Abstract
Pregnancy is a complex physiological process tightly controlled by the interplay among hormones, morphogens, transcription factors, and signaling pathways. Although recent studies using genetically engineered mouse models have revealed that ligands and receptors of transforming growth factor beta (TGFbeta) and bone morphogenetic protein (BMP) signaling pathways are essential for multiple reproductive events during pregnancy, the functional role of SMAD transcription factors, which serve as the canonical signaling platform for the TGFbeta/BMP pathways, in the oviduct and uterus is undefined. Here, we used a mouse model containing triple conditional deletion of the BMP receptor signaling Smads (Smad1 and Smad5) and Smad4, the central mediator of both TGFbeta and BMP signaling, to investigate the role of the SMADs in reproductive tract structure and function in cells from the Amhr2 lineage. Unlike the respective single- or double-knockouts, female Smad1flox/flox Smad5flox/flox Smad4flox/flox Amhr2cre/+conditional knockout (i.e., Smad1/5/4-Amhr2-cre KO) mice are sterile. We discovered that Smad1/5/4-Amhr2-cre KO females have malformed oviducts that subsequently develop oviductal diverticuli. These oviducts showed dysregulation of multiple genes essential for oviduct and smooth muscle development. In addition, uteri from Smad1/5/4-Amhr2-cre KO females exhibit multiple defects in stroma, epithelium, and smooth muscle layers and fail to assemble a closed uterine lumen upon embryo implantation, with defective uterine decidualization that led to pregnancy loss at early to mid-gestation. Taken together, our study uncovers a new role for the SMAD transcription factors in maintaining the structural and functional integrity of oviduct and uterus, required for establishment and maintenance of pregnancy.
Keywords: fertility, mouse models, ovary, oviduct, pregnancy, uterus
INTRODUCTION
Early pregnancy loss is a common occurrence in women and often occurs due to defects during pre-, peri-, and postimplantation periods [1, 2]. In natural conception, the chance of a successful pregnancy occurring in a given menstrual cycle is limited to approximately 30%, and only 50% to 60% of all conceptions advance beyond 20 weeks of gestation [2]. Of those pregnancies that are lost, 75% fail to implant and are not clinically recognized [2]. Multiple factors contribute to implantation failure, including poor embryo quality (e.g., chromosomal abnormalities) or endocrine disturbances resulting in abnormal embryo spacing, decidualization, placentation, and intrauterine embryonic growth [3, 4]. However, in most cases, the cause of implantation failure is unexplained. Defects in fallopian tube structure as well as function can also result in infertility [5]. Recent progress using genetically engineered mouse models indicates that ovarian hormones together with locally produced signaling molecules, including cytokines, growth factors, homeobox transcription factors, lipid mediators, and morphogen genes function through autocrine, paracrine, and juxtacrine interactions to specify the complex processes of embryo implantation and establishment of the uterus-embryo axis during postimplantation period [6, 7]. Because embryo implantation is a dynamic developmental process that integrates multiple signaling molecules into a precisely orchestrated program, it is important to understand the hierarchical landscape of molecular signaling pathways that govern the embryo-uterus interaction in order to generate new strategies to correct and/or identify the causes of implantation failure and improve pregnancy outcome, including pregnancies conceived through assisted reproductive techniques.
Proteins of the transforming growth factor β (TGFβ) superfamily are evolutionarily conserved and essential mediators of cellular growth and differentiation. Members of this family are secreted and bind to type 2 and type 1 cell surface receptors that activate the SMAD transcription factors as the canonical signaling pathway [8]. Based on sequence similarities, the TGFβ superfamily is grossly divided into two main ligand subfamilies: the TGFβ-activin-nodal subfamily and the BMP subfamily [8]. TGFβ, activin, and nodal signal through SMAD2 and SMAD3 (termed AR-SMADs), whereas the bone morphogenetic proteins (BMPs) use SMAD1 and SMAD5 (termed BR-SMADs). Both of these pathways share SMAD4 (the “common” SMAD) to form trimeric protein complexes to initiate signal transduction [9]. Several studies have clearly demonstrated that members of TGFβ superfamily regulate diverse developmental and homeostatic processes and are mutated in numerous human diseases including cancer and infertility [9, 10]. Studies using genetically engineered mouse models demonstrate that ligands and receptors of TGFβ and BMP signaling pathway display spatiotemporal expression patterns in reproductive tissues and are critically involved in multiple reproductive events including germ cell specification, folliculogenesis, embryo implantation, uterine decidualization, and placental development [11, 12]. For instance, progesterone receptor (Pgr) promoter-driven Cre recombinase-mediated conditional deletion of Bmp2 [13], the BMP receptor type I (Bmpr2) [14] or the BMP type 1 receptor, activin-like kinase 2 (Alk2) [15] in the uterine epithelium results in infertility due to abnormalities in uterine functions, including implantation, decidualization, vascular development, and trophoblast invasion during pregnancy. However, ablation of Alk2 in the uterine stromal and myometrial compartment, using anti-Müllerian hormone receptor type 2 (Amhr2) promoter-driven Cre recombinase, does not affect fertility. In contrast, conditional deletion of the TGFβ type I receptor (Tgfbr1) by using Amhr2-cre results in sterility primarily through developmental defects in smooth muscle layers of the oviduct and uterus [16]. Together, these results indicate the compartment-specific (i.e., epithelial/stromal/myometrial) functional role of these genes and highlight the importance of compartment-specific deletion for better understanding the role of TGFβ and BMP signaling pathway during reproductive events.
Mouse models for studying the SMAD transcription factors in pregnancy are limited due to early embryonic lethality of the SMAD homozygous null mutations and are complicated by genetic redundancy between the AR-SMADs or BR-SMADs [17, 18]. Smad4-null embryos arrest during gastrulation at approximately Embryonic Day (E) 7.5 [19], whereas Smad1 null or Smad5 null die at midgestation [17, 20]. Our laboratory has been using tissue-specific conditional gene inactivation by using Cre-loxP technology to expand the understanding of reproductive functions of SMAD transcription factors (Table 1). Disruption of Smad4 using loxP-flanked alleles and Amhr2-cre leads to premature luteinization of ovarian follicles; in contrast, oocyte-specific deletion of Smad4 with the zona pellucida 3 (Zp3)-cre gene or growth differentiation factor 9 (Gdf9)-icre driver lines causes minimal fertility defects in mice but disrupts primordial follicle numbers [21]. Amhr2-cre-mediated deletion of the AR-SMADs (SMAD2 and SMAD3) revealed redundant roles of Smad2 and Smad3 in multiple ovarian processes, including follicular development, ovulation, and cumulus cell expansion [22], but no reported uterine defects. Furthermore, double conditional knockout (KO) of Smad1 and Smad5 using Amhr2-cre reduces litter sizes and causes metastatic granulosa cell tumors [18, 23] (Table 1).
TABLE 1.
Summary of major female reproductive phenotypes in SMAD conditional KO mouse models.a
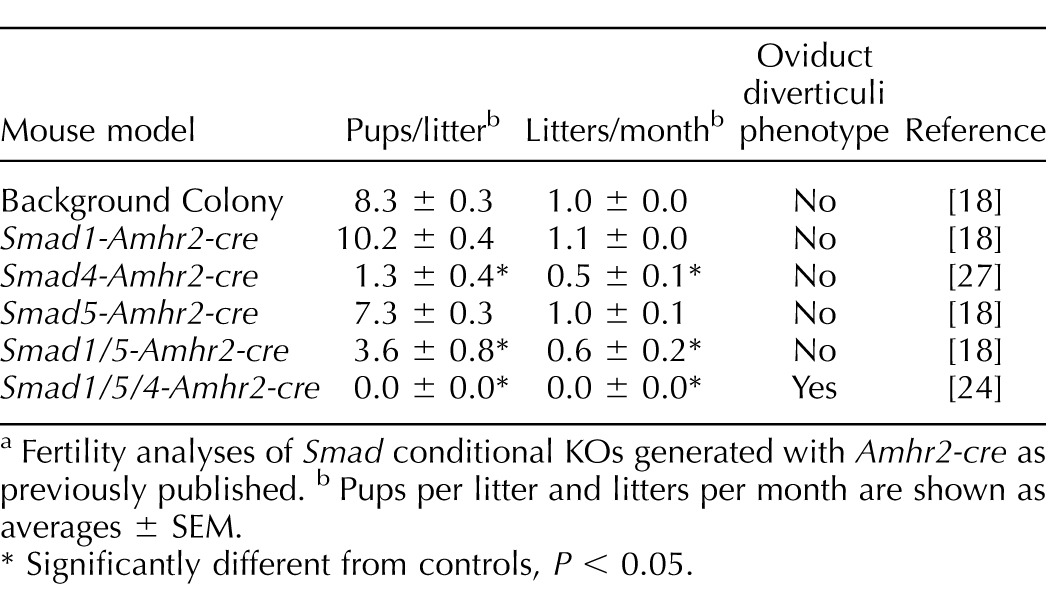
Fertility analyses of Smad conditional KOs generated with Amhr2-cre as previously published. b Pups per litter and litters per month are shown as averages ± SEM.
Significantly different from controls, P < 0.05.
Recently, we generated a triple conditional SMAD KO mouse model by genetically deleting Smad4 from Smad1 Smad5 double-KO mice in the female reproductive tract, using Amhr2-cre [24]. We had generated this mouse model to analyze the role of Smad4 in granulosa cell tumorigenesis. During these studies, we found that surprisingly, Smad1/5/4-Amhr2-cre KO mice were sterile, unlike the single or double Smad1-Amhr2-cre KO, Smad5-Amhr2-cre KO, Smad1/5-Amhr2-cre KO, or Smad4-Amhr2-cre KO mice [24] (Table 1). Our initial findings indicated that the sterility of Smad1/5/4-Amhr2-cre KO females was not fully caused by defects in ovarian function [24], so in this study, we further analyzed the reproductive tract where Amhr2-cre is known to be expressed, that is, in the developing Müllerian ducts as well as the smooth muscle compartments of the oviduct, myometrium, and stroma of uteri in adult mice [25, 26]. We found that Smad1/5/4-Amhr2-cre KO mice have a striking oviductal and uterine phenotype that has not been previously observed in any SMAD mutant mouse models that used Amhr2-cre, thereby uncovering a novel role of SMAD signaling in female reproductive tract development and uterine function. Furthermore, to our knowledge, this is the first study to use the imaging technique of optical coherence tomography for a structural analysis of the reproductive tract in a mouse mutant.
MATERIALS AND METHODS
Animals
Experimental animals were used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with institutionally approved animal protocols at Baylor College of Medicine. Generation and initial characterization of Smad1flox/flox Smad5flox/flox Smad4flox/flox Amhr2cre/+ (referred to throughout as Smad1/5/4-Amhr2-cre KO or Smad1/5/4 tKO) was described previously [24]. Experimental animals were maintained on a C57BL/6J/129S7/SvEvBrd mixed hybrid background. Mice were genotyped using tail genomic DNA and PCR as reported previously [18, 27]. Littermates that were wild type at the Amhr2 locus served as controls (e.g., Smad1flox/flox Smad5flox/flox Smad4flox/flox or their respective single or double lines as appropriate). To control for potential background differences between colonies, we rederived the Smad1/5-Amhr2-cre (double-KO) and Smad4-Amhr2-cre (single-KO) females directly from the Smad1/5/4-Amhr2-cre KO colony.
Fertility Analysis
Fertility of mice was monitored as previously described [18, 27]. Fertility was previously reported in the initial description of each knockout line as shown in Table 1. At 6 wk of age, female control and KO mice were housed individually with wild-type males of proven fertility. Mice were kept in mating pairs for 6 mo with cage enrichment, and the number of pups per litter and litters per month were recorded.
Superovulation
Three- to 4-wk-old control and Smad1/5/4-Amhr2-cre KO females were given i.p. injections of 5 IU of pregnant mare serum gonadotropin (EMD Millipore) for 44 to 46 h, followed by injection of 5 IU of human chorionic gonadotropin (Novarel; Ferring Pharmaceuticals) for 18 h. To collect cumulus-oocyte complexes (COC), the COC were released from the ampulla of the oviduct into minimum essential medium containing bovine hyaluranidase to separate cumulus cells from oocytes for counting. For optical coherence tomography (OCT) imaging, reproductive tracts were dissected intact and imaged (see Optical Coherence Tomography Imaging).
Tissue Collection and Histological Analysis
Mice were anesthetized by isoflurane inhalation (Butler Schein) and euthanized by cervical dislocation prior to tissue collection. For histological studies, oviduct and uterus samples from mice were dissected and fixed in 10% neutral buffered formalin overnight at room temperature. After fixation, formalin-fixed samples were transferred to 70% ethanol for long-term storage at room temperature or processed for embedding. Tissue processing and paraffin embedding were performed by the Human Tissue Acquisition and Pathology Core Services facility at Baylor College of Medicine. Histological sections were cut at 5 μm and stained with hematoxylin and eosin or periodic acid–Schiff (PAS) reaction, using standard procedures. For total RNA isolation, tissue sections were collected and stored in RNAlater solution (Life Technologies) overnight at 4°C to allow thorough penetration of the tissue and then frozen to −80°C for long-term storage or until RNA extraction.
Artificial Induction of Decidualization
The artificial induction of decidualization of the uterus was performed as previously described [28]. Briefly, 8-wk-old control and Smad1/5/4-Amhr2-cre KO mice were ovariectomized via a dorsal incision under anesthesia, and mice were allowed to recover for 2 wk to achieve complete withdrawal of endogenous ovarian hormones. After 2 wk, mice were “primed” with subcutaneous injections of 100 ng of 17β-estradiol (E2) once a day for 3 days. After 2 days, mice were treated daily for 3 days with subcutaneous injections of 1 mg of progesterone (P4) and 6.7 ng of E2 per mouse. One uterine horn was then stimulated to decidualize by traumatizing (“scratching”) the entire length of the uterine endometrium with a large needle 6 h after the last hormone injection. The contralateral horn was left untreated and served as a control. Each day after the decidual stimulus, mice were given subcutaneous injections of 1 mg of P4 and 6.7 ng of E2 per mouse. On the second and fifth days after the decidual stimulus, mice were euthanized 6 h after the last hormone injection. Uterine horns were collected, and the wet weights of the stimulated and control horns were recorded, and tissue was processed for histological and molecular analysis.
Alkaline Phosphatase Staining
Uteri that had undergone artificial decidualization were fixed in 4% paraformaldehyde overnight and immersed in 10% to 30% sucrose solution prior to embedding in optimal cutting temperature compound. Ten-micrometer tissue sections were post-fixed in 0.2% glutaraldehyde and washed in phosphate-buffered saline (PBS). The 5-bromo-4-chloro-3-indoyl phosphate/ nitroblue tetrazolium alkaline phosphotase (BCIP/NBT AP) substrate (Vector) was prepared according to the manufacturer's instructions. Tissues were incubated with the substrate solution for 25 min and rinsed in tap water, nuclei were counterstained with nuclear fast red, and slides were mounted (VectaMount AQ mounting medium; Vector). A dark blue colorimetric reaction indicated stromal cell differentiation.
Optical Coherence Tomography Imaging
For volumetric structural analysis of the reproductive tract, we used a custom-built spectral-domain OCT system that has been described previously [29]. Briefly: the system uses a low-coherence Ti:sapphire laser source (Micra-5; Coherence, Inc.) with a central wavelength of ∼808 nm, a bandwidth of ∼110 nm, and a power output of ∼400 nm. The setup uses a Michelson interferometer to generate interference fringes from the backscattered light collected from the sample and reference arms. The fringes are resolved using a custom-built spectrometer and are converted into in-depth intensity profiles using fast-Fourier transform. The system provides a lateral resolution of ∼4 μm and an axial resolution of ∼5 μm. The A-line (in-depth intensity profile) scan rate was set at 50 kHz. Due to light attenuation in tissue, the imaging depth in reproductive organs is limited to ∼1 to 1.5 mm. For the imaging, reproductive tracts were dissected and placed in a dish with PBS. Freshly excised tissues were imaged with OCT in PBS without any staining. After tissues were imaged, they were collected for further analysis. Data rendering and three-dimensional (3D) visualizations were performed using Imaris software (Bitplane, Switzerland).
Analysis of Implantation
Success of implantation was determined by counting implantation sites at indicated times during early pregnancy (E4.5–E9.5). Eight-wk-old female control and experimental mice were allowed to naturally mate with wild-type males and were examined daily in the morning for the presence of seminal plug; the morning of plug detection was considered E0.5. Implantation of embryos was evaluated at E4.5, E5.5, E6.5, E7.5, and E9.5. For E4.5, implantation sites were visualized by intravenous injection of Chicago Blue dye and euthanized 2 minutes after dye injection [15].
RNA Extraction, Reverse Transcription and Quantitative Real-Time PCR
Total RNA from the oviduct and uterus were extracted using RNeasy micro- or mini-kit (Qiagen) according to the manufacturer's instructions. RNA concentrations were measured using a spectrophotometer (model ND 1000; NanoDrop Technologies). Reverse transcription was carried out using 200 ng of total RNA, using High-Capacity RNA-to-cDNA Master Mix (Life Technologies) according to the manufacturer's instructions. Real-time quantitative PCR (qPCR) was performed using custom-designed primers and Fast SYBR Green Master Mix (Life Technologies). Primer information used in this study is listed in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org). All qPCR assays were performed in triplicate for each sample, using a minimum of three mice per group. Melt curve analysis was performed to verify a single amplification peak for determining the specificity of amplification. Relative mRNA levels of transcript were calculated by the cycle threshold (ΔΔCT) method as described previously [30] and were normalized to the endogenous reference (Gapdh or Rpl19), as indicated in the text. The expression data are presented as relative values ± standard errors of the mean (SEM), using the control mean as the calibrator (set to equal 1) as described previously [31].
Immunofluorescence and TUNEL Assay
Paraffin-embedded sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Antigen retrieval was performed by boiling the sections in a 10 mM sodium citrate antigen unmasking buffer with 0.05% Tween 20 (pH 6.0) for 10 min. Tissue sections were washed for 5 min in PBS with 0.1% Tween-20 (PBS-T), blocked at room temperature for 1 h with 3% bovine serum albumin in PBS-T, and then incubated with the primary antibody in a humidified chamber overnight at 4°C. After tissues were washed with PBS, sections were incubated with Alexa Fluor 546 and 488 conjugated secondary antibodies (Life Technologies) for 1 h at room temperature. Sections were washed with PBS-T and mounted with Prolong Gold Antifade mounting medium with DAPI (Invitrogen). Fluorescent images were viewed and captured using fluorescence microscopy, and all images were compiled using Photoshop CS4 or CS5 (Adobe Systems Inc.). TUNEL assay was performed as previously described [24], using a fluorescein in situ apoptosis kit (ApopTag; Millipore). TUNEL-positive cells were imaged using fluorescence microscopy and defined as apoptotic cells. The following antibodies were used in this study: goat anti-prostaglandin-endoperoxide synthase 2 (PTGS2; 1:200 dilution; Santa Cruz Biotechnology); rat anti-cytokeratin 8 (KRT8; Troma I; 1:200 dilution; Developmental Studies Hybridoma Bank); rabbit anti-alpha smooth muscle actin (α-SMA; 1:200 dilution; Abcam).
Statistical Analysis
Statistical analysis was performed using Prism 5 software (GraphPad) or SPSS statistics 22 (IBM). Multiple comparisons were performed using one-way ANOVA followed by Tukey honestly significant difference (HSD) post hoc test. Single comparisons were carried out using two-tailed, unpaired Student t-tests and a P value of <0.05 was considered statistically significant. Unless otherwise stated, results were obtained from at least three independent biological replicates and carried out in duplicate or triplicate technical replicates.
RESULTS
Triple Conditional Deletion of Smad1, Smad5, and Smad4 using Amhr2-cre Results in Sterility
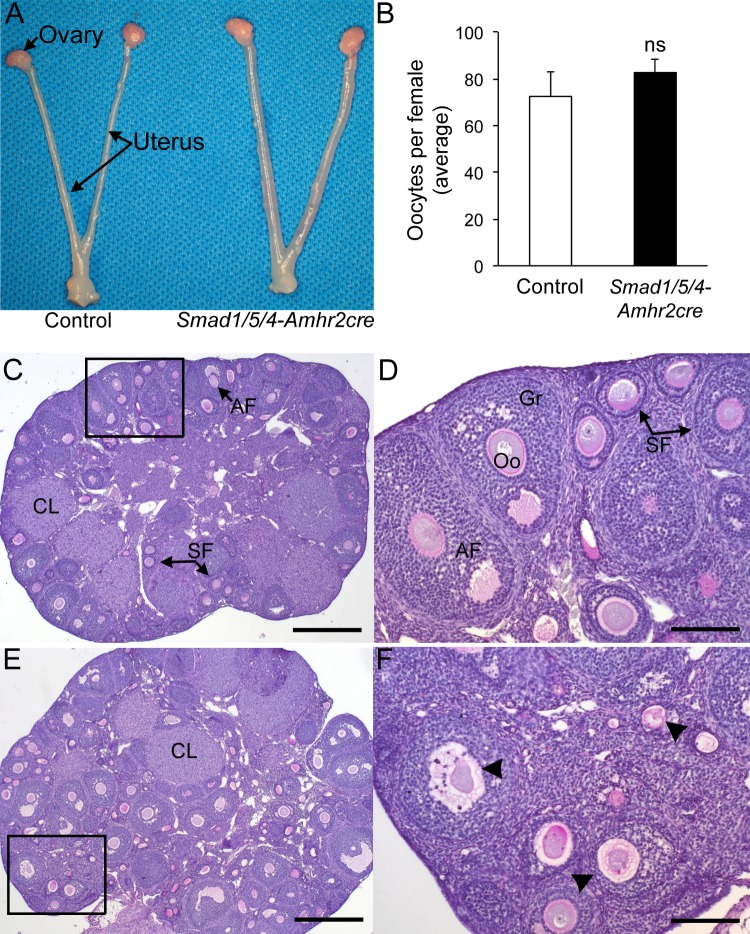
We previously reported the fecundity of conditional deletions for Smad1, Smad5, Smad4, Smad1/5, and Smad1/5/4 mouse models that used Amhr2-cre to delete the respectively floxed alleles [18, 27, 32]. A summary comparison of the fecundity of these models is given in Table 1. Single conditional deletion of Smad1 or Smad5 has no effect on female fertility [18, 23]. In contrast, conditional codeletion of Smad1 and Smad5 by using Amhr2-cre (Smad1/5-Amhr2-cre KO) causes reproductive defects and granulosa tumor development in female mice with full penetration. At younger ages (<4 mo old), female Smad1/5-Amhr2-cre KO mice are subfertile but are unable to produce litters after 4–6 mo of age concurrent with development of large ovarian granulosa cell tumors [18] (Table 1). Young adult Smad4-Amhr2-cre KO mice are subfertile (Table 1), but by 4–6 mo of age, approximately half of the Smad4-Amhr2-cre KO females are infertile [27]. In contrast to these mouse models, we found that triple deletion of Smad1, Smad5, and Smad4 (Smad1/5/4-Amhr2-cre KO) caused sterility (Table 1) [24]. Female Smad1/5/4-Amhr2-cre KO mice did not produce any litters in a 6-mo breeding trial that began at sexual maturity (6 wk of age) [32], but the cause of the infertility was unknown. Tumor development initiates in a similar time frame in Smad1/5/4-Amhr2-cre KO mice, but the tumors grow more slowly, do not metastasize, and have increased apoptosis [24]. Gross morphological examination of reproductive tracts from 6-wk-old female control and Smad1/5/4-Amhr2-cre KO mice appeared normal (Fig. 1A). In our initial characterization of the ovarian defects, we found a significant increase in the proportion of TUNEL-positive follicles in mutant ovaries [24]. However, even with the increased TUNEL-positive follicles, Smad1/5/4-Amhr2-cre KO ovaries contained follicles representing all follicular stages as well as corpora lutea (Fig. 1, C–F). The presence of corpora lutea suggested that despite their inability to produce live pups, Smad1/5/4-Amhr2-cre KO females were capable of ovulation (Fig. 1E). To test this, we pharmacologically ovulated sexually immature control (n = 3) and Smad1/5/4-Amhr2-cre KO females (n = 3) and collected oocytes from the ampulla of oviduct. We did not find statistical differences in the mean number of oocytes ovulated between genotypes of immature mice (Fig. 1B). Thus, the ovarian defects (i.e., increased follicular atresia) are not likely to fully explain the sterility of young adult (6-wk-old) Smad1/5/4-Amhr2-cre KO females. Therefore, we additionally analyzed the reproductive tracts of these mice.
FIG. 1.
Ovarian histology and ovulation in Smad1/5/4-Amhr2-cre KO female mice. A) Reproductive tract of Smad1flox/flox Smad5flox/flox Smad4flox/flox control (left) and Smad1flox/flox Smad5flox/flox Smad4flox/flox Amhr2cre/+ KO mice at 6 wk of age. No gross differences were detected. B) Pharmacologic superovulation of control and Smad1/5/4-Amhr2-cre KO mice at 3 wk compared to control. No statistical differences (ns) were detected in the mean number of oocytes collected in the oviducts of control; n = 3 each genotype. C) PAS stain of an ovary from a 12-wk-old Smad1flox/flox Smad5flox/flox Smad4flox/flox control mouse shows normal follicular development with multiple corpora lutea (CL) and growing follicles (SF, secondary follicles; AF, antral follicle). D) Inset shown in panel C shown at 100x magnification. E) PAS stain of an ovary from a 12-wk-old Smad1flox/flox Smad5flox/flox Smad4flox/flox Amhr2cre/+ KO female. All stages of follicles can be detected, including CLs. However, a large number of atretic follicles (arrowheads) are visible. F) Inset from panel E shown at 100x magnification. Previous quantification of the increased atretic follicles in the Smad1/5/4-Amhr2-cre KO mouse model has been published previously [24]. Bars = 200 μm (C and E) and 50 μm (D and F). Oo, oocyte; Gr, granulosa cell.
Amhr2-cre–Mediated Deletion of Smad1/5/4 Results in Oviduct Abnormalities
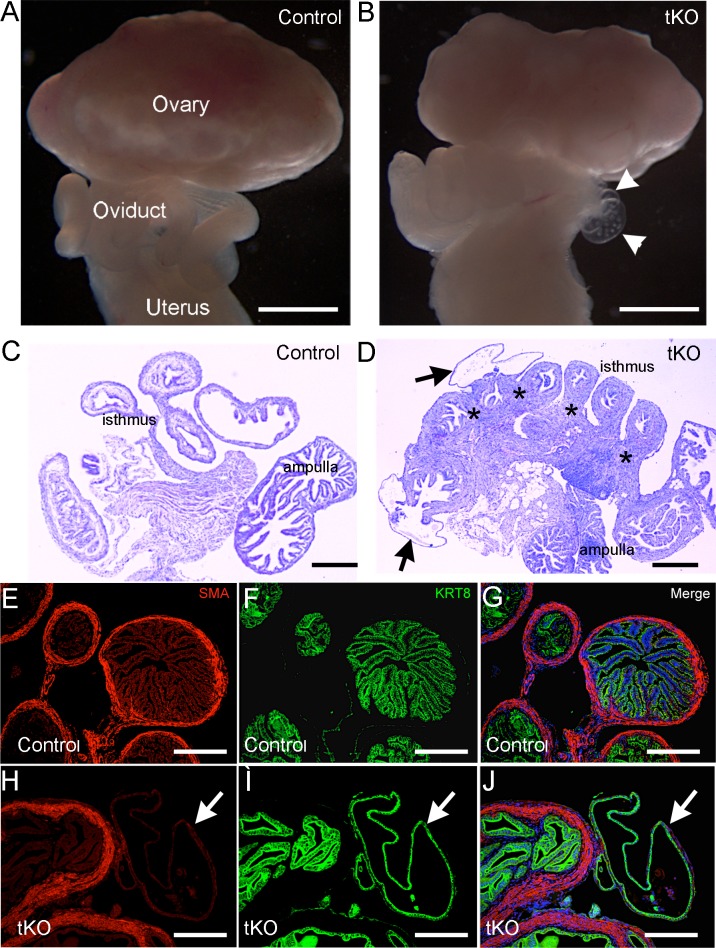
Apart from granulosa cells of growing follicles in the ovary, Amhr2-cre is known to express Cre recombinase in mesenchyme-derived tissues in the reproductive tract [25]. Deletion of genes such as Dicer, tuberous sclerosis 1 (Tsc1), and the TGFβ type I receptor gene Tgfbr1 using Amhr2-cre leads to defects in oviduct development and uterine function [16, 33–35]. To examine the structural integrity of the reproductive tract and determine if any oviductal defect contributes to the sterility in Smad1/5/4-Amhr2-cre KO mice, we performed a morphological and histological analysis of adult Smad1/5/4-Amhr2-cre KO reproductive tracts. In mice at 16 wk of age, we found obvious development of clear fluid-filled bilateral oviductal diverticula throughout the length of each Smad1/5/4-Amhr2-cre KO oviduct in all of the mutant females examined (Fig. 2). Oviductal diverticuli were more abundant near the uterotubal junction and often contained cellular debris and round structures that appeared to be oocytes or embryos (Fig. 2B, Supplemental Fig. S1). Neither Smad4-Amhr2-cre KO nor Smad1/5-Amhr2-cre KO females showed these diverticuli at any age (3 wk–1 yr old) (Table 1) [18, 27].
FIG. 2.
Oviduct abnormalities in Smad1/5/4-Amhr2-cre KO mice. Gross morphology of reproductive tracts from 16-wk-old control mice (A) and 16-wk-old Smad1/5/4-Amhr2-cre KO (tKO) mice (B). Smad1/5/4-Amhr2-cre KO mice display oviduct diverticula, indicated in B (arrowheads). An enlarged image of the diverticuli is shown in Supplemental Figure S1. C and D) Histology of oviducts from 16-wk-old mice showing an increased oviduct thickness and oviduct diverticula (arrows) in Smad1/5/4-Amhr2-cre KOs (D) compared to control (C). D) *Abnormal regions of thickness. E–J) Immunofluorescence of smooth muscle actin (α-SMA) and cytokeratin 8 (KRT8) in the oviducts of 16-wk-old control (E–G) and Smad1/5/4-Amhr2-cre (H–J) mice. White arrowheads indicate KRT8+ oviduct diverticula in Smad1/5/4-Amhr2-cre KO mice (H–J) devoid of α-SMA immunoreactivity. Bars = 500 μm (A and B) and 200 μm (C–J).
Histological examination of oviducts from 16-wk-old Smad1/5/4-Amhr2-cre KO mice revealed abnormalities in tissue morphology compared with controls. The stromal and muscle compartments were disorganized and had an abnormally thickened appearance in all oviduct regions of the Smad1/5/4-Amhr2-cre KO mice compared to those in control (Fig. 2, C and D, Supplemental Fig. S2). Often, histology specimens from random cycling female Smad1/5/4-Amhr2-cre KO mice showed oocytes/embryos within the lumen, which were only rarely seen in control samples (Supplemental Fig. S2). The thickened areas of the oviduct of Smad1/5/4-Amhr2-cre KO mice appeared to consist of mainly additional smooth muscle tissue, and many areas did not have the normal, clearly defined outer and inner smooth muscle layers (Fig. 2, D and H, Supplemental Fig. S2). Immunostaining of smooth muscle cell marker (α-smooth muscle actin [α-SMA]) demonstrated regions of increased thickness in smooth muscle layers within the oviductal ampulla (Fig. 2H). We also examined the epithelial cell marker KRT8; KRT8 staining demonstrated that oviductal diverticula in Smad1/5/4-Amhr2-cre KO oviducts were characterized by a single layer of flattened epithelium (Fig. 2, I and J, arrow), devoid of the smooth muscle layer.
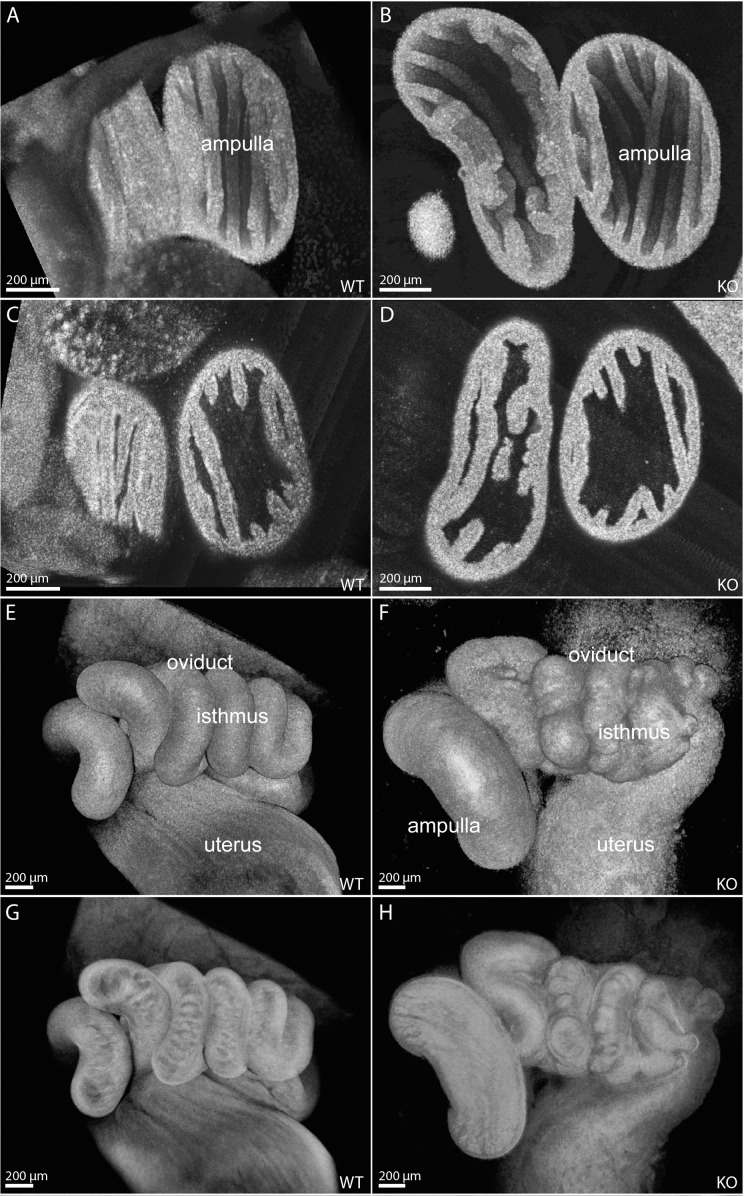
For detailed volumetric structural analysis of the oviduct, we acquired 3D visualizations using OCT. Figure 3 shows representative cross-sections through the structural data sets of the oviduct acquired from 6-wk-old mice, prior to the development of large diverticuli. The mouse oviduct consists of four segments: the infundibulum, ampulla, isthmus, and uterotubal junction. The structure of the ampulla in Smad1/5/4-Amhr2-cre KO oviducts appeared normal. Specifically, the longitudinal epithelial folds in the Smad1/5/4-Amhr2-cre KO ampulla lumen exhibited patterns and density similar to those in controls (Fig. 3, A and B); the thickness of the ampulla wall and of the epithelial folds also appeared normal (Fig. 3, C and D). However, the isthmus of the Smad1/5/4-Amhr2-cre KO oviducts displayed an abnormal phenotype. Figure 3, E and F, shows surface views of the isthmus in controls and Smad1/5/4-Amhr2-cre KO oviducts. In contrast to the smooth appearance of the isthmus surface in control mice (Fig. 3E), the Smad1/5/4-Amhr2-cre KO oviducts appeared rough and uneven (Fig. 3F). Figure 3, G and H, shows corresponding semitransparent view of the same reconstruction. Smad1/5/4-Amhr2-cre KO mice displayed oviduct wall thickening, disorganized luminal folds, and a lack of transverse folding (Fig. 3H) in comparison to those of the control (Fig. 3G). However, the overall coiling pattern of the Smad1/5/4-Amhr2-cre KO oviducts appeared normal with some variation seen in both wild-type and Smad1/5/4-Amhr2-cre KO samples (Fig. 3, E–H).
FIG. 3.
Structural oviduct abnormalities in 6-wk-old Smad1/5/4-Amhr2-cre KO mice visualized with OCT. Three-dimensional reconstructions visualizing the luminal surface of control (A) and Smad1/5/4-Amhr2-cre KO (B) female oviduct ampulla display similar levels of epithelial folding and density. Cross-sectional views through the reconstructions show comparable ampulla wall thicknesses in control (C) and Smad1/5/4-Amhr2-cre KO (D) female oviducts. Surface renderings of volumetric reconstructions of control (E) and Smad1/5/4-Amhr2-cre KO (F) female oviduct isthmus show an abnormal, uneven surface in the mutant. Semitransparent renderings of the corresponding data sets reveal abnormal disorganized luminal folding structures in the isthmus of the mutants (H) in contrast to those in controls (G).
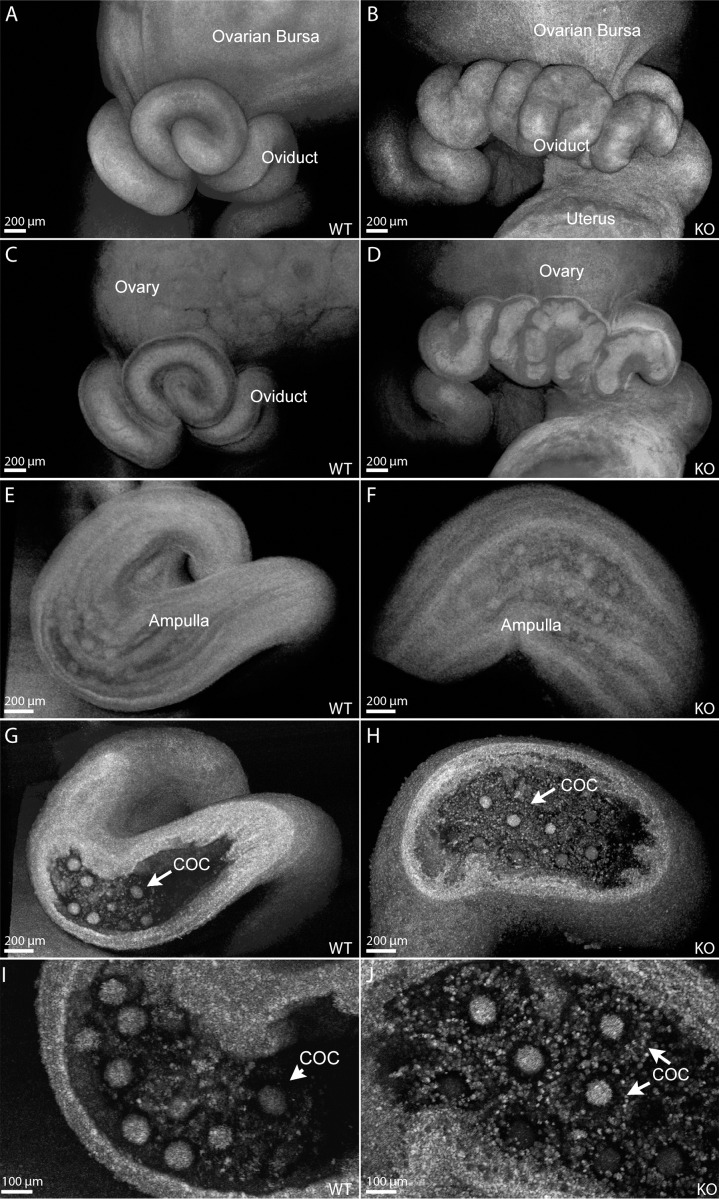
To examine oviduct morphology after ovulation, we acquired OCT visualizations from pharmacologically ovulated 4-wk-old females 18 h after the ovulatory stimulus (injection of human chorionic gonadotropin) (Fig. 4). We observed the same defective appearance of the Smad1/5/4-Amhr2-cre KO oviduct coils at this stage although to a lower extent than at 6 wk of age, which is clearly visible in the surface rendering view of the data (Fig. 4, A and B). Semitransparent renderings of the OCT data sets show the smooth inner lumen of the oviduct in control mice (Fig. 4C) and disorganized lumen in Smad1/5/4-Amhr2-cre KO oviducts (Fig. 4D). Figure 4, E–H, shows structural analysis of the ampulla region. Semitransparent view (Fig. 4, E and F) shows similar epithelial folding patterns and COCs visible through the ampulla wall in control (Fig. 4E) and Smad1/5/4-Amhr2-cre (Fig. 4F) KO mice. In cross-sectional views of the same data sets, oocytes surrounded by cumulus cells are clearly visible in the ampulla of both the control and the Smad1/5/4-Amhr2-cre KO females; COCs appeared morphologically similar and went on to cumulus expansion in both genotypes (Fig. 4, G–J).
FIG. 4.
Structural oviduct abnormalities in pharmacologically superovulated 4-wk-old Smad1/5/4-Amhr2-cre KO mice visualized with OCT. Surface renderings of volumetric reconstructions in control (A) and Smad1/5/4-Amhr2-cre KO (B) 4-wk-old female oviduct isthmus show an uneven appearance to a lower extent than at 6 wk of age. Semitransparent renderings demonstrate smooth luminal surfaces of the isthmus in control animals (C), which are disorganized in the mutants (D). Semitransparent renderings of the ampulla in control (E) and Smad1/5/4-Amhr2-cre KO (F) females show normal overall structure and longitudinal epithelial folding patterns. Cross-sectional views through the corresponding reconstructions reveal COCs in the ampulla of the mutants (H), which appeared similar to those in controls (G). Higher magnifications of G and H are shown in I and J, respectively. Scale bars are indicated in the left corner of each panel.
Loss of Smad1/5/4 Leads to Altered Expression of Genes Essential for Oviduct Development and Differentiation
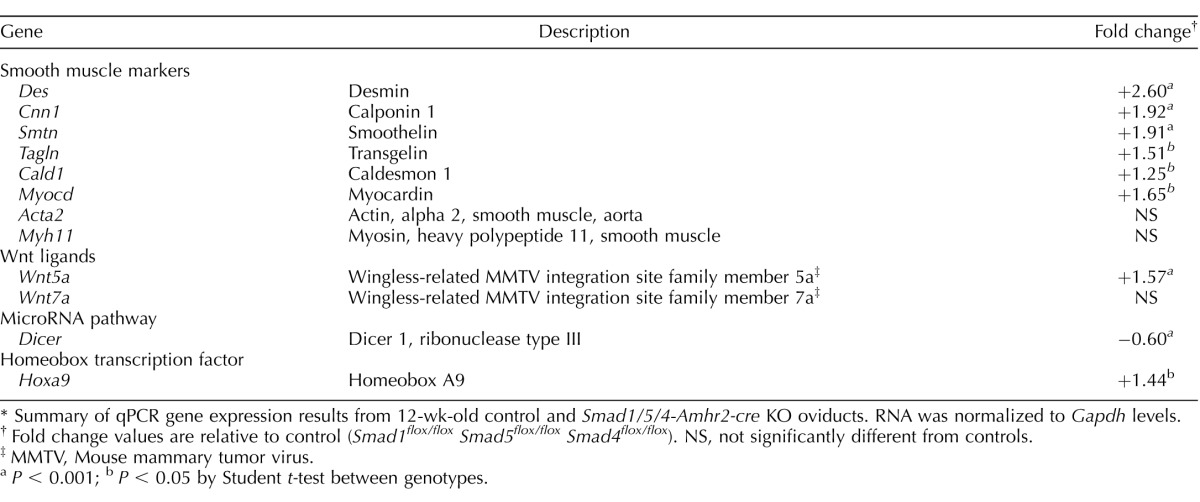
The molecular processes of oviduct development and differentiation are poorly understood. Genetic deletion studies in mice implicate multiple developmental signaling pathways, such as the TGFβ [16], WNT/β-catenin [36], and micro-RNA biogenesis [33] pathways in oviduct formation. To examine whether loss of Smad1/5/4 affected the gene expression pattern in the oviduct, we compared mRNA levels of candidate genes in 4- to 5-wk-old control oviducts with those in 4- to 5-wk-old Smad1/5/4-Amhr2-cre KO mice (Table 2). Of these genes, we observed significantly reduced mRNA levels of Dicer in Smad1/5/4-Amhr2-cre KO oviducts compared to those in controls. Similar to the molecular changes observed in Dicer conditional knockout (cKO) oviduct, we also found significant upregulation of wingless family member 5a (Wnt5a) and homeobox transcription factor 9 (Hoxa9). Wnt5a and the homeobox genes are known to play a pivotal role in patterning of the posterior Müllerian-derived structures [37–39]. However, mouse models with increased levels of Wnt5a, such as those with Müllerian duct mesenchymal stabilization of β-catenin (Ctnnb1) [40] or smoothened (Smo) [41] in the oviduct, displayed uncoiled oviducts and altered radial patterning, unlike the Smad1/5/4-Amhr2-cre KO line.
TABLE 2.
Gene expression changes in Smad1/5/4-Amhr2-cre KO oviducts.*
Summary of qPCR gene expression results from 12-wk-old control and Smad1/5/4-Amhr2-cre KO oviducts. RNA was normalized to Gapdh levels.
Fold change values are relative to control (Smad1flox/flox Smad5flox/flox Smad4flox/flox). NS, not significantly different from controls.
MMTV, Mouse mammary tumor virus.
P < 0.001; b P < 0.05 by Student t-test between genotypes.
As Amhr2 is expressed in the mesenchyme of the Müllerian duct, which gives rise to the smooth muscle of the oviduct and uterus, we further examined whether loss of Smad1/5/4 affects the expression of genes important for smooth muscle development and differentiation (Table 2). Smad1/5/4-Amhr2-cre KO oviducts showed significantly increased expression of the smooth muscle markers desmin (Des) and transgelin (Tagln), the actin-linked regulatory protein calponin 1 (Cnn1), and the cytoskeleton protein smoothelin (Smtn). Caldesmon 1 (Cald1), a protein that interacts with actin, myosin, tropomyosin, and calmodulin, was also significantly upregulated in Smad1/5/4-Amhr2-cre KO oviducts compared to that in controls. Furthermore, the mRNA level of myocardin (Myocd), a smooth muscle and cardiac muscle-specific transcriptional coactivator and master regulator of smooth muscle contractile gene expression [42, 43] was increased in the Smad1/5/4-Amhr2-cre KO oviducts.
Loss of Smad1/5/4 Disrupts Epithelium and Smooth Muscle Development in the Uterus
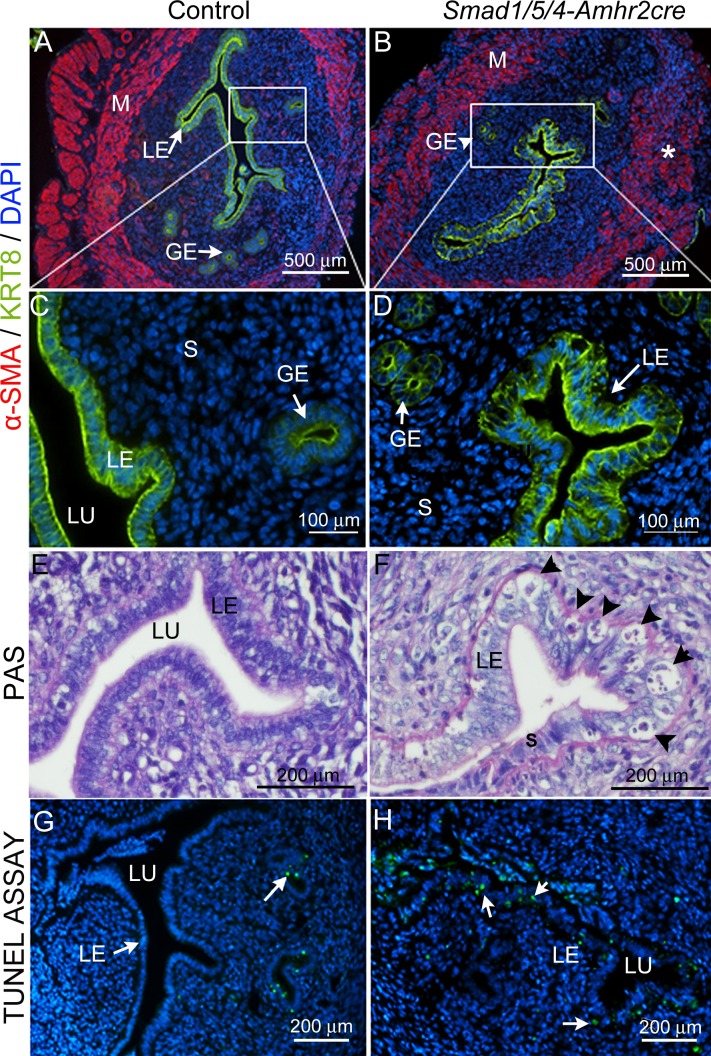
The mouse uterus is composed of two major compartments, the endometrium and myometrium. The endometrial compartment consists of luminal and glandular epithelial cells as well as supporting stromal cells, whereas the myometrium consists of smooth muscle layers. Because the Amhr2 promoter specifically directs expression of Cre recombinase in the stroma and myometrium but not in the epithelium, we next investigated the effect of Smad1/5/4 loss in the uterus. We used co-immunostaining for α-SMA to delineate the muscle layers of the uterus [44] and KRT8 for the epithelium (Fig. 5). The control uteri showed two well-organized layers of longitudinal (outside) and circular (inside) smooth muscles (Fig. 5A, red). In contrast, the Smad1/5/4-Amhr2-cre KO showed a disorganized architecture with variable thickness and an indistinct boundary between the stromal and myometrial layers (Fig. 5B). In addition, in contrast to the simple columnar luminal epithelium of the control uterus (Fig. 5, A and C), the uteri of Smad1/5/4-Amhr2-cre KO female mice had a disrupted luminal epithelium with an irregular pattern of cells and, at times, a hyperplastic phenotype (Fig. 5, D and F). Epithelial cells also showed a high degree of apoptosis, as indicated by histology and TUNEL assay (Fig. 5, F and H).
FIG. 5.
Loss of Smad1/5/4 disrupted smooth muscle development in uterus is shown and causes epithelial hyperplasia and apoptosis. Immunostaining of control (A and C) and Smad1/5/4-Amhr2-cre KO (B and D) in 6-wk-old uterus with the myometrium marker α-SMA (red) and the epithelial marker KRT8 (green). Insets in A and B are shown at 160X magnification for the luminal epithelium in C (control) and D (Smad1/5/4-Amhr2-cre KO). Compared to those in control, Smad1/5/4-Amhr2-cre KO uteri have disorganized myometrium (B*) and large vacuolated apoptotic cells in luminal epithelium (D and F). Six-wk-old sections of uteri were stained with PAS to show the histology of the uterine epithelium in control (E) and Smad1/5/4-Amhr2-cre knockout (F) mice. Vacuolated apoptotic cells in the luminal epithelium are indicated by arrowheads in F. G and H) TUNEL analysis for apoptotic cells (green) in uteri of 6-wk-old control (G) and Smad1/5/4-Amhr2-cre (H) KO mice. Nuclei are counterstained with DAPI (blue). Arrows indicate TUNEL-positive cells (green). GE, glandular epithelium; LE, luminal epithelium; M, myometrium; S, stroma; LU, lumen.
Loss of Smad1/5/4 Causes Defective Implantation and Loss of Embryos During Pregnancy
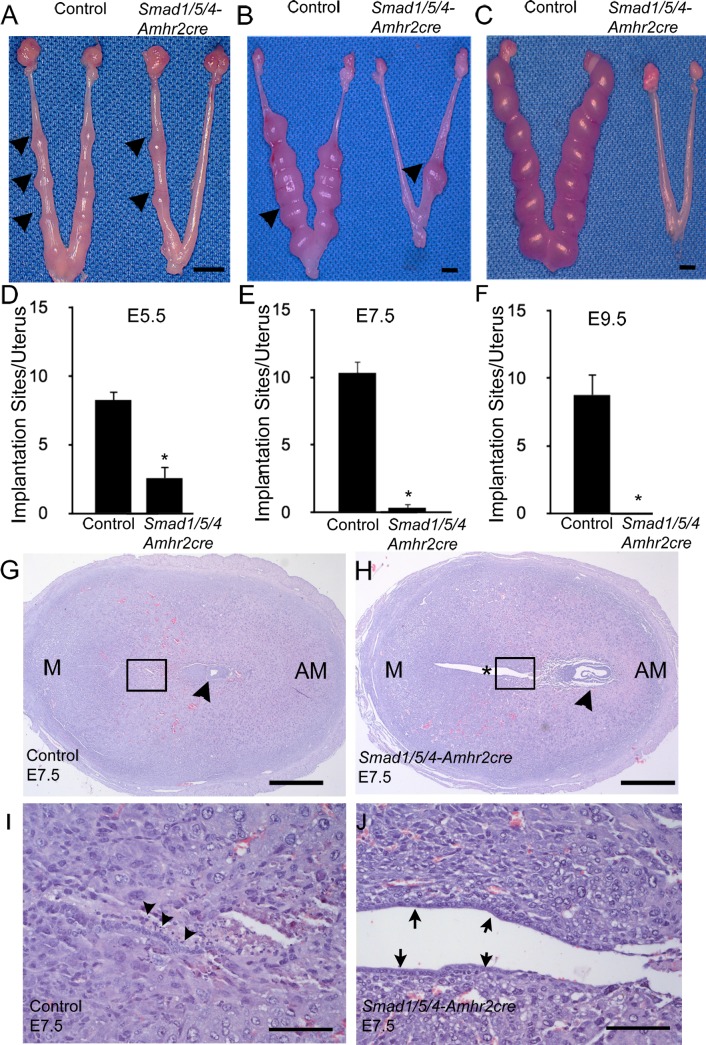
Because the endometrial stromal component of the uterus is derived from Amhr2-expressing Müllerian duct mesenchyme, we next investigated whether loss of Smad1/5/4 in endometrial stroma could lead to defective implantation. Successful implantation requires a series of well-coordinated physical-physiological interactions among the blastocyst and the trophectoderm and various endometrial cell types, including the luminal and glandular epithelium and stromal cells. In mice, the process of implantation is initiated by blastocyst attachment to the uterine luminal epithelium, which occurs on Days 4–5 of pregnancy. To ascertain the stage-specific failure of pregnancy, 8-wk-old control and Smad1/5/4-Amhr2-cre KO females were mated with wild-type males, and natural pregnancy was examined on different embryonic days. Fewer implantation sites were found at E4.5, E5.5, and E7.5 (Fig. 6, A, B, D, and E, Supplemental Figs. S3 and S4), which also appeared smaller in size (Fig. 6, A, B, D, and E, Supplemental Fig. S4). By E9.5, no implantations could be detected in any Smad1/5/4-Amhr2-cre KO uterus (Fig. 6, C and F).
FIG. 6.
Smad1/5/4-Amhr2-cre mutants have implantation failure and defective embryo attachment. A–F) Mice were euthanized at E5.5, E7.5, and E9.5, and the numbers of implantation sites were counted in control and Smad1/5/4-Amhr2-cre KO mice. Arrowheads indicate implantation sites. Data are represented as means ± SEM; at least 3 mice were analyzed per genotype. Two-tailed unpaired Student t test was used to compare the control and Smad1/5/4-Amhr2-cre KO mice (*P < 0.05). G and H) H&E staining of control and Smad1/5/4-Amhr2-cre KO implantation sites at E7.5. G) Control embryo (arrowhead) with well-closed lumen and differentiated surrounding stroma. Insets from G and H are shown in I and J, respectively. H) Smad1/5/4-Amhr2-cre KO embryo (arrowhead) appeared to be degenerating, the uterus showed an *open lumen, and stromal cells have not fully differentiated. I and J) 125x magnification of insets shown in G and H, respectively. The luminal epithelium in control uteri has fully closed, and cells show signs of apoptosis (arrowheads). J). The luminal epithelium in knockout uteri remains visible (arrows). Bars = 5 mm (A–C), 400 μm (G and H), and 100 μm (I and J).
To further characterize the reason for pregnancy failure, we collected uteri from pregnant females at E5.5 and E7.5 and examined the histology of the implantation sites. Control mice exhibited well-attached embryos and induction of a decidual response at E5.5 (Supplemental Fig. S4, A and B). Unlike control mice at E5.5, we observed that Smad1/5/4-Amhr2-cre KO mice failed to assemble a closed uterine lumen, which is essential for successful embryo attachment and critical for establishing intimate contact of the blastocyst and the uterine epithelium that is required for embryo survival (Supplemental Fig. S4, A and B). In control mice by E7.5, the lumen was not visible, and the luminal epithelium had undergone regression (Fig. 6I). In the Smad1/5/4-Amhr2-cre KO, the lumen remained open, and the luminal epithelium was still apparent as a single, organized layer (Fig. 6, G–J). These results suggested that Smad1/5/4-Amhr2-cre KO mice have defects in pregnancy that may be caused, in part, by the failure of closure of the uterine lumen.
Amhr2-cre–Mediated Deletion of Smad1/5/4 in Uterus Compromises Decidualization and Ptgs2 Expression
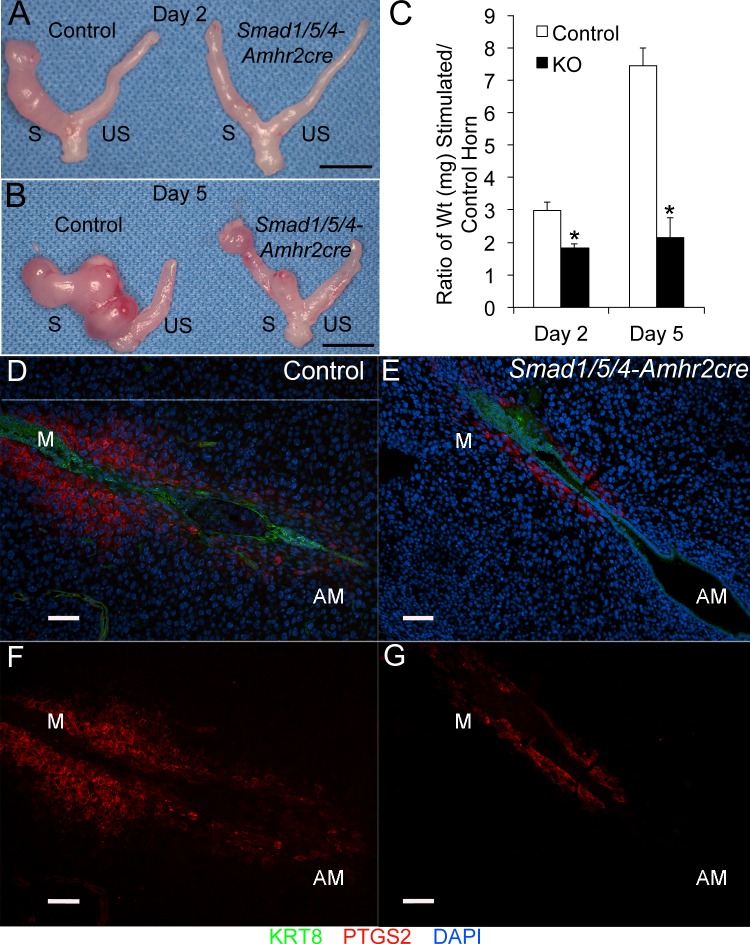
In response to implantation, stromal cells surrounding the implanting embryo undergo extensive proliferation and subsequent differentiation into polyploid decidual cells in a process termed decidualization [4]. Several studies provided direct evidence that a fully developed decidua is mandatory for providing nutrition to the developing embryo and acting as a barrier that prevents uncontrolled trophoblast invasion and protecting the embryo from maternal rejection prior to the formation of functional placenta [4]. We examined decidualization in timed-mated pregnancies by isolating maternal decidua from E6.5 implantation sites in Smad1/5/4-Amhr2-cre KO and control uteri. In Smad1/5/4-Amhr2-cre KO mice, the stroma appeared to only partially decidualize around implanted embryos in comparison to control embryos (Supplemental Fig. S4, C and D). We therefore tested whether Smad1/5/4-Amhr2-cre KO mice were able to undergo decidualization in a well-characterized artificial decidualization assay [28]. Ovariectomized control and Smad1/5/4-Amhr2-cre KO mice were treated with E2 and P4 to induce receptivity and synchronize the estrous cycle. Embryo implantation was mimicked by physically traumatizing the entire length of uterine endometrium of the left uterine horn. The right horn was left unstimulated as a control. Consistent with the reduced size of implantation sites during natural pregnancy, we observed that Smad1/5/4-Amhr2-cre KO females failed to respond to the decidual stimulus 2 days after the trauma compared to control littermates (Fig. 7A). At 4 days, the difference in size of the stimulated to unstimulated horn of Smad1/5/4-Amhr2-cre KO mice to control mice was even greater (Fig. 7B). A quantification of the decidual response by measurement of uterine wet weight showed the stimulated horn of the Smad1/5/4-Amhr2-cre KO mice failed to increase in size in comparison to that in littermate controls (Fig. 7C). Furthermore, we examined alkaline phosphatase activity to assess stromal cell differentiation during decidualization [45]. Compared to controls, which had robust alkaline phosphatase staining, the Smad1/5/4-Amhr2-cre KO showed no reaction (Supplemental Fig. S5). These results confirm that, even when injected with appropriate hormones and given an initiating decidual cue, the uteri of Smad1/5/4-Amhr2-cre KO mice are not able to appropriately decidualize.
FIG. 7.
Smad1/5/4-Amhr2-cre KO mice show defective decidualization and loss of PTGS2. A–C) Uterine decidualization was artificially induced in hormone-stimulated ovariectomized females by traumatizing the antimesometrial luminal epithelium with a needle in control and Smad1/5/4-Amhr2-cre KO uteri. Day 2 (A) and Day 5 (B) uteri collected after the trauma are shown. The stimulated horn is labeled S, and the unstimulated horn is labeled US. C) Ratio of uterine wet weight of stimulated to unstimulated horns collected 2 days and 5 days after the trauma. Smad1/5/4-Amhr2-cre KO females (Day 2, n = 3; Day 5, n = 4) did not show a response to the decidual stimulus compared to controls (Day 2, n = 3; Day 5, n = 3). *Statistical significance, P < 0.05. D–G) Smad1/5/4-Amhr2-cre KO females demonstrate loss of PTGS2 production during decidualization in natural pregnancies. Immunoreactivity of PTGS2 and KRT8 were determined by immunofluorescence analysis at E5.5. Smad1/5/4-Amhr2-cre mutants (E and G) display decreased PTGS2 (D and F) in the decidual cells at the mesometrial pole compared to control mice (A and C). Bars = 1 cm (A and B) and 100 μm (D–G). AM, antimesometrium; M, mesometrium.
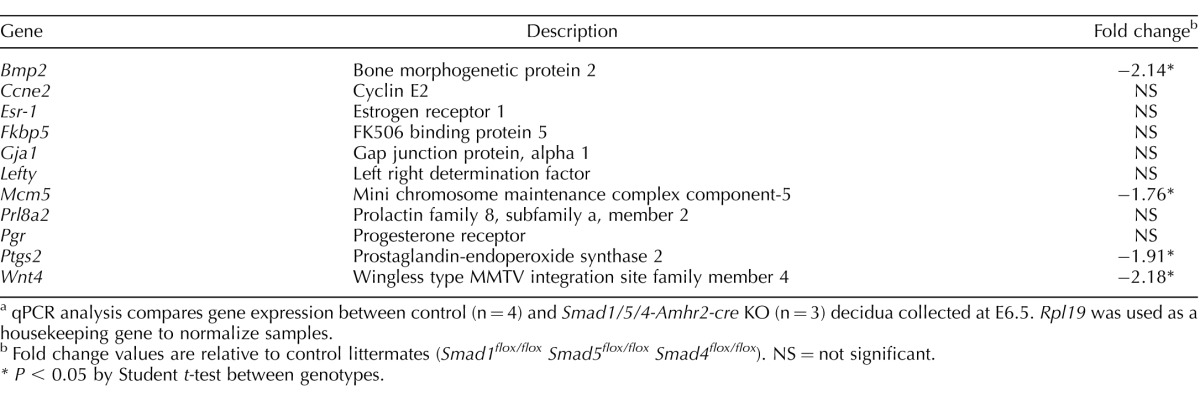
Because of defects in both the natural and artificial decidualization experiments, we analyzed expression of a number of key decidual markers in total RNA extracts prepared from decidua collected at E6.5 from natural pregnancies (Table 3). We did not observe significant changes in expression of cyclin E2 (Ccne2), FK506 binding protein 5 (Fkbp5), estrogen receptor alpha (Esr1), or Pgr, genes known to change expression during decidualization. In contrast, we found significantly decreased expression of Bmp2 and Wnt4, two well-known markers whose expression is greatly induced upon decidualization. We also found a significant reduction in Ptgs2, a marker of implantation and decidualization. Immunofluorescence analysis of PTGS2 demonstrated a reduction in immunoreactivity in Smad1/5/4-Amhr2-cre KO decidual cells at the mesometrial pole at E5.5 in KO mice compared to that in the controls (Fig. 7, D–G). These data indicated that Smad1/5/4-Amhr2-cre KO uteri were unable to undergo normal decidualization at least in part due to decreased expression of known regulators of decidualization including Bmp2, Wnt4, and Ptgs2.
TABLE 3.
Gene expression changes in Smad1/5/4-Amhr2-cre KO decidua collected at E6.5.a
qPCR analysis compares gene expression between control (n = 4) and Smad1/5/4-Amhr2-cre KO (n = 3) decidua collected at E6.5. Rpl19 was used as a housekeeping gene to normalize samples.
Fold change values are relative to control littermates (Smad1flox/flox Smad5flox/flox Smad4flox/flox). NS = not significant.
P < 0.05 by Student t-test between genotypes.
DISCUSSION
The SMAD transcription factors play key developmental and homeostatic roles in multiple tissues. We analyzed the function of the receptor-regulated SMADs SMAD1 and SMAD5, which signal downstream of multiple TGFβ superfamily type I receptors (Alk1, Alk2, Alk3, Alk6, Alk7) and their trimerization partner SMAD4, using the well-characterized Amhr2-cre mouse model [26]. The Amhr2-cre, a knock-in of Cre recombinase into the Amhr2 locus, deletes the loxP-flanked Smad genes in multiple tissues, including the ovary, oviduct, and uterus, and has been a well-established model for deletion in the granulosa cells of the ovary and mesenchymal derivatives of the Müllerian duct that give rise to the cells of the smooth muscle layers of the oviduct and uterus and the uterine endometrial stroma [16, 18, 24, 26, 33, 46, 47]. A number of studies have defined roles for the TGFβ superfamily receptors Bmpr2, Alk2, Alk3, Alk4, and Alk5 during pregnancy by generating cKOs in the uterine epithelium by using Pgr-cre [14, 15, 48–50]. To our knowledge, this is the first cKO for any Smad gene to show a phenotype in the uterus or the oviduct. We previously used the triple Smad1/5/4-Amhr2-cre KO mutant mouse model to compare the phenotypic differences in the double Smad1/5-Amhr2-cre KO mutant mice on granulosa cell tumor development and showed that loss of Smad4 from Smad1/5-Amhr2-cre limits granulosa cell tumor progression and metastasis by increasing granulosa cell and tumor cell apoptosis [24]. In this study, we determined the uncharacterized effects of Smad1/5/4 deletion on female fertility and the female reproductive tract. Unlike the single or double conditional KOs (Table 1), the triple cKO of Smad1/5/4-Amhr2-cre demonstrated sterility and severe developmental defects in the oviduct and uterus. The oviduct defects included disrupted organization and local expansion of the smooth muscle layers of the oviduct, which could lead to impaired oviduct muscular contractions and contribute to the embryo retention phenotype seen in the diverticula, similar to other mouse models [51]. There are also defects found in the uterine smooth muscle development and luminal epithelium of nonpregnant Smad1/5/4-Amhr2-cre KO females, including epithelial hyperplasia. Because Amhr2-cre does not express Cre recombinase in the uterine epithelium, the hyperplasia likely resulted from abnormal paracrine signaling from the uterine stroma, where Cre recombinase is expressed [25, 26]. This hyperplasia may additionally disrupt attachment of the blastocyst during implantation, further contributing to the sterility of the Smad1/5/4-Amhr2-cre KO females. Only a small number of embryos were found in Smad1/5/4-Amhr2-cre KO uteri at E4.5 and E5.5, but no implantations sites were found past mid-gestation. This may be due to a variety of factors in different tissues. First, the embryo quality may be poor perhaps due to improper oocyte development within the Smad1/5/4-Amhr2-cre KO follicles. Second, the environment of the oviduct may disrupt the timing or quality of development. Third, there is incomplete uterine closure and defective decidualization through loss of key factors, including Bmp2, Wnt4, and Ptgs2. Thus, there is probably not a primary defect but compound defects in multiple tissues that lead to the sterility in Smad1/5/4-Amhr2-cre KO females.
In this study, we pioneered the application of OCT for structural phenotyping of the oviduct in mouse mutants. This noninvasive 3D optical imaging technique is based on analysis of interferometry and provides a resolution of approximately 2–10 μm (less than the size of a cell) at a depth of a few millimeters. Because OCT relies on natural tissue contrast and does not require application of contrast agents, it is compatible with live imaging as performed in our study. Recently, we developed methods for structural analysis of mouse reproductive organs in vitro and in vivo and demonstrated that this method can be used for high-resolution volumetric visualization of the reproductive tract, oviduct lumen, and preimplantation embryos as well as for functional analysis of cilia dynamics [52–54]. Using this technique, we were able to clearly visualize the full extent of the defective development of the Smad1/5/4-Amhr2-cre KO model that was not immediately obvious by gross examination or by standard histology. The coiled isthmus at 3 wk of age displayed a rough surface and a disorganized epithelium but no large diverticuli. This phenotype was more severe at 6 wk of age, with a large number of bulges and pockets throughout the isthmus and a highly disorganized lumen. In contrast to luminal longitudinal folds wrapped in a thin layer of smooth muscle as seen in the ampulla, the folding pattern in the healthy isthmus transitions to nodules of folded epithelium arranged in longitudinal rows in the anterior isthmus, followed by ring-like transverse folds in the posterior isthmus and is surrounded by a much thicker smooth muscle layer. These folding patterns are disrupted in the mutant isthmus. The phenotype is more pronounced in the isthmus than the ampulla, possibly due to the known increased smooth muscle content of the isthmus compared to the ampulla. To the best of our knowledge, this is the first time OCT imaging has been implemented for structural phenotyping of the female reproductive tract in a novel mouse mutant. It provides valuable volumetric structural information, which is not accessible through histological analysis or other routinely used imaging methods in reproductive research. OCT imaging has the benefit of being a fast, noninvasive method that does not require any contrast agents or vital reporters and is compatible with live imaging. These features make this imaging approach a unique phenotyping tool for structural defects of reproductive organs with potential for longitudinal studies. Thus OCT will be of added benefit to investigators studying multiple aspects of the ovary and reproductive tract.
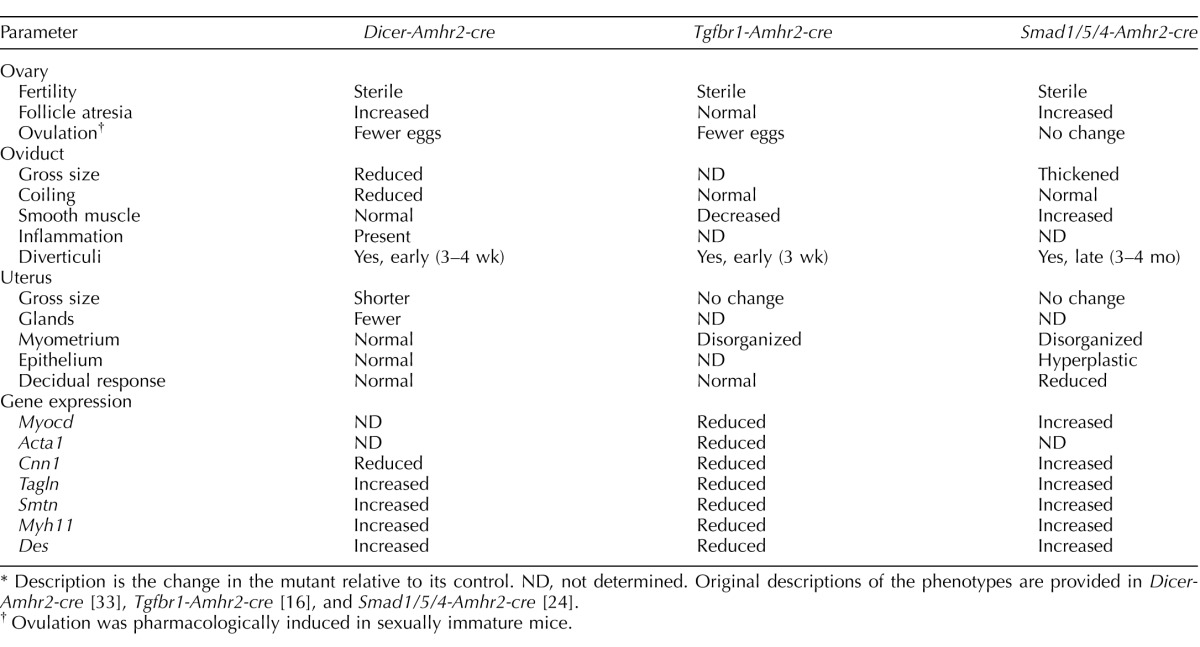
A previous mouse model that deleted the TGFβ type I receptor, Tgfbr1, using Amhr2-cre also has a sterility phenotype with defects in the oviduct and uterus, as outlined in Table 4. Surprisingly, neither the Smad4-Amhr2-cre KO nor the Smad2/3-Amhr2-cre KO mouse model has been reported to show these defects [22, 27]. Adult (16-wk-old) Smad1/5/4-Amhr2-cre KO oviducts also developed diverticuli within the oviduct, although unlike other models (Table 4), there was no evidence of overtly large oviductal diverticuli at 3–6 wk of age by histology or by OCT analysis. This would suggest that the oviductal diverticuli are not the primary cause of the sterility in younger Smad1/5/4-Amhr2-cre KO females. Despite the occurrence of an oviductal diverticuli phenotype in these three mouse models, there are distinct differences between them, including in gene expression changes and effects on the smooth muscle layers (Table 4). Loss of Smad1, Smad5, and Smad4 should limit all TGFβ superfamily signaling that requires SMAD4 as well as abrogate signaling that is dependent upon SMAD1 and SMAD5, such as that for the BMPs. Because loss of Smad1/5, loss of Smad2/3, or loss of Smad4 alone does not have visible or obvious defects in oviductal or uterine smooth muscle layers, it is possible that multiple SMAD pathways may be redundant with respect to its development.
TABLE 4.
Summary of phenotypes of Amhr2-cre mouse models that develop oviduct diverticuli.*
Embryo transit through the oviduct requires at least three related mechanisms, including ciliary motion, muscle contraction, and tubal fluid. The most obvious histologic defect in the Smad1/5/4-Amhr2-cre KO oviduct was disrupted development of the muscle layer, the myosalpinx. Little is known about the molecular mechanisms that control smooth muscle layer development of the oviduct, but both the myosalpinx and the myometrium appear to differentiate after birth [55–57]. In the oviduct, the myosalpinx varies in thickness, thicker in the oviduct isthmus than the ampulla [55]. In the Smad1/5/4-Amhr2-cre KO oviduct, the smooth muscle layer appeared thickened, particularly in the oviduct isthmus, and Smad1/5/4-Amhr2-cre KO oviduct samples abnormally overexpressed a number of smooth muscle markers, such as Des, Tagln, and Myocd. Thus, loss of SMAD signaling may increase the number of smooth muscle cells, as indicated by the increase in Myocd expression and/or their disorganized growth. However, Smad1/5/4-Amhr2-cre KO females did not develop uterine tumors, even though some of the KOs were housed for well over a year [24]. The changes to the smooth muscle layer prior to overt diverticuli formation could alter the kinetics of oocyte/embryo transit and contribute to the sterility phenotype of the Smad1/5/4-Amhr2-cre KO mouse. In support of this, we often found oocytes within most histologic specimens of the Smad1/5/4-Amhr2-cre KO oviduct, in contrast to the control. Thus, the Smad1/5/4-Amhr2-cre KO mutant mouse model would be an excellent model for future functional experiments to determine how structural alterations in the muscle layer of the oviduct can specifically affect oocyte, embryo, or sperm transit through the oviduct and potentially impact fertilization. In addition, it would be interesting to determine if the changes in stromal cells will alter epithelial cell function, for instance, possibly affecting ciliary beating.
While the reduction in the number of implantation sites at E5.5 may be a result of improper transit through the oviducts due to defective structure of the oviductal smooth muscle layer, some embryos clearly transit the oviduct but fail to develop normally as all implantation sites are fully lost by E9.5. Both implantation and decidualization appear to be affected by the loss of Smad1/5/4. During implantation, in a process called apposition, the embryonic trophectoderm becomes closely juxtaposed to the luminal epithelium, followed by closure of the uterine lumen. In the Smad1/5/4-Amhr2-cre KO implantation sites, the lumen only partially closes. This may be due to loss of Ptgs2 expression in decidualizing cells at the mesometrial pole of implantation sites during early pregnancy (E5.5). Furthermore, we found that gene expression of Bmp2 and Wnt4, key regulators of decidualization, were significantly decreased in Smad1/5/4-Amhr2cre KO females (E6.5). Previous studies using an in vitro decidualization system imply that BMP2-mediated SMAD1/5/8 signaling is important for the differentiation of stromal cells into decidual cells during pregnancy in mice and humans [58, 59]. Multiple KO mouse models have also established that Bmp2 induces expression of both Wnt4 and Ptgs2 during decidualization [13, 60]. Thus, loss of Bmp2 appears to be one of the primary causes of the uterine decidualization defect. Interestingly, although decidualization is a largely progesterone (P4)-dependent process, the decrease in expression of Bmp2 in Smad1/5/4-Amhr2-cre KO females does not appear to be due to loss in expression of Pgr, as Pgr and other PGR target genes, such as Fkbp5, are normally expressed. Furthermore, decidualization cannot proceed in the Smad1/5/4-Amhr2-cre KO uteri even during artificial decidualization assays when P4 is exogenously supplied. This may mean that SMAD1/5/4 signaling is required for stromal upregulation of Bmp2, possibly as cofactors in conjunction with PGR signaling for a subset of genes. Other studies have demonstrated that loss of TGF-β signaling using PGR-cre results in defective luminal closure as well as altered PTGS2 expression during peri-implantation [50]. Our mouse model provides further support that both BMP and TGF-β signaling pathways through the common transducer Smad4, are key pathways in stromal cells for uterine decidualization and pregnancy.
In summary, the present study provides the first genetic evidence that Smad1/5/4-mediated signaling is indispensable for structural and functional integrity of the oviduct and uterus. Loss of Smad1/5/4 alters development of the oviduct smooth muscle layer, which likely impedes embryo transit. Disruption of SMAD-mediated signaling also alters development of the myometrium and defects in the uterine stroma leading to early pregnancy loss due to abnormal uterine structure, defective decidualization, and dysregulation of genes of known importance in oviduct development and uterine biology. We provided evidence to support the possibility that the SMAD signaling pathways may control early molecular and cellular changes in the uterus that are required for implantation and decidualization. We also showed that oviduct integrity also involves SMAD signaling in the myosalpinx. An in-depth knowledge of the molecular mechanisms and signaling networks involved in the regulation of early pregnancy events such as these has potential implications in developing new therapies for improving pregnancy rates and treating maternal diseases such as intrauterine growth restriction and preeclampsia. Furthermore, as the implantation process involves numerous signaling pathways common to other systems, this mouse model is a useful biological system for investigating a wide range of biological process beyond reproduction. This includes epithelium-mesenchyme interactions, cell proliferation, differentiation, migration, and invasion in both normal and disease states. Finally, we have pioneered the use of OCT imaging for structural phenotyping of reproductive tract in a novel mutant mouse. Cellular level of resolution, high imaging speed and natural tissue contrast of this approach makes it a unique phenotyping tool. Potentially it can be implemented for in vivo time-lapse analysis and longitudinal studies to investigate functional effects of molecular/genetic manipulations and pharmacological agents, opening a door for variety of innovative studies in reproductive fields.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to thank Drs. Richard Behringer, Elizabeth Robertson, An Zwijsen, Danny Huylebroeck, and Martin M. Matzuk for the mouse lines for these studies. We are grateful to Drs. Francesco J. Demayo, Jia Peng, Diana Monsivais, and Margeaux Wetendorf for helpful discussions and advice, and Hermann Piard for assistance with mouse genotyping. The α-KRT8 antibody (TROMA I) was developed by P. Brulet and R. Kemler and obtained from the Developmental Studies Hybridoma Bank, which was created by the U.S. National Institutes of Health/National Institute of Child Health and Human Development, Department of Biology, University of Iowa, Iowa City, Iowa.
Footnotes
Portions of this research were initially presented as a poster at the 2014 Annual Meeting of the Society for the Study of Reproduction, July 19–23, 2014, Grand Rapids, Michigan.
Supported by U.S. National Institutes of Health grants R01 CA138628, R01 HD076980, T32 HD007165, R01 HL120140, T32 GM008231, and P30 CA125123 and a Burroughs Wellcome Career Award in the Biomedical Sciences.
These authors contributed equally to this work.
REFERENCES
- Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- Koot YE, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822:1943–1950. doi: 10.1016/j.bbadis.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34:939–980. doi: 10.1016/j.mam.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group ECW. Diagnosis and management of the infertile couple: missing information Hum Reprod Update 2004. 10 295 307 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Pangas SA. Regulatory roles of transforming growth factor beta family members in folliculogenesis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:117–125. doi: 10.1002/wsbm.21. [DOI] [PubMed] [Google Scholar]
- Pangas SA. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol Cell Endocrinol. 2012;356:40–47. doi: 10.1016/j.mce.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Transforming growth factor beta signaling in uterine development and function. J Anim Sci Biotechnol. 2014;5:52. doi: 10.1186/2049-1891-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima T, Li Q, Clementi C, Lydon JP, DeMayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA, DeMayo FJ, Matzuk MM. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 2013;9:e1003863. doi: 10.1371/journal.pgen.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Agno JE, Edson MA, Nagaraja AK, Nagashima T, Matzuk MM. Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011;7:e1002320. doi: 10.1371/journal.pgen.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev Biol. 2006;296:104–118. doi: 10.1016/j.ydbio.2006.04.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, Rossant J, Mak TW. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128:3609–3621. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- Li X, Tripurani SK, James R, Pangas SA. Minimal fertility defects in mice deficient in oocyte-expressed Smad4. Biol Reprod. 2012;86:1–6. doi: 10.1095/biolreprod.111.094375. [DOI] [PubMed] [Google Scholar]
- Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28:7001–7011. doi: 10.1128/MCB.00732-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology. 2009;150:5208–5217. doi: 10.1210/en.2009-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri-Attia N, Tripurani SK, Gokul N, Piard H, Anderson ML, Eldin K, Pangas SA. TGFbeta signaling promotes juvenile granulosa cell tumorigenesis by suppressing apoptosis. Mol Endocrinol. 2014;28:1887–1898. doi: 10.1210/me.2014-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20:1406–1422. doi: 10.1210/me.2005-0462. [DOI] [PubMed] [Google Scholar]
- Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7:82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- Syed SH, Coughlin AJ, Garcia MD, Wang S, West JL, Larin KV, Larina IV. Optical coherence tomography guided microinjections in live mouse embryos: high-resolution targeted manipulation for mouse embryonic research. J Biomed Opt. 2015;20:051020–051020. doi: 10.1117/1.JBO.20.5.051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Tripurani SK, Cook RW, Eldin KW, Pangas SA. BMP-specific SMADs function as novel repressors of PDGFA and modulate its expression in ovarian granulosa cells and tumors. Oncogene. 2013;32:3877–3885. doi: 10.1038/onc.2012.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri-Attia N, Tripurani SK, Gokul N, Piard H, Anderson ML, Eldin K, Pangas SA. TGFbeta signaling promotes juvenile granulosa cell tumorigenesis by suppressing apoptosis Mol Endocrinol 2014. me20141217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Yoshie M, Xie H, Sun X, Cha J, Ellenson LH, Dey SK. Conditional deletion of Tsc1 in the female reproductive tract impedes normal oviductal and uterine function by enhancing mTORC1 signaling in mice. Mol Hum Reprod. 2013;19:463–472. doi: 10.1093/molehr/gat016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Park JH, Tanwar PS, Kaneko-Tarui T, Mittal S, Lee HJ, Teixeira JM. Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology. 2012;153:404–416. doi: 10.1210/en.2011-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Mullen RD, Behringer RR. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev. 2014;8:281–296. doi: 10.1159/000364935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines AM, Adam M, Magella B, Meyer SE, Grimes HL, Dey SK, Potter SS. Recombineering-based dissection of flanking and paralogous Hox gene functions in mouse reproductive tracts. Development. 2013;140:2942–2952. doi: 10.1242/dev.092569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Wang Y, Bonilla-Claudio M, Martin JF, Gonzalez G, Taketo MM, Behringer RR. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Müllerian duct and postnatal uterine development. Mol Endocrinol. 2013;27:1442–1454. doi: 10.1210/me.2012-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone FF, Ren Y, Cowan RG, Harman RM, Nikitin AY, Quirk SM. Dominant activation of the hedgehog signaling pathway alters development of the female reproductive tract. Genesis. 2012;50:28–40. doi: 10.1002/dvg.20786. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Bell RD, Gerthoffer WT, Zlokovic BV, Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1505–1510. doi: 10.1161/ATVBAHA.108.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg ME, Stodden GR, King ML, MacLean JA, II, Mann JL, DeMayo FJ, Lydon JP, Hayashi K. Loss of CDH1 and Pten accelerates cellular invasiveness and angiogenesis in the mouse uterus. Biol Reprod. 2013;89:8. doi: 10.1095/biolreprod.113.109462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76:678–688. doi: 10.1002/mrd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Jackson L, Besnard V, Whitsett J, Ellenson LH, Dey SK. Cell-specific conditional deletion of Pten in the uterus results in differential phenotypes. Gynecol Oncol. 2011;122:424–429. doi: 10.1016/j.ygyno.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais D, Clementi C, Peng J, Titus MM, Barrish JP, Creighton CJ, Lydon JP, DeMayo FJ, Matzuk MM. Uterine ALK3 is essential during the window of implantation. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1523758113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Fullerton PT, Jr, Monsivais D, Clementi C, Su GH, Matzuk MM. Uterine activin-like kinase 4 regulates trophoblast development during mouse placentation. Mol Endocrinol. 2015;29:1684–1693. doi: 10.1210/me.2015-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Monsivais D, You R, Zhong H, Pangas SA, Matzuk MM. Uterine activin receptor-like kinase 5 is crucial for blastocyst implantation and placental development. Proc Natl Acad Sci U S A. 2015;112:E5098–5107. doi: 10.1073/pnas.1514498112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- Burton JC, Wang S, Larina IV. Dynamic imaging of preimplantation embryos in the murine oviduct Proc SPIE 2015. 9334 933409. [Google Scholar]
- Burton JC, Wang S, Stewart CA, Behringer RR, Larina IV. High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography. Biomed Opt Express. 2015;6:2713–2723. doi: 10.1364/BOE.6.002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Burton JC, Behringer RR, Larina IV. In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct. Sci Rep. 2015;5:13216. doi: 10.1038/srep13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Behringer RR. Mouse oviduct development. Results Probl Cell Differ. 2012;55:247–262. doi: 10.1007/978-3-642-30406-4_14. [DOI] [PubMed] [Google Scholar]
- Agduhr E. Studies on the structure and development of the bursa ovarica and the tuba uterina in the mouse. Acta Zoologica. 1927;8:1–133. [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat. 1989;186:1–20. doi: 10.1002/aja.1001860102. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–186. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.