thus, immunity is a major defence against metastasis.
Other cell types can also mount a strong defence against metastatic infiltration. Astrocytes, the most abundant cell type in the brain, reject extravasated cancer cells by releasing plasminogen activator (PA). PA generates plasmin that mobilizes the pro-apoptotic cytokine FasL to kill the infiltrated cancer cells. To avert this fate, brain metastatic cells from breast and lung adenocarcinomas produce the PA inhibitors neuroserpin and serpin B29.
Supportive niches
Adult stem cells reside in specialized niches that provide cues to balance stem cell proliferation versus quiescence, and self-renewal versus differentiation. Stem cell niches are rich in developmental and self-renewal signals, such as hedgehog, Wnt, TGF-β family members, and CXCL1263,64. Tumours are thought to arise from mutant stem cells in their native niches or from cell progenies that retain tumour-initiating capacity and benefit from these niche signals65–68. After cancer stem cells disperse to distant sites, their survival and tumour-initiating potential may similarly benefit from interactions with specialized niches69 (Figure 2). Recent evidence suggests that prostate carcinoma stem cells occupy native hematopoietic stem cell niches in the bone marrow70. Indeed, the CXCL12 receptor CXCR4 is a marker and mediator of breast cancer metastasis to CXCL12-rich bone marrow sites71. Breast tumours that are rich in a CXCL12-secreting mesenchymal stroma select for CXCL12 responsive cancer cell populations that are predisposed to survive in the bone marrow72.
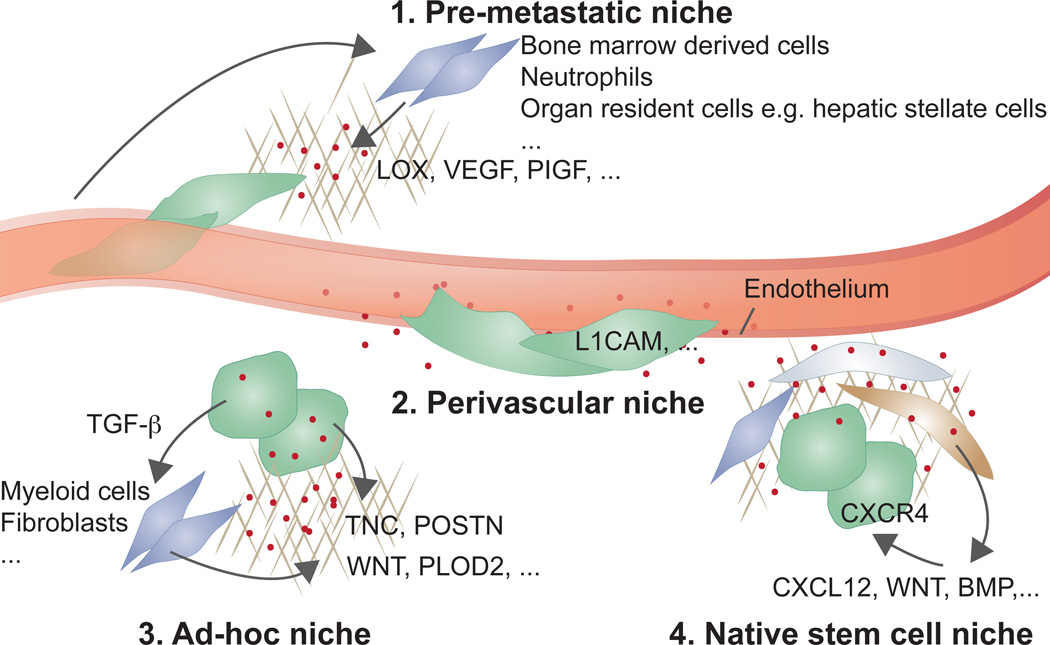
Figure 2. Metastatic niches.
Cancer cells that infiltrate distant tissues survive and retain stem cell potential by situating in supportive niches, akin to the niches that support normal adult stem cells. Different niches have been proposed: (1) pre-metastatic niches formed by systemic signals from the primary tumour that recruit supportive stromal cells before the arrival of cancer cells from the tumour; (2) perivascular niches for cancer cells that spread over the capillary basement membrane right after extravasation, remaining in close apposition to endothelial cells and their paracrine factors; (3) ad-hoc niches constituted by secretory products from the cancer cells themselves that act in an autocrine manner or recruit stromal components as sources of supportive signals; and (4), native stem cell niches of the host tissue, which are invaded by the infiltrated cancer cells to directly occupy a supportive microenvironment. The four entities may be partly overlapping in location or composition. For example, native stem cell niches could be perivascular, or pre-metastatic signals might combine with ad-hoc niches.
The space around small blood vessels is rich in supportive signals and can favour cancer stem cell growth and resistance to therapy73,74. A striking case of metastatic cell interaction with perivascular sites is observed in brain metastasis by breast cancer, lung cancer and melanoma, in which the extravasated cells remain closely associated with capillaries8,75. The cells spread on the basal lamina surrounding the capillaries and proliferate forming a sheath that eventually engulfs and remodels the coopted capillary network, a process mediated by expression of the cell adhesion molecule L1CAM in the metastatic cells9.
DTCs may set an ad-hoc niche by producing stem cell niche components themselves (Figure 2). Lung metastatic breast cancer cells produce the extracellular matrix protein tenascin C, which is deposited in the incipient colony to amplify Notch and Wnt signaling in the cancer cells76. Breast cancer stem cells may also secrete TGF-β, which stimulates stromal fibroblasts to produce periostin, a binding partner of tenascin C that recruits Wnt factors11. Cancer cell secretion of collagen crosslinking enzymes LOX and PLOD2, which stiffen the extracellular matrix, amplifies integrin/focal adhesion signalling, also favouring metastasis77–79.
Studies in experimental models have provided evidence that systemic signals from primary tumours can impact the microenvironment of distant organs, creating ‘pre-metastatic niches’ prior to the arrival of CTCs80,81 (Figure 2). Different classes of systemic mediators, such as tumour-derived inflammatory cytokines, exosomes, and extracellular matrix remodelling enzymes have been shown in breast, lung, and gastrointestinal tumour models to recruit bone marrow derived cells and pre-condition the lung, liver or bone marrow for infiltrating cancer cells82–85. For example, tumour derived PIGF acts on cells in the lung parenchyma to up-regulate chemotactic proteins, activate MMPs, and mobilize bone marrow derived VEGFR1+ cells that increase the survival of infiltrating cancer cells80. Melanoma cells secrete exosomes to induce vascular leakiness, inflammation, and bone marrow progenitor cell recruitment during pre-metastatic niche formation86. Similarly, macrophage inhibitory factor (MIF) containing exosomes from pancreatic cancer cells increase liver metastasis by inducing TGF-β secretion, stimulation of fibronectin production by hepatic stellate cells and the recruitment of bone marrow-derived cells to the liver83. Recently it has been suggested that integrins can target exosomes to specific organs to unload their cargo and prepare the organ for the arrival of tumour cells87, thereby contributing to the organotropism of metastasis.
Observations from the clinic raise questions about how pre-metastatic niches would play out in cancer patients. Most cancer patients develop metastasis months to years after removal of the primary tumour, during which time tumour cells remain largely dormant. Yet pre-metastatic niches, as defined in experimental models, support the immediate outgrowth of disseminated cancer cells that reach the niche. Research will have to address whether pre-metastatic niches remain primed for years after the removal of a primary tumour or, alternatively, whether the role of pre-metastatic niches is to enhance the survival of infiltrating cells to increase their numbers before entering a latent state.
Growth and survival pathways
Acting in specific niches or in undefined spots, a plethora of genes and signals support metastatic cell growth and survival in experimental models, and expression of these genes predicts relapse in the clinic (see also Box1, Origin of metastatic traits). Many of these pro-metastatic stromal mediators ultimately activate stem cell support pathways (Wnt, TGF-β, BMP, Notch, Stat3), pathways that integrate cell metabolism and survival (PI3K/AKT, MAPK, HIF), positional and mechanical pathways (Hedgehog, Hippo), and inflammatory pathways (NFkB, Stat1)69. These pathways also drive development and tissue regeneration, but what is distinctive in the case of metastasis are the strategies that cancer cells employ to ensure sufficient pathway activation in microenvironments with low levels of activating signals (Figure 3). DTCs seem to be selected for the ability to optimize whatever cues the host tissue offers.
Origin of metastatic traits.
Metastasis develops through genetic and epigenetic changes and the subsequent selection for favourable traits under the pressure of successive bottlenecks138,139. Genomic comparisons show close clonal relationships between primary tumours and their metastases. Specific ancestors of metastatic clones can often be identified in the primary tumour30,139,140, supporting the hypothesis that late a clonal expansion in the primary tumour gives rise to metastasis competent clones. These studies also provide evidence for metastases seeding new metastases30. Disseminated cancer cells remain dependent on the oncogenic mutations that underlie the primary tumour, providing a basis for treating metastasis with drugs that target these oncogenic drivers125. In line with this observation, gains in oncogenic mutant alleles occur in metastases, including gains in mutant KRAS in pancreatic cancer metastasis141, and TP53 and androgen receptor mutations in prostate cancer metastasis142. To date, however, no recurrent metastasis-specific mutations have been identified, suggesting that epigenetic alterations and other sources of modified gene expression are the predominant source of selectable pro-metastatic traits during clonal evolution in metastasis139,140.
The cell-autonomous traits that favour cancer cell dissemination, resistance in circulation, extravasation, and initial survival in distant organs matter immediately after cancer cells depart from the primary tumour, and are pre-selected in the primary tumour. For example, certain mediators of neoangiogenesis in breast tumours, including COX2, epiregulin, MMP1, and VEGF are repurposed by cancer cells for extravasation in the lungs and brain42,47,143. Stromal TGF-β in the triple-negative subset of breast carcinomas induces expression of ANGPTL4 in cancer cells, thus priming these cells for extravasation in the lungs41. These early metastatic traits may be selected under the stresses of tissue invasion, immune surveillance, or hypoxia. The evidence favours a model in which a significant proportion of cancer cells in a primary tumour acquire pro-metastatic traits that confer a finite probability of success in the early steps of metastasis. Clones with the most effective combination of pro-metastatic traits stand the highest probability of giving rise to metastatic lesions, and also to re-seeding the primary tumour. Beyond these early steps, cancer cells continue to evolve after dissemination to distant organs, acquiring traits for overt colonization as suggested by the case of bone metastasis114. Cells that disseminate early from a tumour could evolve in parallel with, but independently from the primary tumour144. The origin of metastatic traits remains a fertile area for future research.
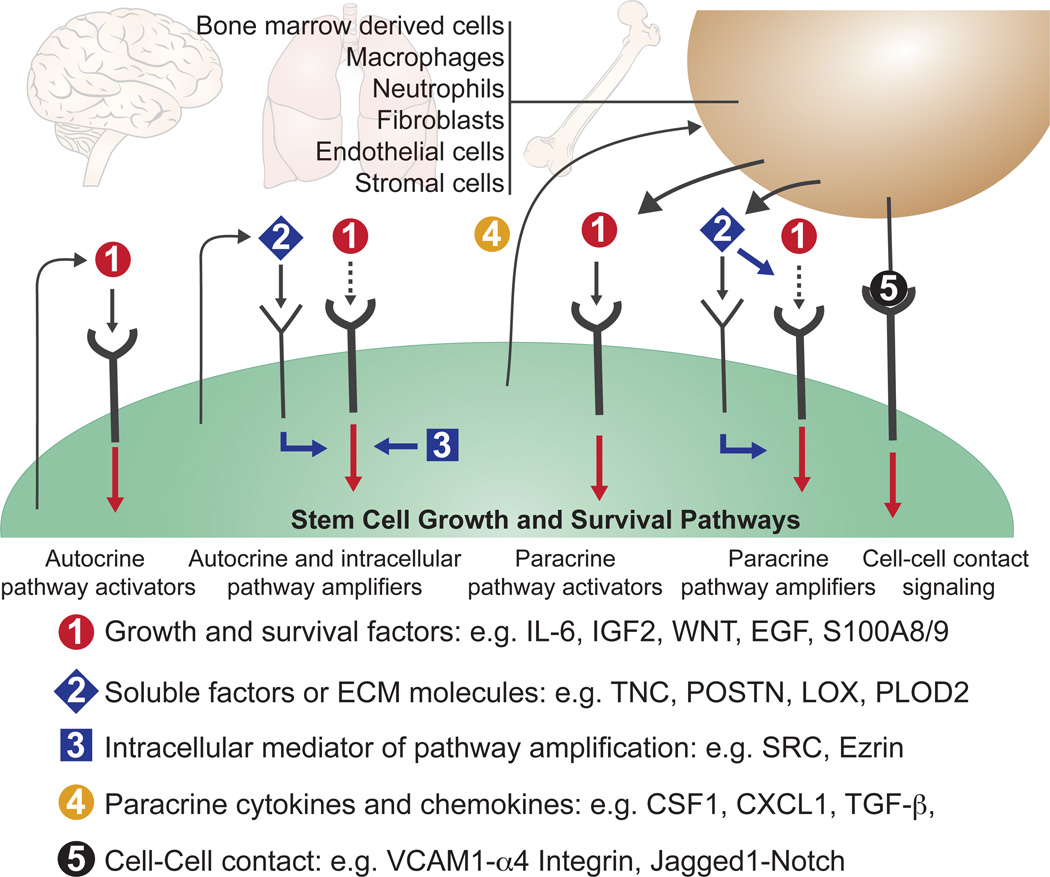
Figure 3. Growth and survival pathway activation by disseminated cancer cells.
During one or more stages of the metastatic colonization process, metastasis-initiating cells require the activity of a common set of pathways that support the growth and survival of stem and progenitor cells. After infiltrating distant tissues that offer limiting levels of pathway activators, the disseminated cancer cells secure pathway activation through autocrine or paracrine mediators that directly activate these pathways (1), or amplify the responsiveness of the pathways to low levels of stromal activators (2, 3). Cancer cells may express autocrine (1, 2) and intracellular (3) pathway activators and amplifiers. Cancer cells may also express paracrine factors (4) that recruit stromal cells as sources of soluble activators and amplifiers, or may achieve pathway activation through direct cell-cell contact. Specific examples of these various mediators are listed in the figure and discussed in the text.
Metastatic cells may achieve stimulation of these pathways by expressing autocrine pathway activators or recruiting stromal cells that produce them (Figure 3). In both cases, the pro-metastatic signal may act by directly stimulating a vital pathway or by amplifying the pathways’ signalling output69. For example, Stat3 stimulation by autocrine IL6 mediates metastasis in prostate, and PI3K/AKT stimulation by autocrine IGF2 mediates metastasis in esophageal cancer cells88,89. The intracellular tyrosine kinase Src amplifies the ability of stromal CXCL12 to activate PI3K/AKT signalling in breast cancer cells that infiltrate the bone marrow90. Breast cancer cells produce CSF-1 to recruit tumour-associated macrophages as a source of EGF91 or CXCL1 to recruit myeloid precursors as a source of S100A8/9 for MAPK activation92. Colorectal cancer stem cells that reach the liver express TGF-β to recruit mesenchymal cells as a source of interleukin-11 (IL-11) for Stat3 activation in the cancer cells12.
Cancer cells may also obtain vital support through contacts with stromal cells (Figure 3). Claudin-2 mediated cell-cell interactions, between breast cancer cells and hepatocytes, induce c-Met signalling and stimulate metastasis to the liver93. Membrane VCAM-1 expressed on breast cancer cells that infiltrate the lungs contacts α4 integrins on stromal monocytes and macrophages to activate PI3K/AKT signalling in the cancer cells94. In contrast, VCAM-1 in breast cancer cells that exit from dormancy in the bone marrow contacts α4 integrins on monocytic precursors to accelerate the differentiation of these cells into osteoclasts that mediate osteolytic metastasis95.
The activity of pro-metastatic pathways may also be increased by epigenetic alterations that expand the range of a pathway’s gene responses. For example, VHL-mutant renal cell carcinoma cells gain metastatic activity in multiple organs by DNA methylation and histone acetylation changes that expand the range of HIF (hypoxia inducible factor) target genes, the dominant oncogenic pathway in these cells96. Additional inputs come from the expression of microRNAs that either promote or suppress metastasis by regulating multiple mediators of tumour-stroma interactions97–100. These examples show that DTCs resort to diverse mechanisms to procure vital inputs for survival and retention of tumour initiating capacity.
Exactly when, where, and how cancer cells resort to these various stromal cues is unclear. Are these niches and pathways important for all stages of metastatic colonization, all the time? Some might be critical only after extravasation, when cancer cells are challenged by tissue defences, or during the latent phase of metastasis, when cancer cells must subsist for years without outgrowth. Yet others might count only for outgrowth as DTCs that exit dormancy. Such questions remain unanswered because most experimental models of metastasis do not incorporate a latency phase. This gap in knowledge is also of concern from a translational perspective. Treating overt metastasis by targeting a survival mechanism that was relevant only during the initial infiltration of distant organs may have no clinical benefit, and targeting a driver oncogenic pathway in latent DTCs may likewise be futile, whereas targeting what supports the viability of latent DTCs could effectively eradicate residual disease.
Latent metastasis
The clinical observation that patients relapse with metastatic disease months to years after removal of the primary tumour, combined with the detection of DTCs in the bone marrow of patients with no evidence of metastatic disease, proves that cancer cells disseminated before treatment of the primary tumour remain competent to re-initiate metastatic growth long thereafter. Some organs are more permissive than others for the accumulation of latent DTCs. For example, patients with colorectal or gastric cancer may harbour DTCs in the bone marrow, yet the incidence of bone metastasis in these patients is low101. The incidence of DTCs in the bone marrow predicts not only for bone metastasis, but also for metastasis to liver, lung and brain2.
Tumour dormancy is thought to occur in two modes (Figure 1). Cellular dormancy involves isolated DTCs that enter a state of proliferative quiescence. Indeed, in patient bone marrow samples most DTCs are found as quiescent single cells101–103. In contrast, tumour mass dormancy involves micrometastases that cease to grow due to insufficient vascularization or to constant culling by immune defences102. What dormancy mode most frequently leads to overt metastases is uncertain.
Despite the biological and clinical relevance of metastatic latency, little is known about how cancer cells enter the dormant state, what signals sustain it, what niches dormant cancer cells inhabit, and what triggers the resumption of aggressive growth. The paucity of experimental model systems that incorporate a latent phase, and the cost of studying a process over extended periods in animal models, have hindered progress. However, recent work has identified stromal signals that impose tumour dormancy in mouse xenograft models. TGF-β and BMPs (bone morphogenetic proteins, members of the TGF-β family) can enforce quiescence and inhibit self-renewal of carcinoma DTCs104–106. The perivascular niche has also been implicated in the induction of cancer cell dormancy107. In contrast, environments that are rich in type 1 collagen108 or fibronectin109 inhibit dormancy.
A scarcity of stromal growth factors and an abundance of growth-inhibitory signals can favour metastatic dormancy in experimental models. Alone, however, these signals may not sustain metastatic latency in the long-term. The tissues hosting DTCs, such as the lungs, liver, or bone marrow, are not in a perpetual state of growth inhibition. On the contrary, these tissues support cell proliferation as part of normal tissue homeostasis and regeneration. This context would regularly stimulate DTCs to enter the cell cycle. Cancer cell-autonomous mechanisms that self-impose quiescence in DTCs may be necessary. It is also not clear how a continuously quiescent DTC population could evolve and acquire the necessary traits for overt metastasis.
Evidence that DTCs are kept latent by the immune system comes from cancer transmission in organ transplantation cases. Kidney, liver, and lung transplants from donors who were cured of melanoma, or who suffered glioblastoma, which is generally considered a non-metastatic tumour, transmitted donor-derived tumour in immunosuppressed recipients110,111. These cases suggest that DTCs are maintained in the latent state by constant pressure from the immune system. Perhaps DTCs intermittently enter the cell cycle and their progeny undergo rapid elimination by the immune system, all the while evolving to acquire traits for eventual metastatic outbreak.
Overt metastasis
Breaking through growth inhibitory or immune barriers might be sufficient for the initiation of aggressive metastatic outgrowth in some organs. However, organs markedly differ in tissue structure and composition, and their overt colonization involves distinct organ-specific metastatic traits112. This translates into a remarkable variation of metastasis distribution patterns depending on the tumour type. For example, prostate cancer has a marked propensity to relapse in bone, uveal melanoma in the liver, and sarcomas in the lungs. In contrast, melanomas, breast carcinomas, and lung adenocarcinomas tend to relapse in multiple organs. The kinetics of relapse also varies. For example, lung cancer frequently relapses in brain and other sites early, whereas brain relapse is typically a late event in metastatic breast cancer. Certain oncogenic mutations appear to affect metastatic tropism. For example, KRAS-mutant colon cancer secondarily colonizes the lungs from established liver metastases113.
Bone metastasis is the best-understood case of overt colonization, and offers clear examples of the nature of organ-specific metastasis traits that determine this final stage of the metastatic process (Figure 3). Osteolytic bone metastasis results from an altered balance of bone generating osteoblasts and bone-resorbing osteoclasts, in favour of the latter. Numerous mediators of osteoclast activation have been implicated in this process114. Cancer cell-derived parathyroid hormone-related protein (PTHrP), IL-11, and tumour necrosis factor α (TNF-α) cue osteoblasts to release RANKL, which stimulates osteoclast maturation114–116. Bone metastatic cells also produce MMPs, which increase RANKL activity117 and reduce the levels of the RANKL antagonist osteoprotegrin118. Expression of the Notch ligand Jagged1 and cell adhesion molecules VCAM-1 and sICAM-1 also contribute to osteoclast mobilisation95,119,120. Bone matrix degradation by the hyperactivated osteoclasts releases TGF-β, which in turn augments the production of PTHrP, IL-11 and Jagged1 in the cancer cells, driving a vicious cycle of bone destruction114–116.
Interestingly, prostate cancer cells metastatic to bone alter the homeostatic balance in favour of osteoblastic activity, stimulating bone matrix deposition with eventual displacement of the bone marrow. Cancer-cell factors implicated in osteoblastic metastasis include fibroblast, insulin-like and vascular endothelial growth factors (FGFs, IGFs, VEGF), as well as endothelin 1, Wnt factors and BMPs114. Thus, bone metastasis provides a compelling example of how cancer cells engage the host microenvironment in overt metastasis. Specific stromal components may similarly be engaged in other organs by metastatic cells with the necessary organ-specific colonization traits. For example, in the case of brain metastasis of breast and lung carcinomas, the cancer cells can profitably engage astrocytes and microglia through the expression of endothelin-1121. However, our knowledge of overt colonization traits specific for organs other than bone is woefully limited and needs further investigation.
In certain subsets of patients metastasis is mainly confined to a particular organ that resists therapy better than others. A prime example is the current rise in the incidence of late brain and leptomeningeal metastasis in HER2+ breast cancer patients. These patients benefit from advances in targeted therapies that suppress extracranial metastasis. However, this success is short lived in many cases, owing to the eventual emergence of brain metastasis. Brain metastasis is a major cause of morbidity and mortality, with an overall incidence ten-fold higher than that of all primary brain tumours combined, and it has few therapeutic options. A better understanding of its underlying mechanisms is urgently needed.
After therapy
The surgical removal of a malignant tumour is often complemented with radiotherapy and systemic adjuvant chemotherapy to suppress relapse. If metastasis becomes clinically manifest, most systemic treatments target metastasis irrespective of organ site. Treatments include classical chemotherapy, targeted therapy against oncogenic drivers, immunotherapeutic agents that leverage the antitumour power of the immune system, and increasingly, a combination of all of the above. Treatments that target metastasis in a particular organ, by taking aim at cancer cell interactions with the host tissue, would be indicated when metastasis is confined to that organ, as is the case with bone metastasis in some breast cancer patients. A meta-analysis suggests that adjuvant therapy with osteoclast inhibitory bisphosphonates suppresses bone metastasis and prolongs survival in postmenopausal women122. Denosumab, an antibody that targets RANKL, reduces the incidence of bone fractures associated with metastasis in patients receiving aromatase inhibitors123.
In spite of these advances, current therapies frequently achieve only partial tumour shrinkage, leaving behind substantial residual disease. Continued treatment may keep the residual tumour indolent for some time. However, from the residual cancer cell population, drug-resistant clones eventually emerge that drive rapid relapse124,125. As a result, cure rates of patients with metastasis remain disappointingly low.
Current research has begun to focus on the biology of residual metastatic cells after therapy, with the aim of better suppressing its re-emergence (Figure 4). The cancer cell population may resist treatment through alterations of negative feedback signalling loops126 and supportive interactions with the tumour microenvironment. For example, DNA damaging agents induce the secretion of trophic factors including IL-6 and Timp-1 in normal cells of the thymus, creating a chemoprotective niche for the survival of residual cancer cells and eventual relapse127. Similarly, stromal fibroblasts secrete Wnt16b in response to chemotherapy, promoting therapy resistance in prostate cancer128. Chemotherapy induces the expression of TNF-α in tumour associated endothelial and mesenchymal cells, amplifying the expression of the pro-metastatic cytokine CXCL1 in cancer cells92. In BRAF-mutant melanomas treated with RAF inhibitors, tumour-associated macrophages secrete TNF-α and VEGF129,130 and tumour-associated fibroblasts secrete HGF131, which protect the cancer cells and limit the effectiveness of therapy.
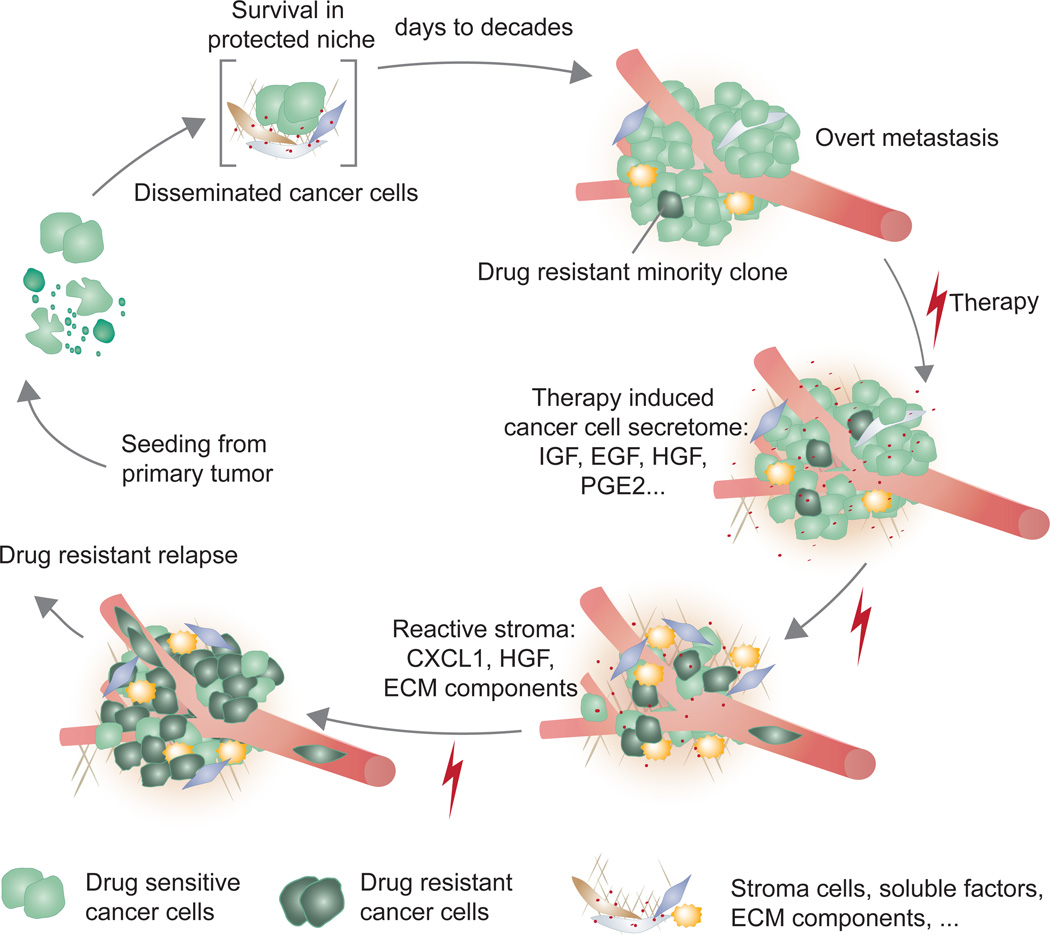
Figure 4. Metastasis biology before and after cancer therapy: A model.
Latent metastasis results from conditions that preserve the survival and tumour-initiating ability of disseminated cancer cells. Eliminating latent metastasis by targeting these survival mechanisms would prevent metastasis. Once cancer cells break out of the latency state and form manifest metastasis, the condition is treated with combinations of conventional chemotherapy, targeted therapy, and/or immunotherapy. The treatment may dramatically reduce the metastatic burden, but tumour elimination is frequently incomplete. Significant segments of the tumour cell population withstand treatment by adaptation of their intracellular pathways or activation of supportive paracrine inputs. Under the stress of targeted therapy, drug-sensitive cancer cells express a large number of secreted factors (therapy-induced secretome) that salvage drug-sensitive cells and accelerate the growth of minority drug-resistant clones. The accelerated growth leads drug-resistant clones to drive relapse as a drug-resistant tumour. The growth and survival mechanisms utilized by residual cancer cells under treatment might resemble those utilized by their predecessors during the latent phase before overt colonization in this model.
Under the stress of therapy, the cancer cells themselves can be a source of survival signals54,132,133. Targeted therapy with tyrosine kinase inhibitors against melanoma (vemurafenib, dabrafenib) or lung adenocarcinoma (erlotinib, crizotinib) triggers the production of a complex secretome (“therapy-induced secretome”) that activates multiple survival pathways in the remaining, drug-sensitive cancer cells54. Furthermore, this secretome can stimulate the selective outgrowth of drug resistant clones, their dissemination, and further metastatic reseeding. Collectively, these findings reveal a complex biology of cancer cell populations that remain after the treatment of metastatic tumours, finally contributing to tumour relapse.
Future Directions
An important target of future research is the identification of mediators of metastasis that are common to different organ sites and tumour types. Although the topic of organ-specific metastasis has intrigued researchers for over a century, the reality is that many patients suffer from, or are at risk of metastasis in multiple organs. For these cases, identifying common mediators of metastatic colonization as therapeutic targets would be of value. For example, current checkpoint immunotherapy and its encouraging clinical success are based on the premise that immune evasion is a shared feature of metastatic disease irrespective of organ site. More knowledge on common mediators of metastatic colonization and regrowth after therapy would provide clues for the improved elimination of residual disease.
The advent of single-cell analysis techniques, in particular single cell RNA sequencing and signalling pathway profiling, is allowing functional and phenotypic analysis of heterogeneous cell populations with unprecedented detail134–137. The application of these techniques to residual disease and overt metastases will allow a better definition of tumour heterogeneity, cell population structure and evolution, cell type-specific response patterns to stromal cues and therapeutic agents, and other parameters at a level never before possible. Furthermore, the ability to analyse circulating tumour DNA in the blood of cancer patients will allow the monitoring of therapy responses, the emergence of distinct resistant clones, and the patterns of early disease recurrence.
In the end, preventing metastasis in high-risk patients would be far better than having to treat it later. The systemic nature of metastatic disease, the heterogeneity of metastatic tumours, the multitude of genes and pathways involved in different organs, and the many mechanisms of drug resistance, paint a sobering picture of the problem and prospects of addressing overt metastatic disease. Prevention of relapse ostensibly is the goal of systemic therapy delivered after the removal of a primary tumour. However, most agents used in the adjuvant therapy setting target growing cancer cells, not quiescent DTCs that predominate during metastatic latency. A better understanding of the basis for metastatic colonization, in particular of its latent phase, is therefore needed in order to develop better treatments. Research on the mechanisms that support the viability of latent metastatic cells should yield clues for targeting residual disease with the goal of preventing metastasis.
Acknowledgments
We thank K. Ganesh and T. Wiesner for useful input. J.M. was supported by the National Institutes of Health (NIH) (grants CA163167 and CA129243), the Congressionally Directed Medical Research Program of the Department of Defense, the Cancer Center Support (grant P30 CA008748), and the Memorial Sloan Kettering Cancer Center (MSKCC) Metastasis Research Center. A.C.O. was an Erwin Schroedinger Fellowship awardee (J3013, FWF, Austrian Science Fund).
Footnotes
The authors declare no competing financial interests.
References
- 1.Nagrath S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 3.Wong CW, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333–338. [PubMed] [Google Scholar]
- 4.Minn AJ, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 6.Luzzi KJ, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron MD, et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- 8.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 9.Valiente M, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyn C, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 11.Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 12.Calon A, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 14.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 15.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. doi: 10.1007/s10555-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 16.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giampieri S, et al. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh-Johnson M, et al. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33:4203–4212. doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 21.Magnon C, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 22.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer KR, et al. EMT is not required for lung metastasis but contributes to chemoresistance. Nature. doi: 10.1038/nature15748. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, et al. Epithelial to Mesenchymal Transition is dispensable for metastasis but confers chemoresistance in pancreatic cancer. Nature. doi: 10.1038/nature16064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 27.Marusyk A, et al. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–58. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508:113–117. doi: 10.1038/nature13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calbo J, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–256. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Gundem G, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Gal K, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7:308re308. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 34.Piskounova E, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015 doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deneve E, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem. 2013;59:1384–1392. doi: 10.1373/clinchem.2013.202846. [DOI] [PubMed] [Google Scholar]
- 36.Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 37.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 38.Budczies J, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6:570–583. doi: 10.18632/oncotarget.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11:R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 43.Tichet M, et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun. 2015;6:6993. doi: 10.1038/ncomms7993. [DOI] [PubMed] [Google Scholar]
- 44.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sevenich L, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16:876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tominaga N, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 53.Kim MY, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obenauf AC, et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature. 2015;520:368–372. doi: 10.1038/nature14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyles J, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bidwell BN, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012;18:1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- 57.Smyth MJ, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 58.Paolino M, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milsom CC, Lee CR, Hackl C, Man S, Kerbel RS. Differential post-surgical metastasis and survival in SCID, NOD-SCID and NOD-SCID-IL-2Rgamma(null) mice with parental and subline variants of human breast cancer: implications for host defense mechanisms regulating metastasis. PLoS One. 2013;8:e71270. doi: 10.1371/journal.pone.0071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda K, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 61.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schepers AG, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 69.Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 72.Zhang XH, et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao Z, et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell. 2014;25:350–365. doi: 10.1016/j.ccr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as"soil" in brain metastasis. PLoS One. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 78.Gilkes DM, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Eisinger-Mathason TS, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wculek SK, Malanchi I. Pre-metastatic neutrophils support highly lung metastatic breast cancer cells. Nature. in press. [Google Scholar]
- 86.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. doi: 10.1038/nature15756. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nowak DG, et al. MYC Drives Pten/Trp53-Deficient Proliferation and Metastasis due to IL6 Secretion and AKT Suppression via PHLPP2. Cancer Discov. 2015;5:636–651. doi: 10.1158/2159-8290.CD-14-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li B, et al. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance--implications for IGF-II and IGF-IR-targeted therapy. Clin Cancer Res. 2014;20:2651–2662. doi: 10.1158/1078-0432.CCR-13-2735. [DOI] [PubMed] [Google Scholar]
- 90.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 92.Acharyya S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tabaries S, et al. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol Cell Biol. 2012;32:2979–2991. doi: 10.1128/MCB.00299-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu X, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanharanta S, et al. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat Med. 2013;19:50–56. doi: 10.1038/nm.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tavazoie SF, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 99.Korpal M, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pencheva N, et al. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068–1082. doi: 10.1016/j.cell.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 102.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573–581. doi: 10.1016/j.ccr.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao H, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bragado P, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi A, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12:863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 2010;11:790–796. doi: 10.1016/S1470-2045(10)70024-3. [DOI] [PubMed] [Google Scholar]
- 111.Collignon FP, Holland EC, Feng S. Organ donors with malignant gliomas: an update. Am J Transplant. 2004;4:15–21. doi: 10.1046/j.1600-6143.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 112.Obenauf AC, Massague J. Surviving at a distance: Organ specific metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Urosevic J, et al. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol. 2014;16:685–694. doi: 10.1038/ncb2977. [DOI] [PubMed] [Google Scholar]
- 114.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin JJ, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 117.Lynch CC, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 118.Lu X, et al. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–1894. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ell B, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24:542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim SW, et al. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol. 2014;16:1585–1598. doi: 10.1093/neuonc/nou128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Early Breast Cancer Trialists' Collaborative G, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 123.Gnant M, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 124.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 125.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lito P, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun Y, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359-+. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith MP, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov. 2014;4:1214–1229. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang T, et al. BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth. Clin Cancer Res. 2015;21:1652–1664. doi: 10.1158/1078-0432.CCR-14-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurtova AV, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang Q, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Macosko EZ, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levine JH, et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lawson DA, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat Rev Clin Oncol. 2015;12:258–272. doi: 10.1038/nrclinonc.2014.238. [DOI] [PubMed] [Google Scholar]
- 141.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–554. doi: 10.1038/ncb2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]