Abstract
Objective
Autologous chondrocyte implantation (ACI) has not been proven to be durable over the long-term. The purpose of this systematic review was (1) to evaluate activity level and knee function, (2) to evaluate reoperation and failure rates, and (3) to analyze risk factors for reoperation and failure of ACI at minimum long-term follow-up.
Design
A comprehensive review was performed for studies with long-term outcomes after ACI for cartilage defect repair. Studies reported outcome scores such as Tegner score, Lysholm score, and International Knee Documentation Society (IKDC) score along with rates of failure and reoperation. Modified Coleman Methodology Scores were calculated to assess study methodological quality.
Results
Nine studies with a total of 771 patients with a mean age of 33.4 ± 2.5 years, mean defect size of 5.9 ± 1.6 cm2, and mean follow-up of 11.4 years were included. Tegner score, Lysholm score, and IKDC score change from preoperative to final follow-up was 1.1 (95% CI 0.8-1.4, P < 0.001), 24.9 points (95% CI 18.8-31, P < 0.001), and 16.5 points (95% CI 5.4-27.5, P < 0.01), respectively. The mean failure and reoperation rates were 18% and 37%, respectively. Increased age and lesion size (>4.5 cm2) were significantly correlated with increased risk of reoperation and failure.
Conclusions
Overall, ACI demonstrated successful outcomes in 82% of patients over the long-term. Increased patient age and lesion size greater than 4.5 cm2 were risk factors for a higher reoperation and failure rate. Nonetheless, this review is limited by heterogeneity in surgical technique, and lesion and patient characteristics.
Keywords: cartilage, autologous chondrocyte implantation, long-term
Introduction
A variety of cartilage repair techniques—such as osteochondral allograft (OCA), osteochondral autograft transfer (OAT), microfracture (MFX), and autologous chondrocyte implantation (ACI)—are being utilized to restore function and increase activity in patients with symptomatic cartilage defects. These chondral lesions can otherwise progress to early degenerative joint disease, with an associated major source of functional disability and economic cost.1,2 Even with these restoration techniques, biomechanical features of hyaline cartilage have been difficult to replicate with currently available procedures, especially when evaluated over the long term.3-5 In addition to improvement of short-term symptoms, an important goal of these procedures is to avoid joint replacement in young patients. Therefore, it is critical to demonstrate long-term durability of these restoration techniques.
Autologous chondrocyte implantation (ACI), currently the only Food and Drug Administration–approved biological technique which uses autologous cells to repair cartilage defects, has gained widespread use over the past decade. It employs a 2-stage technique to harvest and implant cultured chondrocytes with a periosteal membrane patch over the defect, and was popularized by Peterson.6 More recently, the availability of collagen membranes in second-generation ACI and biomaterials in third-generation ACI create a stable scaffold for chondrocyte implantation. Disadvantages of ACI include multiple operations, longer time to return to activity, and complications such as periosteal graft hypertrophy. Still, ACI has shown favorable results in clinical outcomes scores over the short term (1-4 years) to mid-term (5-8 years) with regard to return to activity and subjective patient outcomes.7-13 Few studies have compared long-term (9 or more years) survivorship and functional outcomes of ACI due to insufficiency of patients, and variation in defect size, number, and location.14-17
Therefore the purpose of this systematic review was (1) to evaluate activity level and knee function, (2) to evaluate reoperation and failure rates, and (3) to analyze risk factors for reoperation and failure of ACI at minimum long-term follow-up. This would allow a comprehensive and balanced view of the strengths and weaknesses of ACI over the long term and improve surgical indications and patient expectations.
Methods
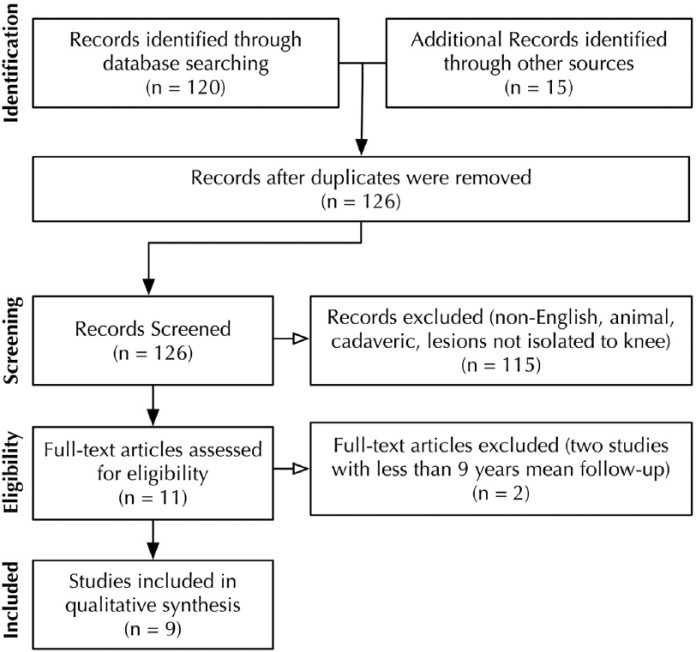
To address the study hypothesis, a comprehensive review of the literature was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( Fig. 1 ).18 Our search was conducted using the following databases: PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials, CINAHL (Cumulative Index for Nursing and Allied Health Literature). The search was conducted on June 1, 2015. The search period was from January 1, 1995 to June 1, 2015. Only abstracts for articles published in English were reviewed. Studies that did not report on the knee were excluded. The electronic search strategy used was “((cartilage OR articular cartilage OR chondral OR chondrocyte OR articular OR osteoarticular OR osteochondral) AND (autograft OR autologous OR implantation OR implant OR aci OR caci OR restore OR repair) AND (long OR long term OR long-term OR year OR years) AND (knee)).” Full-text was obtained and reviewed for the studies meeting inclusion and exclusion criteria. The reference list for each included article was manually crosschecked to avoid missed articles. A consensus was reached on the explicit classification of short-, mid-, and long-term follow-up. Based on current literature terminology for cartilage repair in the human knee, short term was defined to be follow-up from 1 to 4 years,19-23 with mid-term defined as 5-8 years,24-28 and long term as greater than 9 years.15,17,29-34 Articles in question of inclusion and exclusion criteria were discussed among the authors to reach a consensus decision.
Figure 1.
Search strategy according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 Nine studies identified for inclusion. Two studies excluded.8,36
Studies were included if they used autologous chondrocyte implantation with a minimum mean long-term follow-up (9 years) as determined by previous cartilage repair studies using ACI.8,35,36 Studies included reported clinical outcomes such as International Knee Documentation Committee (IKDC) score,37 Tegner Activity Scale score,38 and Lysholm score.39 Studies of all levels of evidence were included. Studies were excluded if they were not in English, had a mean follow-up of less than 9 years, were isolated to lesions not involving the knee, or did not report clinical outcomes.
A total of 126 studies were identified for the inclusion in the review after removing of duplications. The 126 articles were reviewed, and 11 articles matched the inclusion criteria of English-language articles containing clinical outcome data for cartilage defects of the knee. Two of the 11 studies were excluded because of mean follow-up of less than 9 years, leaving 9 studies to be included for systematic analysis.8,36
Study methodological quality was assessed via the Modified Coleman Methodology Score (MCMS), where scores are designated as following: excellent (>85), good (70-84), fair (55-69), poor (<55).40 In addition, systematic extraction of data included study characteristics and design, patient demographic parameters, level of evidence, cartilage defect characteristics, surgical technique, and clinical follow-up. Primary clinical outcomes (activity-related scores, and patient-reported clinical scores) and secondary clinical outcomes (failure rate, complication rate, and reoperation rate as defined by the authors) were extracted to determine knee function at long-term follow-up.
Statistical Analysis
Data were extracted and standardized to arithmetic means and standard deviations as a measure of variance, taking sample size into account. Unavailable values of means and standard deviations were calculated from standard error or 95% confidence intervals (CIs) and estimated from given figures and graphs when possible. The standard deviations of difference in means were imputed using a pretest/posttest correlation of 0.5.41 Descriptive statistics were calculated for each ACI cohort in all studies. Continuous variables were reported as mean ± standard deviation with the mean weighted for sample size where applicable. Categorical variables were reported as frequencies with percentages. Differences between continuous variables were evaluated using a 2-sample, 2-tailed, Wilcoxon rank sums test. Differences between categorical variables were evaluated using a chi-square (χ2) analysis or a Fisher’s exact test to account for small sample bias. Multivariate correlation analyses were conducted using Pearson’s correlation coefficient. All data were analyzed using SPSS software (version 18.0; IBM, Armonk NY) and Excel (2003; Microsoft Corp, Redmond, WA). Differences with P values <0.05 were considered significant
Results
Study Characteristics
Level-of-evidence rating was extracted for the included studies. Included studies had zero Level I studies, one Level II study, one Level III study, and seven Level IV studies ( Table 1 ). One of the 9 studies was a prospective, randomized controlled trial.29 The average MCMS was 63.2 ± 14.1 (range, 46-87, fair). This was significantly higher than the score of 43.5 ± 1.6 (P < 0.01) reported for methodological quality of cartilage repair studies.42 MCMS did not show a statistically significant correlation with level-of-evidence rating (r = −0.140, P = 0.91).
Table 1.
Summary of Included Studies.
| Study | Year of Publication | Journal | Intervention | Type of Membrane | Subjects | Follow-Up(Range, Years) | Level of Evidence | MCMS Score | Outcome Measures Reported | Time Points Reported (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aldrian et al.14 | 2014 | AJSM | ACI | Collagen (MACI) and Hylograft-C | 16 | 10.8 (10-11) | 4 | 69 | IKDC-S, KOOS-S, Tegner, Brittberg, Noyes, VAS-P, VAS-S, Hop Test, Stability Test, MRI MOCART | Preop, 3, 6, 12, 24, 60, 120 |
| Bentley et al.29 | 2012 | JBJS Br | ACI (vs M-OAT) | Collagen and periosteum | 58 | (10-12) | 2 | 70 | Cincinnati, Stanmore-Bentley, K-M | Final |
| Biant et al.15 | 2014 | AJSM | ACI | Collagen and periosteum | 104 | 10.4 (10-12) | 4 | 87 | Cincinnati, Stanmore-Bentley, VAS-P, K-M | Preop, Final |
| Martinčič et al.16 | 2014 | KSSTA | ACI | Periosteum | 33 | 10 | 4 | 67 | IKDC, Lysholm, Tegner, K-L XR, | Preop, 24, 60, 120 |
| Minas et al.17 | 2014 | CORR | ACI | Periosteum | 210 | 12 | 4 | 76 | Cincinnati, WOMAC, KSS, SF-36, K-M | Preop, Final |
| Moradi et al.43 | 2012 | Arthroscopy | ACI | Periosteum | 23 | 9.9 (7-14) | 4 | 47 | Lysholm, Tegner, IKDC, Pain Rating Scale, SF-36, MRI MOCART | Preop, 12 (T1), Final (T2) |
| Moseley et al.33 | 2010 | AJSM | ACI | Periosteum | 72 | 9.2 (6-10) | 4 | 51 | Cincinnati, K-M | Preop, 12-60, 72-120 |
| Peterson et al.34 | 2010 | AJSM | ACI | Periosteum | 224 | 12.8 (9.3-20.7) | 4 | 56 | Lysholm, Tegner, Brittberg-Peterson, Cincinnati, KOOS, Noyes | Preop, Final |
| Vasiliadis et al.35 | 2010 | AJSM | ACI | Periosteum | 31 | 12.9 (9-18) | 3 | 46 | MRI, KOOS | Final |
MCMS = Modified Coleman Methodology Score; ICRS = International Cartilage Repair Society Score; IKDC = International Knee Documentation Committee Score; RTS = return-to-sport; VAS = visual analog scale; HSS = Hospital of Special Surgery Score; KOOS = Knee Injury and Osteoarthritis Outcome Score; KSS = Knee Society Score; SF-36 = Short Form–36 Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; K-M = Kaplan-Meier; MFX = microfracture; MRI, magnetic resonance imaging; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; ACI = autologous chondrocyte implantation; OAT = osteochondral autograft transplantation; OCA = osteochondral allograft transplantation; AJSM = American Journal of Sports Medicine; JBJS Br = Journal of Bone and Joint Surgery, British Edition; CORR = Clinic Orthopedics and Related Research; KSSTA = Knee Surgery, Sports Traumatology, Arthroscopy.
Patient Characteristics
Nine studies with total of 771 patients (56% male, 44% female) with an average age of 33.4 ± 2.5 years and an average body mass index of 26.5 ± 0.7 kg/m2 at the time of surgery were included in this systematic review ( Table 2 ). The average postoperative follow-up time was 11.4 years (range, 9.2-18 years). Seven studies had a minimum follow-up greater than 9 years14-17,29,34,35, while 2 studies included patients with minimum follow-ups of 6 and 7 years.33,43 The mean defect size was 5.9 ± 1.6 cm2 (range, 0.6-15.8 cm2). Mean duration of symptoms before surgery was 4.8 ± 2.5 years (range, 1.8-7.6 years). Eight out of the 9 studies reported information on the location of the lesion with 48% of all lesions present on the medial femoral condyle (MFC), 15% on the lateral femoral condyle (LFC), 18% on the patella, and 19% on the trochlea. Seven studies explicitly reported on rate of previous surgery. Of those that reported this rate, the mean proportion of patients having previous surgery on the same joint was 40%. Only 4 studies reported specifically on rates of previous cartilage surgery on the same joint, and of those that reported this rate, the mean proportion of patients having previous cartilage surgery was 35%.
Table 2.
Patient and Defect Characteristics.
| Aldrian et al. (2014)14 | Bentley et al. (2012)29 | Biant et al. (2014)15 | Martinčič et al. (2014)16 | Minas et al. (2014)17 | Moradi et al. (2012)43 | Moseley et al. (2010)33 | Peterson et al. (2010)34 | Vasiliadis et al. (2010)35 | |
|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | 16 | 58 | 104 | 33 | 210 | 23 | 72 | 224 | 31 |
| No. of knees (or lesions) | (23 lesions) | 58 | 104 | 33 | 210 | 23 | (84 lesions) | 224 | 36 |
| Subjects | |||||||||
| M/F, n | 11/5 | 33/25 | 55/49 | NR | 113/97 | 19/4 | 44/28 | NR | 15/16 |
| Mean age at time of surgery, years | 33.3 | 30.9 | 30.2 | 30.5 | 36 | 30.5 | 37 | 33.3 | 29.4 |
| Mean BMI, kg/m2 | NR | NR | NR | 24.8 | 26.7 | 25.3 | 27.2 | NR | NR |
| Mean preoperative duration of symptoms, years | NR | 7.2 | 7.8 | 17/33 (with more than 1 year of symptoms) | NR | 3.9 | NR | NR | NR |
| Patients with previous surgery, n | 13/16 | 52/56 | 104/104 with debridement (76/104 w/ cartilage surgery) | 16/33 (previous cartilage surgery only) | 89/210 with prior cartilage repair by marrow stimulation | 20/23 | 53/72 with previous surgery (49/72 with previous cartilage repair) | NR | NR |
| Defect size, cm2 | 3.8 (1.7) | 4.41 | 4.77 | 4.32 (2.43) | 8.4 (5.5) | 4.3 (2) | 5.2 (4.15) | 5.3 | 5.14 (2.70) |
| Defect size range, cm2 | 1.2-6.7 | 1.0-10.5 | 1.2-25.0 | NR | NR | 2.5-11.5 | 0.4-23.5 | 0.6-15.8 | NR |
| Defect location | |||||||||
| Medial femoral condyle, % | 48 | 41 | 44 | 94 | NR | 87 | 73 | 45 | 56 |
| Lateral femoral condyle, % | 9 | 22 | 16 | 6 | NR | 13 | 18 | 18 | 19 |
| Patella, % | 26 | 34 | 35 | 0 | NR | 0 | 0 | 21 | 22 |
| Trochlea, % | 17 | 2 | 5 | 0 | NR | 0 | 10 | 16 | 3 |
M = Male, F = Female, BMI = Body Mass Index, NR = Not Reported.
Surgical Characteristics
Of the studies included, 2 studies used both collagen and periosteal membranes.15,29 One study used MACI and hyalograft-C membranes.14 All other studies used first-generation ACI with periosteal membranes. Every study conducted ACI via an open arthrotomy. One study used only fibrin glue for closure of membrane.14 Five studies used sutures without a mention of fibrin glue.16,17,33,35,43 Three studies used both sutures and fibrin glue.15,29,34
Of the 7 studies that reported on concomitant surgical procedures, 30% of the ACI cases underwent a total of 201 concomitant procedures. Of these procedures, 33% were ACL reconstructions, 2% were meniscal allograft transplantations, 12% were meniscectomies, 24% were tibial tubercle osteotomies, and 27% were high tibial osteotomies.
Postoperative Rehabilitation
Seven studies reported explicit rehabilitation protocol. Of those patient populations, only 4 cohorts used continuous passive motion (CPM). These studies reported time to full weightbearing with an average of 7.0 weeks for a total of 441 patients.
Activity-Related Outcomes and Return-to-Sport
Four of the 9 studies reporting long-term outcomes post ACI used Tegner scores to report activity related outcomes.14,16,34,43 Because of heterogeneity of reported time points, preoperative and final postoperative scores were used to assess delta values (difference in means from preoperative to final values), which were pooled accounting for sample size and standard deviations ( Fig. 2 ). Overall, the improvement in Tegner score (10-point scale) for a total of 296 patients from preoperative to final values was 1.1 (95% CI 0.8-1.4, P < 0.001).
Figure 2.
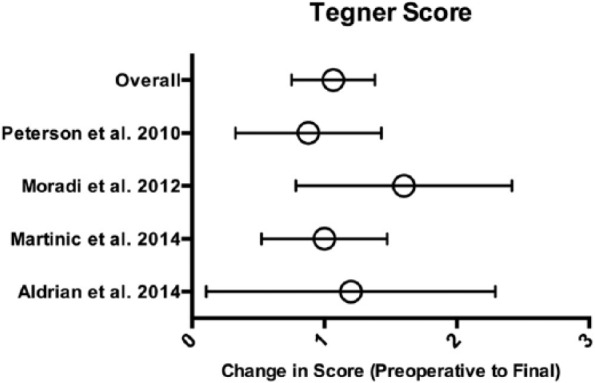
Tegner Activity Scale scores. Means with error bars representing 95% CI (confidence interval).
Clinical and Functional Outcomes
Because of significant variability in time points for reported outcomes, only preoperative and final postoperative scores were assessed for studies using patient-reported clinical and functional outcomes scores. Three studies reported clinical outcomes using the IKDC score (100-point scale)14,16,43 and 3 studies used the Lysholm score (100-point scale).16,34,43
With respect to IKDC score, the mean improvement in a total of 72 patients from preoperative to final values was 24.9 (95% CI 18.8-31, P < 0.001; Fig. 3 ). With regard to Lysholm score, the mean improvement in a total of 280 patients from preoperative to final values was 16.5 (95% CI 5.4-27.5, P < 0.01; Fig. 4 ).
Figure 3.
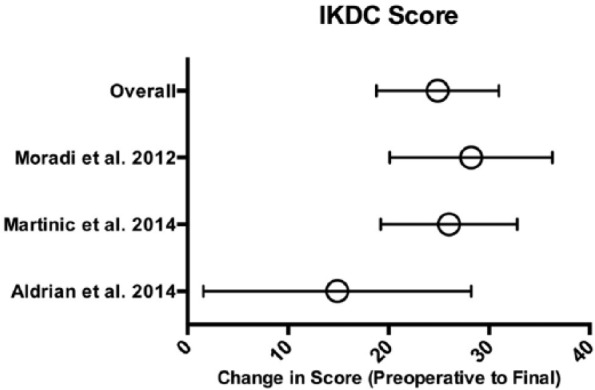
IKDC scores. Means with error bars representing 95% CI (confidence interval). IKDC = International Knee Documentation Committee.
Figure 4.
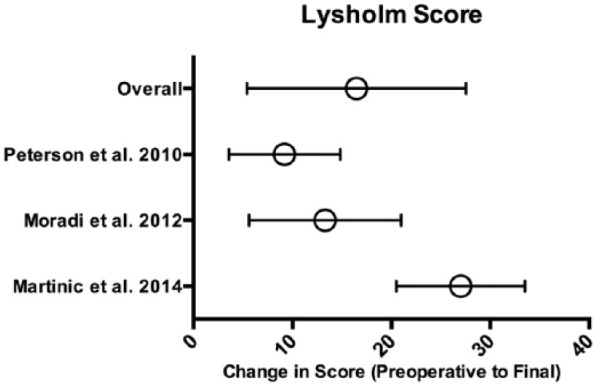
Lysholm scores. Means with error bars representing 95% CI (confidence interval).
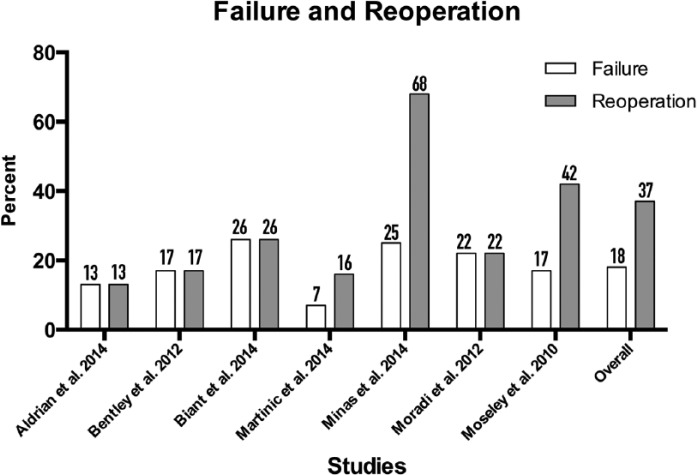
Reoperations and Failure
Reoperations and failures, as defined by each author, were reported in 7 of the 9 studies ( Fig. 5 ).14-17,29,33,43 Failures consisted of surgery due to evidence of failure of ACI on imaging or arthroscopy, revision cartilage surgery (such as repeat ACI, or conversion OAT or OCA), and progression to unicompartmental or total knee arthroplasty. The mean failure rate across all studies was 18%; the mean reoperation rate across all studies was 37%. There were a total of 217 reoperations as reported in 8 out of the 9 studies ( Table 3 ). Of all the reoperations, the largest proportion belonged to surgery unrelated to original defect at 35%, 29% due to lysis of adhesions, 19% for conversion to knee arthroplasty (total or unicompartmental), 19% for revision cartilage surgery, and 5% for planned second look for arthroscopic or histological evaluation. Because of 1 study reporting graft hypertrophy as “substantial number” of reported 72 complications, graft hypertrophy percentage may vary from 6% (if zero of those complications were hypertrophy) to 39% (if all 72 contained graft hypertrophy). Only 3 studies reported explicitly on postoperative infection—all 3 studies reported no incidences of either superficial or deep infection status-post cartilage repair with ACI.14-16
Figure 5.
Failure and reoperation rate. Numbers atop the bars represent percent scores of failure and reoperation.
Table 3.
Complications and Reoperations after Surgical Procedures.a
| Aldrian et al. (2014)14 | Bentley et al. (2012)29 | Biant et al. (2014)15 | Martinčič et al. (2014)16 | Minas et al. (2014)17 | Moradi et al. (2012)43 | Moseley et al. (2010)33 | Peterson et al. (2010)34 | Vasiliadis et al. (2010)35 | |
|---|---|---|---|---|---|---|---|---|---|
| Concomitant surgical procedures | |||||||||
| ACL reconstruction | 0 | 0 | NR | 10 | 11 | 0 | NR | 46 | NR |
| Meniscal allograft transplant | 0 | 0 | NR | 0 | 4 | 0 | 1 | b | NR |
| Meniscectomy | 0 | 0 | NR | 8 | 14 | 2 | 1 | b | NR |
| High tibial osteotomy | 0 | 0 | NR | 0 | 33 | 0 | 1 | 21 | NR |
| Tibial tubercle osteotomy | 0 | 0 | NR | 0 | 49 | 0 | NR | NR | NR |
| Complications and reoperations | |||||||||
| Total | 2 | 10 | 27 | 17 | 142 | 12 | 7 | NR | NR |
| Planned second look | 0 | 0 | 0 | 10 | 0 | NR | 0 | NR | NR |
| Graft hypertrophy | 0 | 0 | 0 | 1 | 72c | 8 | 3 | NR | 0 |
| Graft delamination | 0 | NR | 0 | 0 | 12 | NR | NR | NR | 2 |
| Revision cartilage surgery | 0 | 4 | 7 | 27 | NR | 3 | NR | NR | |
| Lysis of adhesions | 2 | 0 | 0 | 0 | 61 | NR | 0 | NR | NR |
| Unrelated to index defect | 0 | 0 | 0 | 5 | 70 | NR | 0 | NR | NR |
| TKA/UKA | 0 | 5 | 12 | 0 | 19 | 1 | 4 | NR | NR |
| Infection | |||||||||
| Deep | 0 | NR | 0 | 0 | NR | NR | NR | NR | NR |
| Superficial | 0 | NR | 0 | 0 | NR | NR | NR | NR | NR |
| Deep vein thrombosis | 0 | NR | 1 | 0 | NR | NR | NR | NR | NR |
ACL = anterior cruciate ligament; TKA = total knee arthroplasty; UKA = unicompartmental knee arthroplasty; NR = not reported.
All reported complications are reported as number of events.
Meniscus surgery reported as “before or during” autologous chondrocyte implantation (ACI) surgery
Graft hypertrophy reported as “substantial number” out of 72 complications.
Risk Factor Analysis
Of the 7 studies that reported reoperation and failure rates, a multivariate regression analysis was conducted between failure rate, reoperation rate, average age, and lesion size accounting for sample size of each cohort. Significant positive correlation was found when failure rate was compared with mean age of patient cohort (r = 0.149, P < 0.001) and mean lesion size (r = 0.574, P < 0.001). A stronger positive correlation was found when reoperation rate was compared to mean age (r = 0.811, P < 0.001). The correlation between reoperation rate and lesion size had the strongest correlation of almost 1 (r = 0.974, P < 0.001).
Four studies, each with a mean lesion size of less than 4.5 cm2 (230 patients) were compared to 3 studies, each with a lesion size of greater than 4.5 cm2 (386 patients). The failure rate in in those with mean lesion greater than 4.5 cm2 was significantly greater than those with a lesion size smaller than 4.5 cm2 with a odds ratio (OR) of 3.3 (less than 4.5 cm2 = 8.7%, greater than 4.5 cm2 = 23.8%, OR = 3.3, P < 0.001). Similar results were found for reoperation rate where the odds ratio for lesion size greater than 4.5 cm2 compared with less than 4.5 cm2 was 8.8 (less than 4.5 cm2 = 10.8%, greater than 4.5 cm2 = 51.6%, OR = 3.3, P < 0.001).
Discussion
Though authors have reported good short- to long-term outcomes for cartilage repair procedures, there have been few studies examining these outcomes over the long term to determine the procedure’s durability in restoring a functional cartilage surface. The current systematic review represents a comprehensive assessment of all studies with long-term follow-up of patients treated with ACI to repair cartilage defects. Based on this review, ACI results in significant increases in activity level and subjective patient outcomes from preoperative to postoperatively with an 82% rate of long-term success. Additionally, this durability may be higher (up to 92%) for lesions less than 4.5 cm2 and in younger patients, with a lower reoperation rate.
The current study found a significant improvement in Tegner score from preoperative to postoperative levels. Previously, a return-to-sport rate of 70% and 86% has been reported after ACI by Kon et al.44 and Niemeyer et al.45 at short- to mid-term follow-up. This agrees with previous findings by Mithoefer and Della Villa46 in which it was determined that ACI, compared with other cartilage restoration techniques, had the highest activity level as determined by Tegner score after ACI surgery. This rate of return-to-sport compares to rates of 93% after OAT and 79% after OCA, but these studies reported only short-term follow-up.47,48 In a study assessing follow-up longer than 2 years, rate of return-to-sport rate for MFX decreased from 80% at 2 years to 55% by the 6-year time point, likely representing deterioration of fibrocartilage over time,49 whereas the cartilage repair tissue in ACI may be more hyaline-like and have greater durability.50
This systematic review found significant improvement from preoperative to final IKDC and Lysholm scores of 24.9 and 16.5, respectively. The reported minimal clinically important differences (MCID) for the IKDC and Lysholm scores are 11.5 and 10.1, respectively.37,38 Therefore, at long-term, there was a greater than 200% MCID improvement in the IKDC score and greater than 150% in the Lysholm score. Interestingly, previous studies have demonstrated that an early positive response to improvement of symptoms predicts successful outcomes at mid-term follow-up, or similarly that an early poor response can persist over time.51 Pestka et al.51 reported an 81% chance of having a poor IKDC and Lysholm score after 36 months following ACI if a good score after 6 months was not achieved. On the other hand, Peterson and colleagues52 reported good or excellent clinical results in 50 of 61 patients after 2 years, which persisted in all 50 patients 5 to 11 years later. As reported by this group of authors, stiffness measurements were 90% or more of those of normal cartilage in 8 of 11 patients that were evaluated with an electromechanical indentation probe.52 This likely means that obtaining a durable cartilage repair tissue translated into a functional bearing surface that was long-lasting and resilient. In their systematic review of return to sport, Mithoefer and Della Villa46 have shown that ACI was the only cartilage restoration technique that was able to maintain function over time compared to 40% decline in MFX and 20% in OAT. Alternatively, patients may decrease their activity level to accommodate their level of symptoms, as has been suggested previously.53 Pelissier et al.54 reported a decreased activity level from a preoperative Tegner score of 7 to 6 at 2.5 years, and 4 at 10 years, even though improvement was seen in IKDC and Lysholm scores.
In the current review, patients had a rate of reoperation of 37% over the long term. This rate dramatically increased to more than 50% if the lesions treated by ACI were larger than 4.5 cm2. Specifically, most of the reoperations consist of operations due to ACI graft hypertrophy and lysis of adhesions, but a significant percentage were surgeries that represented failure of the graft or progression of osteoarthritis over time and included knee arthroplasty or revision cartilage surgery. Of the studies included, 6 studies only used periosteal membranes for their cartilage repair technique,16,17,33-35,43 and all studies used arthrotomy to perform the repair. The studies using periosteum had a reoperation rate of 52.6% compared with a reoperation rate of 15.1% for the studies that used collagen membranes. In a systematic review, it has been shown that ACI performed arthroscopically has a lower reoperation rate compared to those performed with an arthrotomy.55 In addition, the same review demonstrated that the reoperation rate of periosteum-ACI (24%) to be more than 4 times greater than either that of collagen membrane-ACI (5%).55 Therefore, with currently used techniques, the reoperation rate of ACI should be significantly lower than what is reported in the current study that includes primarily first generation techniques.
The long-term failure rate in this systematic review was 18%. It is important to emphasize that more than 50% of these patients had concomitant osteotomies, which may have allowed for such durable results. The rate of osteotomy (high tibial osteotomy or tibial tubercle osteotomy) was 39% by Minas et al.17 in 2014 compared with 9% for Peterson et al.34 in 2010. These osteotomies, while potentially increasing rehabilitation time and increasing morbidity, can be valuable if they increase durability by correcting varus or valgus deformities that may have contributed to the development of the cartilage lesion. Minas et al.,17 in their long-term follow-up of ACI, demonstrated that concurrent osteotomy significantly increased graft survivorship, with an impressive 15-year survival rate with proximal tibial osteotomy of 88%. On the other hand, failure rates can be higher than expected if preoperative background factors such as malalignment are not respected, such as in the prospective randomized trial comparing MFX with ACI.9 In that study, preoperative alignment radiographs were not obtained, and corrective osteotomy was performed only after failure of ACI or MFX. 9 This highlights the importance of concomitant alignment procedures to increase the long-term durability of the cartilage repair.
This study found that larger lesions and older patients had increased risk of reoperation and failure. This agrees well with previous studies. Age has been demonstrated to be an independent risk factor for outcome and return to activity following cartilage repair procedures.56,57 In addition, smaller lesion size has also been demonstrated to correlate with a higher level of postoperative activity.56,57 Minas et al.17 also reported that very large lesion size portended a worse outcome at long-term follow-up. Perhaps smaller lesion size represents a true focal lesion, whereas larger and multifocal lesions with a larger total surface area represent early osteoarthritis and degenerative change.58 Certainly, bipolar changes can represent advanced disease and have inferior outcomes when compared with unipolar lesions.34 A modifiable factor that may play a role in outcome is the surgical method of ACI utilized. As noted above, the failure rate was higher for periosteum-ACI (21%) compared with the studies that utilized collagen-ACI (15%). Similar results were found in a study by Harris et al.,55 where periosteum-ACI had a failure rate (8%) more than 6 times greater than collagen-ACI (1.5%). This may suggest that long-term follow-up of more contemporary techniques using collagen membranes may even have better results than in this systematic review of primarily first generation techniques.
There are strengths and limitations of this systematic review. Even though the mean time to follow-up is long term at 11.4 years and the mean MCMS score for our included studies is higher than that reported previously for cartilage procedures,42 most of the studies are Level IV retrospective studies due to the lack of long-term follow-up for Level I and Level II studies. The inclusion of 771 patients provides this review with statistical power, but the reporting of nonstandardized outcomes (such as only 4 studies using Tegner score) creates a difficulty in exhibiting statistical and clinical significance. Moreover, the heterogeneity in inclusion and exclusion criteria, concomitant procedures, lesion sizes, lesion types, and surgical technique (types of membranes used) create a limitation in our analysis for this current review with regards to patient outcomes and ACI durability.
Conclusion
Overall, ACI demonstrated good durability with successful outcomes in 82% of patients at long-term follow-up. Increased patient age and lesion size greater than 4.5 cm2 were risk factors for a higher reoperation and failure rate. Nonetheless, this systematic review is limited by heterogeneity in surgical technique, lesion and patient characteristics, and reporting of nonstandardized outcome measures.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Michael J. Stuart is a paid consultant for Arthrex, Inc and receives research support from Stryker. Aaron J. Krych is a paid consultant for Arthrex, Inc, and receives research support from Arthritis Foundation and Histogenics.
References
- 1. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231-7. [DOI] [PubMed] [Google Scholar]
- 2. Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartha L, Vajda A, Duska Z, Rahmeh H, Hangody L. Autologous osteochondral mosaicplasty grafting. J Orthop Sports Phys Ther. 2006;36:739-50. [DOI] [PubMed] [Google Scholar]
- 4. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994-1009. [DOI] [PubMed] [Google Scholar]
- 5. Hangody L, Dobos J, Balo E, Panics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125-33. [DOI] [PubMed] [Google Scholar]
- 6. Peterson L. Articular cartilage injuries treated with autologous chondrocyte transplantation in the human knee. Acta Orthop Belg. 1996;62(Suppl 1):196-200. [PubMed] [Google Scholar]
- 7. Basad E, Wissing FR, Fehrenbach P, Rickert M, Steinmeyer J, Ishaque B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg Sports Traumatol Arthrosc. 2015;23:3729-35. [DOI] [PubMed] [Google Scholar]
- 8. Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40:562-7. [DOI] [PubMed] [Google Scholar]
- 9. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 10. Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39:1668-75. [DOI] [PubMed] [Google Scholar]
- 11. Mithofer K, Minas T, Peterson L, Yeon H, Micheli LJ. Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33:1147-53. [DOI] [PubMed] [Google Scholar]
- 12. Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42:150-7. [DOI] [PubMed] [Google Scholar]
- 13. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 14. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42:2680-8. [DOI] [PubMed] [Google Scholar]
- 15. Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RW. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. 2014;42:2178-83. [DOI] [PubMed] [Google Scholar]
- 16. Martinčič D, Radosavljevič D, Drobnič M. Ten-year clinical and radiographic outcomes after autologous chondrocyte implantation of femoral condyles. Knee Surg Sports Traumatol Arthrosc. 2014;22:1277-83. [DOI] [PubMed] [Google Scholar]
- 17. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-75. [DOI] [PubMed] [Google Scholar]
- 20. Kon E, Filardo G, Venieri G, Perdisa F, Marcacci M. Tibial plateau lesions. Surface reconstruction with a biomimetic osteochondral scaffold: Results at 2 years of follow-up. Injury. 2014;45(Suppl 6):S121-5. [DOI] [PubMed] [Google Scholar]
- 21. Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M. Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop. 2014;38:1905-12. [DOI] [PubMed] [Google Scholar]
- 22. Dhollander AA, Verdonk PC, Lambrecht S, Verdonk R, Elewaut D, Verbruggen G, et al. Short-term outcome of the second generation characterized chondrocyte implantation for the treatment of cartilage lesions in the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:1118-27. [DOI] [PubMed] [Google Scholar]
- 23. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30-5. [DOI] [PubMed] [Google Scholar]
- 24. Marquass B, Mahn T, Engel T, Gossner J, Theopold JD, von Dercks N, et al. Clinical and radiological mid-term results after autologous osteochondral transplantation under consideration of quality of life [in German]. Z Orthop Unfall. 2012;150:360-7. [DOI] [PubMed] [Google Scholar]
- 25. Viste A, Piperno M, Desmarchelier R, Grosclaude S, Moyen B, Fessy MH. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop Traumatol Surg Res. 2012;98:737-43. [DOI] [PubMed] [Google Scholar]
- 26. Erdil M, Bilsel K, Taser OF, Sen C, Asik M. Osteochondral autologous graft transfer system in the knee; mid-term results. Knee. 2013;20:2-8. [DOI] [PubMed] [Google Scholar]
- 27. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96:824-30. [DOI] [PubMed] [Google Scholar]
- 28. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456-64. [DOI] [PubMed] [Google Scholar]
- 29. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504-9. [DOI] [PubMed] [Google Scholar]
- 30. Filardo G, Kon E, Perdisa F, Tetta C, Di Martino A, Marcacci M. Arthroscopic mosaicplasty: long-term outcome and joint degeneration progression. Knee. 2015;22:36-40. [DOI] [PubMed] [Google Scholar]
- 31. Solheim E, Hegna J, Øyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20:287-90. [DOI] [PubMed] [Google Scholar]
- 32. Ulstein S, Årøen A, Røtterud JH, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22:1207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moseley JB, Jr, Anderson AF, Browne JE, Mandelbaum BR, Micheli LJ, Fu F, et al. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in US patients. Am J Sports Med. 2010;38:238-46. [DOI] [PubMed] [Google Scholar]
- 34. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-24. [DOI] [PubMed] [Google Scholar]
- 35. Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943-9. [DOI] [PubMed] [Google Scholar]
- 36. Løken S, Ludvigsen TC, Høysveen T, Holm I, Engebretsen L, Reinholt FP. Autologous chondrocyte implantation to repair knee cartilage injury: ultrastructural evaluation at 2 years and long-term follow-up including muscle strength measurements. Knee Surg Sports Traumatol Arthrosc. 2009;17:1278-88. [DOI] [PubMed] [Google Scholar]
- 37. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Neyret P, Richmond JC, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34:1567-73. [DOI] [PubMed] [Google Scholar]
- 38. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37:890-7. [DOI] [PubMed] [Google Scholar]
- 39. Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am. 2004;86-A:1139-45. [DOI] [PubMed] [Google Scholar]
- 40. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2-11. [DOI] [PubMed] [Google Scholar]
- 41. Higgins JPT, Green S and. Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 42. Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-9. [DOI] [PubMed] [Google Scholar]
- 43. Moradi B, Schönit E, Nierhoff C, Hagmann S, Oberle D, Gotterbarm T, et al. First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years’ clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy. 2012;28:1851-61. [DOI] [PubMed] [Google Scholar]
- 44. Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549-57. [DOI] [PubMed] [Google Scholar]
- 45. Niemeyer P, Kostler W, Salzmann GM, Lenz P, Kreuz PC, Sudkamp NP. Autologous chondrocyte implantation for treatment of focal cartilage defects in patients age 40 years and older: a matched-pair analysis with 2-year follow-up. Am J Sports Med. 2010;38:2410-6. [DOI] [PubMed] [Google Scholar]
- 46. Mithoefer K, Della Villa S. Return to sports after articular cartilage repair in the football (soccer) player. Cartilage. 2012;3(1 Suppl):57S-62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gudas R, Stankevicius E, Monastyreckiene E, Pranys D, Kalesinskas RJ. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14:834-42. [DOI] [PubMed] [Google Scholar]
- 48. Krych AJ, Robertson CM, Williams RJ., 3rd Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053-9. [DOI] [PubMed] [Google Scholar]
- 49. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213-21. [DOI] [PubMed] [Google Scholar]
- 50. Vasara AI, Nieminen MT, Jurvelin JS, Peterson L, Lindahl A, Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005;(433):233-42. [DOI] [PubMed] [Google Scholar]
- 51. Pestka JM, Bode G, Salzmann G, Steinwachs M, Schmal H, Südkamp NP, et al. Clinical outcomes after cell-seeded autologous chondrocyte implantation of the knee: when can success or failure be predicted? Am J Sports Med. 2014;42:208-15. [DOI] [PubMed] [Google Scholar]
- 52. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 53. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971-8. [DOI] [PubMed] [Google Scholar]
- 54. Pelissier A, Boyer P, Boussetta Y, Bierry G, Van Hille W, Hamon P, et al. Satisfactory long-term MRI after autologous chondrocyte implantation at the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:2007-12. [DOI] [PubMed] [Google Scholar]
- 55. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19:779-91. [DOI] [PubMed] [Google Scholar]
- 56. Mithoefer K, Hambly K, Della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(Suppl 1):167S-76S. [DOI] [PubMed] [Google Scholar]
- 57. Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34:1413-8. [DOI] [PubMed] [Google Scholar]
- 58. Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465-80. [PubMed] [Google Scholar]