Abstract
Migraine headache is among the most prevalent neurologic disorders. Status migrainosus often leads to hospitalization, and multiple medications are sometimes required for symptomatic relief. In 2008, neurologists at our institution started using the atypical antipsychotic ziprasidone as an abortive medication for status migrainosus. The Clinical Investigation Data Exploration Repository was used to search for patients admitted to the Barnes-Jewish Hospital inpatient neurology service with diagnoses of “headache” or “migraine.” Patients were identified as having status migrainosus if they met the International Headache Society criteria for a migraine lasting >72 hours. Clinical records of identified patients were then entered into a secure online database (REDCap). Between 2008 and 2015, a total of 34 patients received 10 to 40 mg of ziprasidone for the treatment of status migrainosus. Among patients who received ziprasidone, headache severity decreased 5.68 ± 3.0 points on a 10-point scale, from admission to discharge. Ziprasidone was the last abortive medication added prior to discharge in 65% of cases. The 30-day readmission rate for migraine headache in patients who received ziprasidone was 12%. Ziprasidone was well tolerated, with side effects limited to a mild dystonic reaction (n = 1), rhinorrhea (n = 1), and a prolonged QTc of 495 milliseconds (n = 1). This observational study suggests that ziprasidone may be a safe, effective abortive medication for the treatment of status migrainosus. Further studies comparing ziprasidone to standard of care are warranted.
Keywords: migraine disorders, headache disorders, ziprasidone
Background and Purpose
The International Headache Society (IHS) defines status migrainosus (SM) as an unremitting migraine lasting greater than 72 hours.1 Status migrainosus often leads to hospitalization at an average cost of US$5000 per admission.2 Patients with SM may fail first-line (e.g., acetaminophen, nonsteroidal anti-inflammatory drugs, caffeine) and second-line abortive therapies (eg, triptans, ketorolac, prochlorperazine, metoclopramide). Practitioners often resort to third-line treatments such as neuroleptics, intravenous valproate, or dihydroergotamine (DHE) in the inpatient setting. Clinical trials suggest that droperidol, haloperidol, and other dopaminergic antagonists may be effective abortive therapies.3–5 However, these medications are reserved for the emergency room or the inpatient setting where patients can be monitored for dangerous adverse effects including cardiac ischemia (DHE), prolongation of the QTc interval, oversedation, or extrapyramidal symptoms (neuroleptics).
Given an overlapping dopaminergic mechanism of action with droperidol and haloperidol, and a small case series showing efficacy in chronic daily headache,6 we hypothesized that intramuscular (IM) ziprasidone (quicker onset of action of <60 minutes and potential added placebo effect of the invasiveness of IM injection) may be an effective and safer alternative third-line treatment for SM. In 2008, neurologists at Barnes-Jewish Hospital (BJH) started using ziprasidone as an abortive therapy in patients with SM. This retrospective chart review of the use of ziprasidone at BJH is an attempt to examine its efficacy and safety profile as an abortive therapy for SM.
Methods
This is a retrospective chart review (class IV evidence) of patients admitted to BJH with SM over a 7-year period. This study was approved by the Washington University Institutional Review Board. A waiver of informed consent was granted. Patients admitted to the neurology inpatient service meeting the IHS criteria for a migraine lasting > 72 hours were identified using the Clinical Investigation Data Exploration Repository (patients were identified with the primary diagnosis of “headache” or “migraine” and cross matched with the search term “ziprasidone” or “Geodon”). Patients were excluded if they received ziprasidone for any other indication or were taking any other D2 antagonists on admission. All clinical data were entered into a secure online database (REDCap).
Results
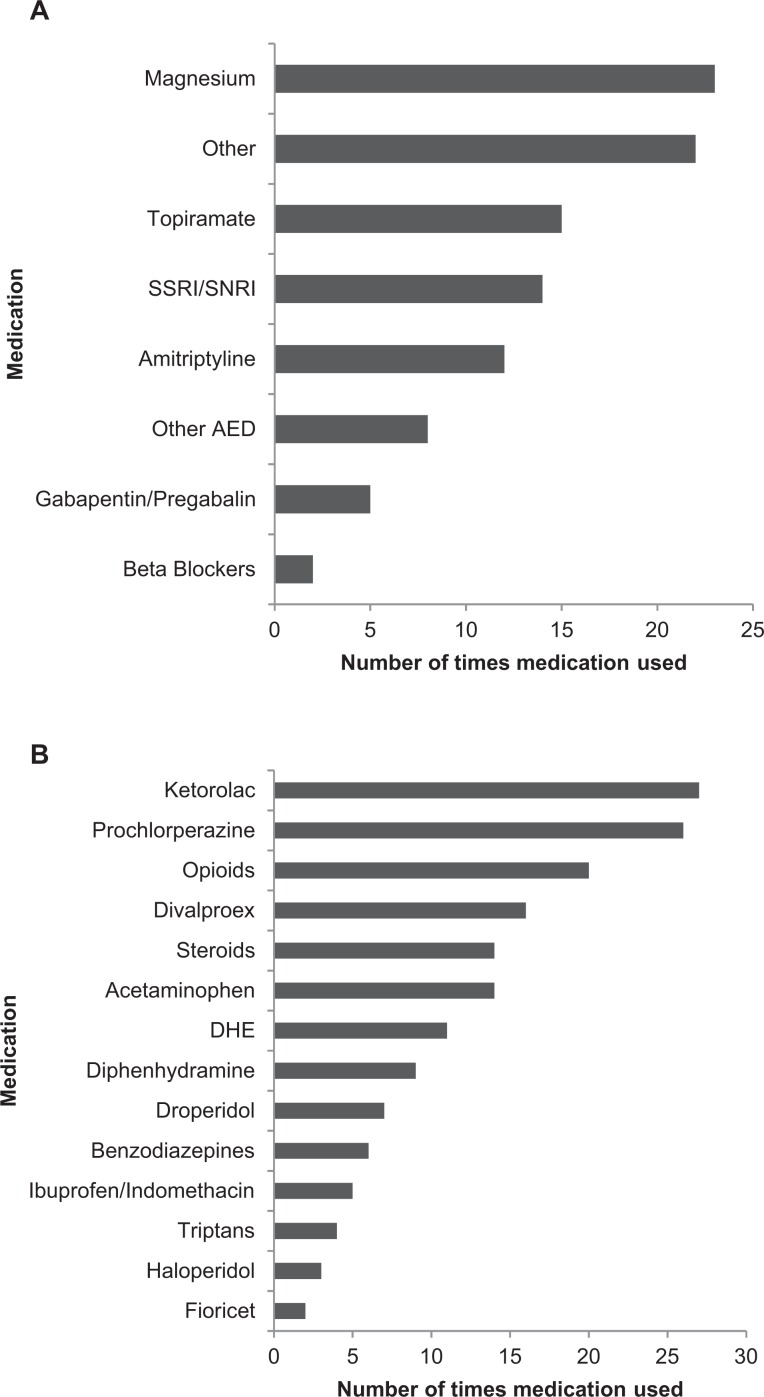
Between 2008 and 2015, a total of 43 patients with SM were treated with ziprasidone. Typical dosing was 10 to 20 mg IM × 1 to 2 doses, with a few exceptions (40 mg IM × 2, 20 mg IV × 2, 20 mg orally × 1-2). See Table 1 for patient demographics and for a comparison to other treatment cohorts evaluating the efficacy of neuroleptics in migraine. On admission, patients were taking 1.7 ± 1.3 (mean ± standard deviation [SD]); 1, 0 to 4 (median, range) classes of medicine for migraine prophylaxis (Figure 1A), and 0.9 ± 1.2 (mean ± SD) abortive therapies (Figure 1B).
Table 1.
Demographics and Comparison to Other Studies.
| Ziprasidone, Landsness et al (2016), n = 43 | Droperidol, Silberstein et al (2003), n = 61 | Haloperidol, Honkaniemi et al (2006), n = 40 | |
|---|---|---|---|
| Age, mean ± SD; min-max (years) | 39 ± 9; 21-57 | 42 ± 10 | 36 |
| Female (%) | 84 | 77 | 85 |
| Race (%) | |||
| Caucasian | 80 | ||
| Black/African American | 16 | ||
| Unknown | 4 | ||
| Percentage with prior admissions (median # of admissions) | 57% (3) | 4 Attacks/month | |
| Duration (mean) | 42 days (17.5 median) | 3.5 hours | 75 hours |
| Photo/phonophobia (%) | 87 | 100/98 | |
| Nausea/emesis (%) | 80 | 98/43 | |
| Aura/complicated (%) | 40 | ||
| Prior head trauma to current presentation (%) | 36 | ||
| Psychiatric disease (%) | 46 | ||
| Any caffeine use (%) | 48 | ||
| Headache-related sleep problems (%) | 41 | ||
| Family history (%) | 31 | ||
| Headache-related work impairment (%) | 70 |
Abbreviation: SD, standard deviation.
Figure 1.
A, Medications prior to admission for migraine prophylaxis. Other (magnesium, acetazolamide, botulinum toxin). Other anti-epileptic drugs (AED) (carbamazepine, lamotrigine, levetiracetam, zonisamide). B, Medications on admission used for migraine abortive therapy.
Headache severity was measured on a standard 0 to 10 Likert scale, with maximum severity equal to 10. As this was a retrospective chart review, the electronic medical record (EMR) lacked adequate documentation of headache severity on admission (n = 8) or on discharge (n = 4) for some patients. Headache severity on admission was 8.9 ± 1.5 (n = 35, mean ± SD), and 3.0 ± 2.9 (n = 39, mean ± SD) on discharge from the hospital. Thirty-four patients had both admission and discharge severity scales, with an average decrease in pain by 5.7 ± 3.0 (mean ± SD; Figure 2). Based on the wording of the discharge summaries, ziprasidone was deemed helpful in reducing headache severity 81% of the time and was the definitive medication leading to discharge in 65% of cases. Almost all patients reported a period of consolidated sleep following administration of ziprasidone. See supplemental material for excerpts from patient discharge summaries.
Figure 2.

Change in pain scale for individual patients. Line thickness is proportional to the number of patients.
Given that ziprasidone was being used off-label, we confirmed that it was given no earlier than as a third-line agent. On average 4.7 ± 2.5 (mean ± SD) different types of medication were used prior to ziprasidone (Figure 3). Of these prior medications, 10 patients failed treatment with droperidol and haloperidol prior to receiving ziprasidone. Adverse events and medication side effects with ziprasidone were not systematically documented. However, there were single reports of an asymptomatic prolongation of the QTc, upper back “stiffness” that was treated as a dystonic reaction, and rhinorrhea.
Figure 3.
A, Number of patients receiving a medication for migraine prophylaxis while admitted for status migrainosus. In almost all instances outpatient medications were continued during hospitalization and some additional medications were started. Other (acetazolamide, muscle relaxants, botulinum toxin, riboflavin, trazodone, verapamil). Other anti-epileptic drugs (AED) (carbamazepine, lamotrigine, levetiracetam, zonisamide). (B) Number of patients who received a medication class for the acute treatment of status migrainosus. DHE indicates dihydroergotamine.
In order to assess whether ziprasidone may have shortened length of stay, we identified a comparison group of 20 patients with SM who were evaluated during the same time period and received droperidol along with a comparable number of abortive therapies but did not receive ziprasidone. In the ziprasidone group, the length of stay was 3, 1 to 8 (median, range), days in comparison to the droperidol group, which was 4, 2 to 17 (median, range). Similar to those receiving ziprasidone, the droperidol group received 4.3 ± 1.4 (mean ± SD) different abortive medications. Headache recurrence is common with SM. While there was no systematic, scheduled 48-hour follow-up to assess for the return of symptoms, 12% (n = 5) were readmitted for migraine to BJH within 30 days of discharge.
Discussion
This study suggests that ziprasidone may be an effective abortive medication for SM. There are several limitations including its retrospective nature, the lack of a control group, and lack of a systematic collection of adverse events. Nonetheless, these findings, and those of a small case series,6 warrant cautious optimism. Additional studies examining both the mechanism of action of ziprasidone and its efficacy in the treatment of SM in a prospective, placebo-controlled fashion are justified.
Beneficial effects of neuroleptics on migraine are thought to be mediated via the D2 dopamine receptor. D2 receptors are found in the brain stem nuclei and sympathetic ganglia and nerves and may regulate autonomic visceral, gastrointestinal, and hemodynamic responses frequently affected by migraine.3 Ziprasidone is an antagonist of D2 and serotonin type 1 and 2 (ie, 5HT1D, 5HT2A) receptors. It is also an agonist at the serotonin 5HT1A receptor, moderately inhibits reuptake of norepinephrine and serotonin, and has alpha-blocking and antihistaminic activity.6 While D2 receptor blockade may account for some of ziprasidone’s efficacy in migraine, other neurotransmitter systems are likely involved. This study only looked at ziprasidone and it is possible that many of the other second-generation D2 antagonists may have a similar effect.
In addition to an improvement in their headache, most patients reported restorative sleep after receiving ziprasidone. Two studies have shown that ziprasidone is associated with improvement in sleep continuity and sleep efficiency, reduced REM sleep, and significant increases in REM latency and percentage of stage 2 sleep and slow-wave sleep.7,8 In the migraine literature, there is a clear association with sleep disturbances triggering attacks and sleep being used as an abortive intervention.9 While this study did not control for sleep as a factor, future studies should specifically address whether ziprasidone-induced sleep is responsible for its apparent efficacy as an abortive therapy for SM.
We report a median length of inpatient hospital admission of 3 days, which is similar to the national average.1 While ziprasidone was often the last medication tried and led to discharge in 65% of cases, it is possible that the patient’s migraine would have resolved on its own on a similar time scale or with sleep. Therefore, we retrospectively identified a group of 20 patients who were given droperidol for the treatment of SM and also received a similar number of additional abortive medications. The length of stay was longer in the droperidol group (4 days vs 3 in the ziprasidone group). However, the results of this retrospective comparison should be interpreted cautiously as there are many confounding variables inherent to this type of analysis. Future controlled studies comparing early ziprasidone administration to standard of care are needed. Additionally, the placebo effect (especially the invasive nature of an IM injection) is well documented in the headache literature and could confound our results; although in the clinical setting, any beneficial effect is advantageous.
If a drug is to be of value in the acute treatment of SM, it must act rapidly. The IM form of ziprasidone reaches peak availability within 60 minutes in comparison to the oral formulation (6-8 hours). A subgroup of patients in this study did receive oral ziprasidone (n = 5), all of which showed improvement in their headache pain scale. Unfortunately, due to the nature of this retrospective chart review, there was no systematic temporal measurement of pain scales immediately before and after medication administration. Therefore, only the pain scales on admission and discharge could be used. Future studies will undoubtedly contain this temporal data and will help address appropriate dosage (ie, dose response), and whether the oral formulation can be used in the outpatient setting.
While adverse events were not systematically documented at the time of ziprasidone administration, there appears to be a lower incidence of adverse events with ziprasidone in this study when compared to prior migraine studies utilizing neuroleptics, consistent with the lower incidence of extra-pyramidal side effects seen with ziprasidone.10
Another well-documented risk with ziprasidone is QTc prolongation and rarely torsade de pointes.11 In this study, there was 1 documented case of asymptomatic QTc prolongation. Therefore, it is recommended that any patient with SM receiving ziprasidone have a baseline QTc of less than 500 milliseconds and that doses do not exceed 20 mg IM (as the lower doses of 10-20 mg seemed equally effective).
Another major limitation of this study is the heterogeneity of the patient population receiving ziprasidone. All patients who received ziprasidone were enrolled regardless of whether they were first-time versus chronic migraineurs (average of 1.7 prophylactic migraine medications on admission); and whether there was a component of analgesia overuse (average of 0.9 abortive medications used prior to admission). To place our findings in context of the headache literature, we compared our findings to 2 prior studies using the neuroleptics droperidol4 and haloperidol3 (see Table 1). Of note, 3 of the 4 nonresponders to ziprasidone were chronic migraineurs with multiple prior admissions who had already failed treatment with droperidol and haloperidol, suggesting a shared mechanism. All 3 studies showed a comparable therapeutic response (see Table 2) and the comparison also suggests that ziprasidone may have a better side effect profile than the other 2 neuroleptic medications.
Table 2.
Headache Outcomes and Side Effects.
| Ziprasidone, Landsness et al (2016), n = 34 | Droperidol, Silberstein et al (2003), n = 61 | Haloperidol, Honkaniemi et al (2006), n = 40 | |
|---|---|---|---|
| Pain before | 9.0 + 1.4 | 64/36% (Mod/Severe) | 7.7 |
| Pain after | 3.3 + 3.0 | 39% (No pain/mild) | 2.3 |
| Side effects | Prolonged QTc (2%),* dystonia (2%),* rhinorrhea (2%)* | Anxiety (28%), akathisia (16%-32%), somnolence (25%) | Akathisia (50%), sedation (53%) |
| Recurrence | N/A | 69% at 24 hours | 16% at 72 hours |
| 30-Day readmission (%) | 12%* |
*Calculated for n = 43 participants.
Conclusions
In this open-label, retrospective study, ziprasidone was associated with a significant therapeutic benefit in 65% of all patients and may be a safe, third-line treatment for SM. However, until additional studies are performed the efficacy of ziprasidone in SM remains unknown.
Acknowledgments
We thank the neurology residents and attending physicians between the years 2008 and 2013, especially Arun Varadhachary, Allan Azarion, Enrique Alvarez III, Richard Sohn, Joseph Black, and Sylvia Awadalla.
Footnotes
Authors’ Note: Dr. Landsness was involved in designing the study, data collection and analysis, and writing the paper. Dr. Wang and Dr. Bucelli were involved in designing and writing the paper.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
References
- 1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 2. Lucado J, Paez K, Elixhauser A, Headaches in U.S. Hospitals and Emergency Departments, 2008. HCUP Statistical Brief #111. May 2011. Agency for Healthcare Research and Quality; Web site http://www.hcup-us.ahrq.gov/reports/statbriefs/sb111.pdf [PubMed] [Google Scholar]
- 3. Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S, Haloperidol in the Acute Treatment of Migraine: A Randomized, Double-Blind, Placebo-Controlled Study. Headache. 2006;46(5):781–787. [DOI] [PubMed] [Google Scholar]
- 4. Silberstein SD, Young WB, Mendizabal JE, Rothrock JF, Alam AS, Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial. Neurology. 2003;60(2):315–321. [DOI] [PubMed] [Google Scholar]
- 5. Mascia A, Afra J, Schoenen J. Dopamine and migraine: a review of pharmacological, biochemical, neuropsychological, and therapeutic data. Cephalalgia. 1998;18(4):174–182. [DOI] [PubMed] [Google Scholar]
- 6. Cahill CM, Hardiman O, Murphy KC. Treatment of refractory chronic daily headache with the atypical antipsychotic ziprasidone – a case series. Cephalalgia. 2005;25(10):822–826. [DOI] [PubMed] [Google Scholar]
- 7. Cohrs S, Meier A, Neumann AC, Jordan W, Rüther E, Rodenbeck A. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: a randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66:989–996. [DOI] [PubMed] [Google Scholar]
- 8. Baskaran A, Summers D, Willing SL, Jokic R, Milev R. Sleep architecture in ziprasidone-treated bipolar depression: a pilot study. Ther Adv Psychopharmacol. 2013;3(3):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holland PR, Headache and Sleep: shared pathophysiological mechanisms. Cephalalgia. 2014;34(10):725–744. [DOI] [PubMed] [Google Scholar]
- 10. Satterthwaite TD, Wolf DH, Rosenheck RA, Gur RE, Caroff SN. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment of agitation. J Clin Psychiatry. 2008;69(12):1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC, QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13. [DOI] [PubMed] [Google Scholar]