Abstract
We describe video electroencephalography (video-EEG) correlates of transient neurological attacks due to plateau waves—paroxysmal elevations in intracranial pressure—in patients with leptomeningeal metastases. We identified 3 patients with leptomeningeal metastases, intracranial hypertension, and transient neurological attacks captured on video-EEG without evidence of seizures or epileptiform activity. We identified all clinical events on video and reviewed the corresponding EEG data for evidence of abnormalities. All 3 patients had mild to moderate slowing and 2 had frontal intermittent rhythmic delta activity during background EEG recording. There were 33 clinical events recorded and stereotyped for each patient. All 33 events were associated with an increase in delta range slowing of ≥30% compared to the background. This abnormality started ≤2 minutes before the onset of clinical symptoms and persisted for minutes after clinical resolution. This study is the first to carefully describe the electrographic correlates of transient neurological attacks due to plateau waves in patients with leptomeningeal metastasis. Clinical attacks were consistently associated with a possible EEG signature of diffuse delta range slowing. Future studies can validate the sensitivity and specificity of these EEG changes as a prognostic and/or response biomarker in patients with leptomeningeal metastases with or without intracranial hypertension.
Keywords: leptomeningeal metastases, electroencephalography, intracranial hypertension, plateau waves
Introduction
Leptomeningeal metastases (LM) are defined by direct invasion of the subarachnoid space of the central nervous system (CNS) by metastatic cancer cells. This is seen in 1% to 8% of solid and hematologic malignancy deaths.1 Clinical concern for LM is raised when a patient with cancer develops signs or symptoms referable to multifocal CNS lesions. Diagnosis is confirmed via cerebrospinal fluid (CSF) and/or brain and spine magnetic resonance imaging (MRI).2 Despite treatments, median survival remains dismal at 2.4 to 4.8 months.2,3
In a large series, 26% of patients with LM developed increased intracranial pressure (ICP) with or without hydrocephalus.2 Patients with increased ICP had significantly poorer survival.2 Some patients with LM and increased ICP experience transient neurological attacks attributable to plateau waves.4 First described in 1960, plateau waves are paroxysmal ICP elevations measuring 25% to 72% above mean ICP with sudden onset and offset.5,6 Cerebral perfusion pressure (CPP) may decrease when ICP suddenly rises, leading to decreased consciousness, tonic posturing of the arms and legs, neck and back arching, nausea and vomiting, or urinary and fecal incontinence.7 Attacks may be induced by changes in position, typically last minutes, and resolve between episodes.
Transient neurological attacks due to plateau waves must be distinguished from seizures reported in 14% of patients with LM.8 Prolonged video-electroencephalography (video-EEG) monitoring is frequently utilized to capture clinical and/or electrographic seizures. A transient neurological event without epileptiform discharges may exclude seizure and often leads to a diagnosis of plateau waves. Plateau waves are presumed, and not definite, due to the absence of invasive ICP monitoring, which is rarely utilized in patients with LM since many have normal cognition between attacks.
To our knowledge, careful descriptions of video-EEG correlates of definite or presumed plateau waves in patients with LM have not been reported. Our objective was to characterize EEG correlates of transient neurological attacks due to suspected plateau waves in patients with LM.
Methods
Study Population
Patients included in this retrospective study were followed by Neuro-Oncology at New York-Presbyterian Hospital/Columbia University Medical Center (CUMC) in 2013 and 2014. Inclusion criteria were age >18 years, clinically documented LM, transient neurological attacks captured on video-EEG without evidence of seizures, and clinical diagnosis of plateau waves. Three patients met these criteria. Our retrospective case series of 3 patients was exempt from institutional review board approval at CUMC. The single living patient provided consent for reporting of her clinical data.
Video-EEG Recordings
Two epileptologists, a fellow and a board-certified electroencephalographer, independently reviewed video-EEGs and then compared results. Particular attention was given to background findings and any transient neurological events identified by the patient event button or described by clinical notation. When available, the video was analyzed to determine the event characteristics. An event was considered to have an EEG correlate if there was a clear change from baseline by visual inspection defined as a change in frequency and/or amplitude of ≥30% lasting for ≥10 seconds. This change must only be seen immediately preceding, during, or after a typical clinical event, not occurring otherwise in the background, and not explained by a seizure, state change, electrocardiogram (ECG) changes, or artifact. This change was differentiated from state changes by the absence of expected EEG changes, including roving eye movements and sleep transients. Only those events agreed upon independently by both epileptologists were considered. Quantitative EEG was attempted but technically limited primarily due to artifact.
Results
Clinical Findings
Patients 1 and 2 had metastatic lung adenocarcinoma, and patient 3 had metastatic T-cell lymphoma (Table 1). All had neurological signs or symptoms of LM with malignant cells on CSF cytology (patients 1 and 2) or flow cytometry (patient 3). Patients 1 and 2 underwent MRI revealing leptomeningeal enhancement consistent with LM. All patients had increased ICP measured when at their clinical baseline. Patients 1 and 2 had multiple clinical events with stereotypic features specific to each patient.
Table 1.
Clinical, Laboratory, and Imaging Findings.
| Patient | 1 | 2 | 3 |
|---|---|---|---|
| Age | 71 years | 42 years | 48 years |
| Sex | Male | Female | Female |
| Primary malignancy | Lung adenocarcinoma | Lung adenocarcinoma | T-cell lymphoma |
| Onset of transient neurological attacks from LM diagnosis | Immediate | 3.5 months | 5 months |
| Neurological deficits | No neurological deficits or papilledema | Diplopia, increased tone in legs; no evidence of papilledema | Left abducens nerve palsy, left facial weakness, drift of right arm, and left leg with full strength to confrontation. Funduscopic examination not performed |
| CSF profile | RBC 11, WBC 11, protein 82, glucose 38 | RBC 5, WBC 1, protein 16, glucose 90 | RBC 252, WBC 601, protein 752, glucose 31 |
| Source of CSF | Lumbar puncture | Ommaya reservoir | Lumbar puncture |
| MRI brain findings | Right cerebellar intraparenchymal metastasis; evidence of transependymal flow | Enhancement of bilateral parietal leptomeninges | No contrast imaging performed |
| MRI spine findings | Enhancement of cauda equina nerve roots | Enhancement of leptomeninges in the lower thoracic cord, conus medullaris, and cauda equina | No contrast imaging performed |
| Notable background EEG characteristics | Low-voltage mild diffuse slowing | Mild to moderate diffuse slowing and FIRDA | Mild to moderate diffuse slowing and FIRDA |
| Seizures or epileptiform discharges | No | No | No |
| Number of clinical attacks captured on EEG | 8 | 24 | 1 |
| Clinical characteristics of attacks | Unresponsiveness, upward eye rolling, irregular mouth movements, neck and back arching | Nausea and dizziness | Period of unresponsiveness, rigidity, eyes rolled up |
| Physiologic changes during clinical attacks | Tachypnea, hypertension | Bradycardia—vitals not recorded during all attacks | (Relative) bradycardia, tachypnea, hypertension |
| Duration of clinical events | 1 to 6 minutes | Minutes, exact durations unclear | Minutes, exact duration unclear |
| Position-dependent attacks | Yes | Not captured on video but reported clinically | No |
| Onset of EEG changes compared to onset of clinical symptoms | 6 to 90 seconds prior to push button event | 22 to 120 seconds prior to push button event | Not clear |
| EEG attenuation | Yes | No | No |
| Duration of EEG changes | 50 seconds to 6.5 minutes | 10 seconds to 9 minutes | 7 minutes |
Abbreviations: CSF, cerebrospinal fluid; EEG, electroencephalography; FIRDA, frontal intermittent rhythmic delta activity; LM, leptomeningeal metastases; MRI, magnetic resonance imaging; RBC, red blood cells; WBC, white blood cells.
The clinical events for patient 1 were dramatic, with the patient transitioning from speaking with family to sudden unresponsiveness and tonic posturing. After video-EEG did not suggest seizure, he had a ventriculoperitoneal shunt (VPS) placed with complete resolution of these attacks. He received palliative whole brain radiation (WBRT) and conformal spine radiotherapy. He died 13 days after VPS placement from respiratory failure due to his lung malignancy. Patient 2 was treated for 2 months after diagnosis before developing high ICP requiring VPS placement. Neurologic symptoms consisted of transient dizziness and nausea that was position dependent. The VPS placement leads to resolution of neurological symptoms. She underwent WBRT followed a year later by radiotherapy to the lumbosacral spine for recurrent LM. She remained on maintenance with epidermal growth factor receptor (EGFR) inhibitors therapy, then initiated chemotherapy for recurrent systemic disease. She has survived for >28 months since LM diagnosis. Video-EEG monitoring was not repeated after VPS placement. Due to severe intracranial hypertension, patient 3 deteriorated to persistent coma hours after the single event captured on video-EEG. She was admitted to intensive care, when family elected comfort measures prior to her death.
Video-EEG Findings
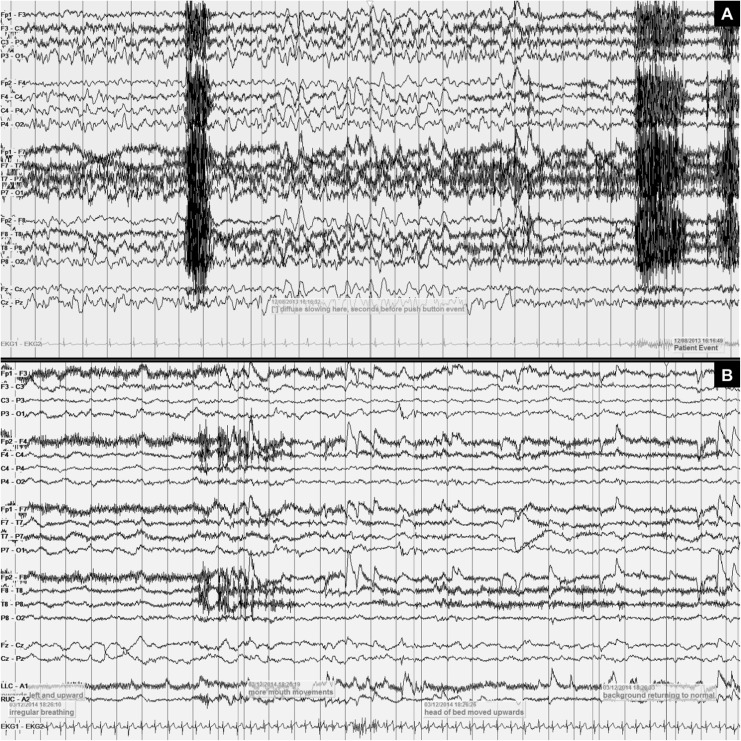
A total of 33 clinical events were captured on video-EEG (Table 1). Video-EEG captured clinical attacks of unresponsiveness, arm posturing, eyes rolling upward, irregular mouth movements, and behavioral arrest, none explained by a state change. All clinical events were associated with an increase in delta range slowing. In patients 1 and 2, the increase in delta slowing clearly started 90 to 120 seconds prior to clinical events and persisted up to minutes after clinical remission. There were no seizures or epileptiform discharges throughout the video-EEG. No significant arrhythmias were noted on single-lead ECG. Figure 1 shows a sample EEG during an acute neurologic attack. Additional sample EEG tracings are included as Supplementary Materials.
Figure 1.
Examples of electroencephalography (EEG) findings during acute neurological event. A, Depiction of 30-second EEG recording of a transient neurological event for patient 2, showing mild diffuse background slowing followed by 11 seconds of more prominent diffuse slowing, in the absence of electrocardiogram (ECG) abnormalities. The EEG changes were immediately followed by activation of the push button event. The corresponding neurological event consisted of eye deviation and tonic posturing along with loss of consciousness, returning to baseline immediately after the event. B, Depiction of 29-second EEG recording for patient 1, showing persistent attenuation during episode of irregular breathing and unresponsiveness. As the head of the bed is moved upward, there is an improvement in attenuation and slowing, followed by improved neurological function.
Discussion
To our knowledge, this is the first careful description of video-EEG correlates of presumed plateau waves in patients with LM and intracranial hypertension. The transient neurologic events captured were stereotyped in each patient and consistent with plateau waves.4,7 Video-EEG revealed an increase in delta range slowing of ≥30% that temporally correlated with clinical events. Thus, video-EEG was not just negative for electrographic seizures but suggestive of a possible electrographic signature of plateau waves.
Previous descriptions of EEG findings during plateau waves are limited. In 1961, Ingvar and Lundberg gathered EEG data from 6 patients with increased ICP and paroxysmal neurologic events.7 They found an association between elevated pressure and arousal reactions with increased alpha activity. In their study, offset of increased pressure correlated with slow waves. That study differed in that plateau waves were defined as elevations in intraventricular fluid pressure noted by continuous monitoring, rather than clinically. Moreover, only 1 patient had LM, whereas 5 had intraparenchymal neoplasms. In these cases, localized pressure recordings from the anterior ventricular horns may not reflect the pressure at the cortex.
Plateau waves are thought to result from low CPP as a result of increased ICP or decreased systolic blood pressure. Low CPP may result in the slowing patterns on EEG. Specifically, attenuation may result from impaired brain perfusion in the setting of low CPP and impaired autoregulation in poorly compliant brain and/or due to poor CSF flow in the setting of leptomeningeal disease. The variable duration of EEG changes among patients may relate to the duration of CPP deficiency and reflect vascular dysregulation.9 Cerebral vascular dysregulation has been demonstrated in multimodal monitoring of patients with traumatic brain injury at the peak of plateau waves.5 Specifically, EEG revealed longer duration of delta range slowing due to less hyperemia at the end of plateau waves, resulting in less cerebral blood flow and brain oxygenation.
Our findings add to the limited literature on video-EEGs in patients with LM. The EEG abnormalities were revealed in a series of 21 patients with LM, though it is not clear how many, if any, had increased ICP or transient neurological attacks.10 Electrographic abnormalities included delta slowing in 52% of patients, nonspecific generalized dysrhythmia in 71%, and focal epileptiform discharges in 19%.10 Patients 2 and 3 had frontal intermittent rhythmic delta activity (FIRDA), not previously specifically reported in LM. Frontal intermittent rhythmic delta activity is often a nonspecific correlate of encephalopathy but has been associated with hydrocephalus in patients with posterior fossa tumors and in patients with tuberculosis meningitis.11–13 Although elevated ICP alone proves insufficient to produce FIRDA, EEG pattern may theoretically result from periventricular edema as a result of transependymal CSF flow.14 The current study has several potential limitations. Only 3 patients are reported, one of which had a single clinical event captured. Also, none had invasive ICP monitoring, so plateau waves are presumed and not definite. Finally, video-EEG metrics used for quantifying the degree of delta range slowing associated with plateau waves are not standardized in the literature.
Our findings serve to generate hypotheses for future studies. Concurrent video-EEG and noninvasive ICP monitoring, such as transcranial Dopplers, in patients with LM with or without clinical symptoms of high ICP, may validate the sensitivity and specificity of these EEG findings as an early biomarker of CSF flow dysregulation. The EEG changes could be quantified by the assessment of delta power. Resting blood oxygen level-dependent measures may assess neurovascular decoupling and its correlation with EEG findings and/or clinical plateau waves. The EEG changes in suspected or confirmed LM may precede and potentially predict time to symptomatic high ICP. Resolution of EEG changes, as a measure of improved CSF flow and ICP, would objectively assess the benefit of acute treatment for symptomatic plateau waves, including steroids and diuretics. Resolution of EEG changes after whole brain irradiation, VPS, and/or medical therapies for LM could serve as an objective measure of response. The utility of a putative EEG biomarker is not to simply distinguish from seizures clinically but additionally for early detection of CSF flow and ICP abnormalities preceding the onset of clinical symptoms. Electroencephalography correlates would provide an easy noninvasive measure of CSF flow dysregulation and, thus, serve as a practical biomarker of prognostication and/or response in patients with LM.
Conclusion
This is the first detailed description of electrographic correlates of transient neurological attacks due to plateau waves in patients with LM. Clinical attacks were consistently associated with a possible EEG signature of diffuse delta range slowing by visual inspection. Future studies can utilize quantitative EEG measures and prospective cohorts to validate the sensitivity and specificity of these EEG changes as a prognostic and/or response biomarker in patients with LM with or without intracranial hypertension.
Supplementary Material
Footnotes
Authors’ Note: C.A.G. provided clinical care for patients 1 and 3, conceptualized the project, and authored the manuscript. N.O. devised the methods for EEG review, expertly reviewed the EEG data for all 3 patients, and contributed to the Methods section of the manuscript. S.S. expertly reviewed the EEG and contributed to the Methods, Results, and Discussion section of the manuscript. L.S. and A.H. gathered clinical and EEG data and contributed to the Discussion section of the manuscript. Y.O. provided clinical care for all 3 patients, conceptualized the project, and critically revised the entire manuscript.
Our case series of 3 patients is exempt from IRB approval at CUMC. The single living patient provided consent for report of data.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online supplemental materials are available at http://nhos.sagepub.com/supplemental.
References
- 1. Clarke JL. Leptomeningeal metastasis from systemic cancer. Continuum (Minneap Minn). 2012;18(2):328–342. doi:10.1212/01.CON.0000413661.58045.e7. [DOI] [PubMed] [Google Scholar]
- 2. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. doi:10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167–178. doi:http://dx.doi.org/10.1016/j.jns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 4. Hansen K, Gjerris F, Sørensen PS. Absence of hydrocephalus in spite of impaired cerebrospinal fluid absorption and severe intracranial hypertension. Acta Neurochir (Wien). 1987;86(3-4):93–97. doi:10.1007/BF01402291. [DOI] [PubMed] [Google Scholar]
- 5. Lang EW, Kasprowicz M, Smielewski P, Pickard J, Czosnyka M. Changes in cerebral partial oxygen pressure and cerebrovascular reactivity during intracranial pressure plateau waves. Neurocrit Care. 2015;23(1):85–91. doi:10.1007/s12028-014-0074-9. [DOI] [PubMed] [Google Scholar]
- 6. Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36(149):1–193. [PubMed] [Google Scholar]
- 7. Ingvar DH, Lundberg N. Paroxysmal symptoms in intracranial hypertension, studied with ventricular fluid pressure recording and electroencephalography. Brain. 1961;84(3):446–459. [Google Scholar]
- 8. Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9(3):225–229. doi:10.1007/BF02341153. [DOI] [PubMed] [Google Scholar]
- 9. Rosner MJ, Becker DP. Origin and evolution of plateau waves. J Neurosurg. 1984;60(2):312–324. doi:10.3171/jns.1984.60.2.0312. [DOI] [PubMed] [Google Scholar]
- 10. Balm M, Hammack J. Leptomeningeal carcinomatosis: presenting features and prognostic factors. Arch Neurol. 1996;53(7):626–632. [DOI] [PubMed] [Google Scholar]
- 11. Daly D, Whelan JL, Bickford RG, Maccarty CS. The electroencephalogram in cases of tumors of the posterior fossa and third ventricle. Electroencephalogr Clin Neurophysiol. 1953;5(2):203–216. [DOI] [PubMed] [Google Scholar]
- 12. Accolla EA, Kaplan PW, Maeder-Ingvar M, Jukopila S, Rossetti AO. Clinical correlates of frontal intermittent rhythmic delta activity (FIRDA). Clin Neurophysiol. 2011;122(1):27–31. doi: http://dx.doi.org/10.1016/j.clinph.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 13. Kalita J, Misra UK, Das BK. SPECT changes and their correlation with EEG changes in tuberculous meningitis. Electromyogr Clin Neurophysiol. 2002;42(1):39–44. [PubMed] [Google Scholar]
- 14. Leschey WH, Jr, Briggs RC, Altemus LR. Frontal intermittent rhythmic delta activity (FIRDA), periventricular edema and normal pressure hydrocephalus. Clin Electroencephalogr. 1978;9(3):110–117. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.