Abstract
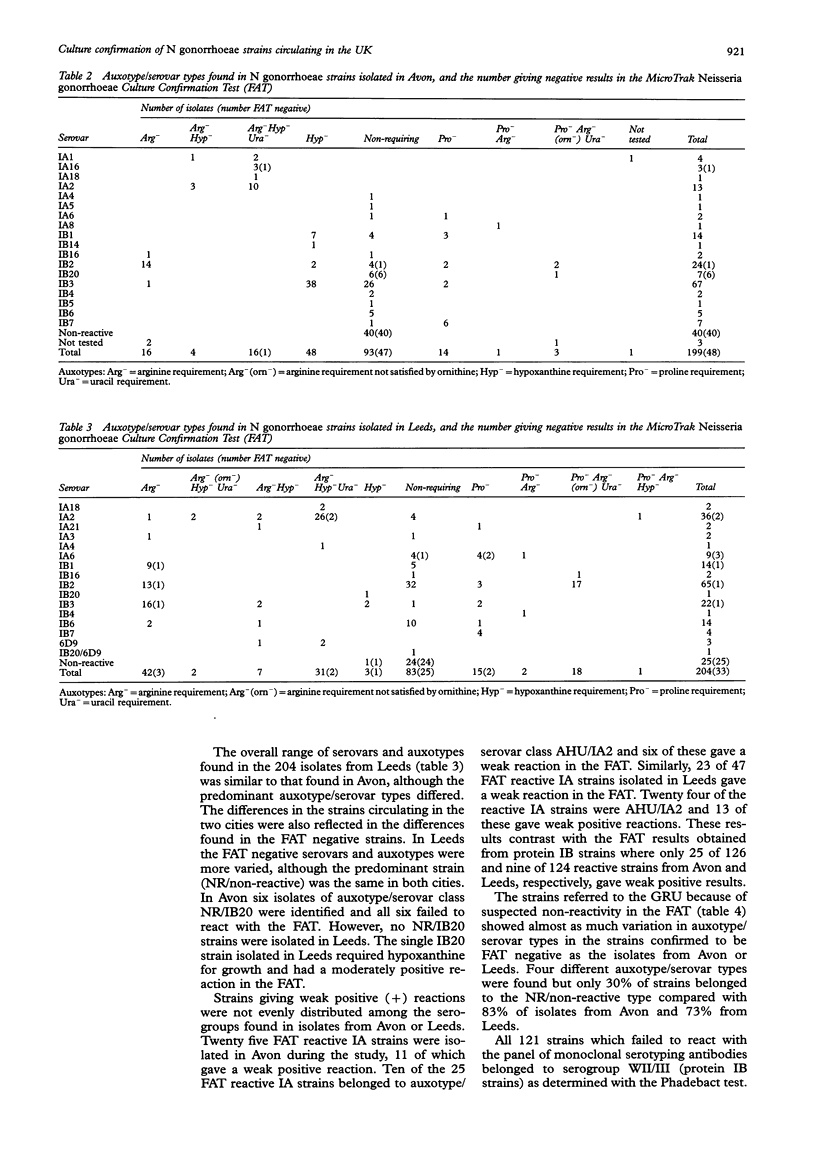
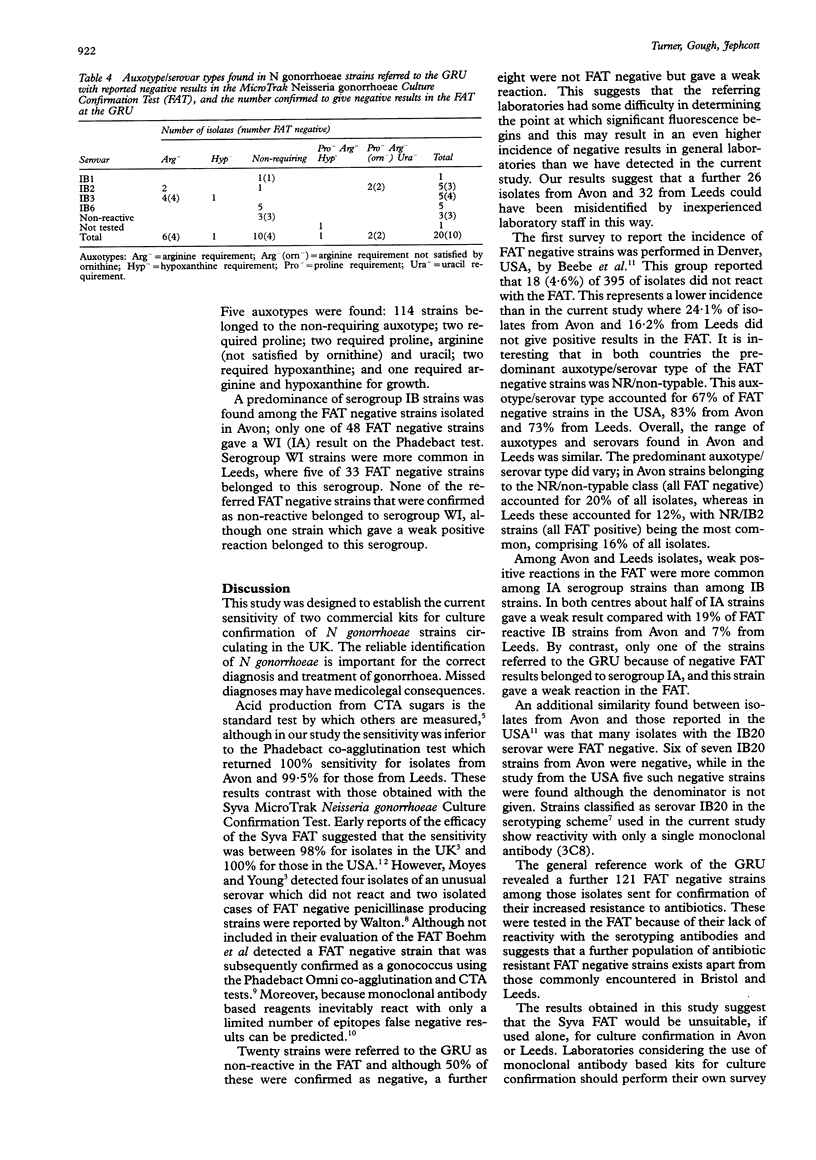
AIMS--To establish the current sensitivity of two commercial kits for culture confirmation of Neisseria gonorrhoeae strains circulating in the UK. METHODS--A total of 544 strains were studied (199 gonococci from male patients attending hospitals in the county of Avon, 204 unselected N gonorrhoeae isolates from male patients in Leeds, 20 strains referred to the Gonococcus Reference Unit because of difficulties with the Syva fluorescent antibody test (FAT), and 121 strains collected over a four year period which had not reacted with serotyping antibodies). Strains were tested by sugar utilisation in cysteine trypticase base agar (CTA test), the Phadebact Monoclonal GC Test and Syva MicroTrak Neisseria gonorrhoeae Culture Confirmation Test. The auxotype and serovar of each strain were also determined. RESULTS--The sugar utilisation test confirmed the identity of 99% (197/199) of gonococci from Avon and 97% (198/204) of those from Leeds. The Syva FAT confirmed 76% (151/199) of isolates from Avon and 84% (171/204) of those from Leeds. The Phadebact test confirmed all but one isolate from the 403 strains from both cities. Half of the 20 referred FAT negative isolates also give a negative result in the Syva FAT; however, only 10% of the remainder gave a strong reaction in our laboratory. All serotyping antibody negative strains were negative in the FAT, although all these and all of the 20 strains that give a negative result in the FAT gave positive reactions in the other culture confirmation tests. Typing tests revealed a greater diversity amongst the FAT negative strains from Leeds than those from Avon. CONCLUSIONS--Considerable differences in the sensitivity of the MicroTrak but not with the Phadebact or CTA tests were found for the identification of isolates from two geographically distinct areas of the UK. Our results suggest that the Syva FAT would not be suitable, if used alone, for culture confirmation in Avon or Leeds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe J. L., Rau M. P., Flageolle S., Calhoon B., Knapp J. S. Incidence of Neisseria gonorrhoeae isolates negative by Syva direct fluorescent-antibody test but positive by Gen-Probe accuprobe test in a sexually transmitted disease clinic population. J Clin Microbiol. 1993 Sep;31(9):2535–2537. doi: 10.1128/jcm.31.9.2535-2537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. M., Bernhardt M., Kurzynski T. A., Pennell D. R., Schell R. F. Evaluation of two commercial procedures for rapid identification of Neisseria gonorrhoeae using a reference panel of antigenically diverse gonococci. J Clin Microbiol. 1990 Sep;28(9):2099–2100. doi: 10.1128/jcm.28.9.2099-2100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley C. G., Egglestone S. I. Auxotyping of Neisseria gonorrhoeae isolated in the United Kingdom. J Med Microbiol. 1983 Aug;16(3):295–302. doi: 10.1099/00222615-16-3-295. [DOI] [PubMed] [Google Scholar]
- Ison C. A. Laboratory methods in genitourinary medicine. Methods of diagnosing gonorrhoea. Genitourin Med. 1990 Dec;66(6):453–459. doi: 10.1136/sti.66.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Ehret J. M., Tanino T. T., Van der Pol B., Handsfield H. H., Jones R. B., Judson F. N., Hook E. W., 3rd Fluorescent monoclonal antibody for confirmation of Neisseria gonorrhoeae cultures. J Clin Microbiol. 1987 Dec;25(12):2388–2390. doi: 10.1128/jcm.25.12.2388-2390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes A., Young H. Fluorescent monoclonal antibody test for the confirmation of Neisseria gonorrhoeae. Med Lab Sci. 1989 Jan;46(1):6–10. [PubMed] [Google Scholar]
- Walton D. T. Fluorescent-antibody-negative penicillinase-producing Neisseria gonorrhoeae. J Clin Microbiol. 1989 Aug;27(8):1885–1886. doi: 10.1128/jcm.27.8.1885-1886.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. D., Cartwright G. Fluorescent monoclonal antibody compared with carbohydrate utilization for rapid identification of Neisseria gonorrhoeae. J Clin Microbiol. 1988 Feb;26(2):293–296. doi: 10.1128/jcm.26.2.293-296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H., Moyes A. Utility of monoclonal antibody coagglutination to identify Neisseria gonorrhoeae. Genitourin Med. 1989 Jan;65(1):8–13. doi: 10.1136/sti.65.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]



