Abstract
Objectives
Conventional spinal cord stimulation (SCS) delivers a tonic waveform with consistent stream of pulses; burst delivers groups of pulses separated by short pulse‐free periods. The current study compared the short‐term safety and efficacy of burst with tonic stimulation in subjects already receiving SCS.
Materials and Methods
At 4 IRB‐approved sites, 22 subjects previously implanted with an SCS device for intractable, chronic pain gave informed consent and received burst stimulation for 14 days. Subjects reported average daily Visual Analog Scale (VAS) for overall, trunk, and limb pain using tonic stimulation and after 7 and 14 days of burst stimulation. Thoughts about pain were assessed using the Pain Catastrophizing Scale. Areas of paresthesia were assessed during tonic and burst stimulation using body maps. Assessment of patient satisfaction and preferred stimulation occurred after 14 days of burst.
Results
Average daily overall VAS reduced 46% from a mean of 53.5 (±20.2) mm during tonic SCS to 28.5 (±18.1) mm during burst (p < 0.001); trunk and limb VAS scores were also reduced by 33% and 51%, respectively. During burst, 16 subjects (73%) reported no paresthesia, 5 (23%) reported a reduction, and 1 (4%) reported increased paresthesia. After 14 days, 21 subjects (95%) reported being very satisfied or satisfied with burst. Burst was preferred by 20 subjects (91%), tonic by 1 (5%), and 1 (5%) reported no preference. Better pain relief was the most common reason cited for preference.
Conclusions
A majority of subjects reported improved pain relief using burst compared with tonic stimulation. Most subjects experienced less paresthesia during burst and preferred burst citing better pain relief.
Keywords: Burst stimulation, chronic pain, spinal cord stimulation, tonic stimulation
Introduction
Published literature indicates that spinal cord stimulation (SCS) reduces pain and medication use, improves quality of life, allows some patients to return to work, and provides cost savings with minimally significant adverse events (AE) for refractory neuropathic back and leg pain 1. However, an estimated 30–50% of patients receiving permanent SCS fail to achieve and/or maintain a 50% reduction in pain intensity on the Visual Analog Scale (VAS), indicating that a subset of patients may benefit from technological developments in SCS therapy 2, 3, 4.
Conventional SCS uses a tonic waveform that delivers a consistent stream of pulses at a pre‐set amplitude, frequency and pulse width creating paresthesias intended to cover most or all of the areas of pain. By making adjustments to frequency, pulse width, and amplitude, or by using cycle mode programs, patients can maximize the area of paresthesia coverage over the painful area. The burst waveform has been previously described in detail 5, 6. Briefly, burst programming delivers groups of pulses, called burst trains, which are separated by quiescent periods, called interburst intervals. Each burst train contains a series of pulses at constant pulse amplitude, pulse width, and interpulse frequency delivered in a pattern similar to that of burst firing neurons that are known to exist, alongside tonic firing neurons, in some pain pathways 7.
A form of burst stimulation for the treatment of pain has been used with transcutaneous electrical nerve stimulation devices 8, 9. More recently, de Ridder and colleagues compared the burst and tonic waveforms for subjects with chronic pain during an SCS trial period 6, 10. In both de Ridder studies, burst provided statistically superior pain relief, and all subjects preferred burst mode over tonic stimulation. AEs were not reported. Together, the work of de Ridder and colleagues suggests that burst stimulation is effective at reducing pain in SCS‐naïve subjects.
de Vos and colleagues reported the use of burst stimulation in 48 subjects who had six months of experience with tonic stimulation 11. VAS scores were reduced by 37% from baseline using tonic stimulation. After two weeks using burst stimulation, VAS scores were reduced by 62% from baseline. Subjects with failed back surgery syndrome (FBSS) whose response to tonic stimulation waned over time reported a 10% improvement in pain with burst, and FBSS subjects who maintained their response to tonic stimulation reported a 41% reduction in pain. Subjects with diabetic neuropathy reported a 58% reduction with burst. Three subjects reported mild AEs while using burst stimulation similar in type and severity to those reported in tonic SCS studies. The study by deVos and colleagues indicates that burst stimulation provided pain relief superior to tonic with limited side effects for subjects who were accustomed to tonic stimulation.
In a prospective, randomized trial, Schu and colleagues compared pain outcomes for one week each of tonic stimulation, burst stimulation, and placebo stimulation in subjects with FBSS who had at least three months of experience using tonic stimulation 6. Subjects reported the lowest pain scores while using burst stimulation, and 80% of subjects reported a preference for burst. No AEs were reported during the study.
This evidence suggests that burst stimulation may provide improved pain relief compared with tonic stimulation. The safety profile for burst, however, is also not well documented in the literature. The current study sought to evaluate the short‐term safety and efficacy of burst stimulation compared with tonic stimulation in subjects who were receiving tonic stimulation for at least 90 days.
Methods
This open‐label, multi‐site study compared burst stimulation to tonic stimulation. Subjects with a previously implanted SCS device were programmed with burst stimulation for 14 days. Subject assessment occurred while using tonic stimulation at the start of the study and again during burst stimulation at 7‐ and 14‐day visits. Subject enrollment occurred at 4 investigational sites in Australia from November 27 to December 5, 2013. All sites obtained appropriate institutional review board approval prior to recruitment.
Subjects
The number of subjects at each site was not controlled; consecutive, potentially eligible subjects were approached for participation in the study until 22 subjects, the maximum defined by the study protocol, were enrolled. Before enrollment, subjects provided written informed consent in which they were informed that the form of programming they would receive during the study may or may not produce paresthesia while providing pain relief. Subjects were not required to discontinue other current treatments, to modify current medications, or to undergo additional surgical procedures to participate in the study.
Subjects were enrolled if they: 1) provided informed consent and were willing to participate in study visits, 2) were at least 18 years of age, 3) had a diagnosis of chronic intractable pain of the trunk and/or limbs, 4) were previously implanted with an SCS device that was compatible with the investigational programmers, and 5) had at least 90 days with tonic stimulation. Subjects were not eligible for enrollment if they: 1) were currently participating in another study with an active study arm, 2) had SCS components that were incompatible with the investigational programmers, 3) had more than one IPG, 4) had peripheral leads, 5) had an infusion pump, or 6) were pregnant.
Devices and Stimulation Parameters
At the start of the study, subjects were assessed while using their favorite tonic stimulation program and cathode‐anode configuration. For burst programming, investigational device programmers were used in conjunction with commercially available SCS system components and leads (St. Jude Medical, Plano, TX). The investigational programmer allowed programming of burst stimulation mode, collection and transfer of stimulation parameters, and control of the patient programmer options. At the initial burst programming, the clinician programmed the electrode configurations and amplitude for burst stimulation based on the subject's effective tonic parameters and clinician discretion for achieving pain coverage. Programming sessions, initial or at seven days, were aimed at optimizing pain relief. For all subjects, burst programming contained burst trains occurring with a frequency of 40 Hz. Within each burst train, 5 pulses occurred at a frequency of 500 Hz with a pulse width of 1000 μs.
The patient programmer provided one burst program, allowed the subject to turn the stimulation on or off, and allowed the subject to adjust amplitude for the burst program. Subjects were unable to switch to tonic stimulation for the duration of the study. At the end of the study, subjects were returned to tonic stimulation programming.
Assessment Tools
Pain intensity was assessed using the VAS. Subjects indicated pain intensity by drawing a line on a 100 mm horizontal axis anchored by descriptors on each end (no pain and worst imaginable pain). Pain intensity was measured in mm from the left side of the scale (no pain) to the location indicated by the subject. Higher scores indicated greater pain intensity. Subjects were asked to complete three VAS scales at each of the three study visits recalling their average overall daily pain, average daily trunk pain, which includes back pain, and average daily limb pain during the previous week.
Subjects reported paresthesia using a standard body map labeled with numbered sections. The participant was instructed to shade in or place an X in the area(s) where sensations of stimulation/paresthesia were present. If marking the body map was not applicable due to a total lack of paresthesia, subjects were asked to indicate this in a check box on the body map form. Subject's paresthesia was classified as “no paresthesia” or by the percentage of change in the number of paresthesia areas during burst stimulation compared to the number of areas of paresthesia reported during tonic stimulation at the start of the study.
Subjects completed the validated Pain Catastrophizing Scale (PCS), which assesses feelings and thoughts experienced when a patient is experiencing pain (i.e., not at the current moment) 12. Pain catastrophizing, as a psychosocial aspect of the pain experience, has been linked to functional disability and may lead to delayed recovery for chronic pain patients 13. The scale includes 13 statements concerning pain experiences that are rated by the subject on a scale between 0 (“not at all”) and 4 (“always”). The scale is self‐administered and examines the following three domains: Rumination, Magnification and Helplessness. A higher score indicates a higher level of catastrophizing.
Subject satisfaction with burst stimulation was assessed using a 5‐item Likert scale (ranging from greatly dissatisfied to greatly satisfied) and by asking their preferred stimulation type (burst or tonic). Information about device usage and programming was collected via subject report.
Study Visits
Site investigators conducted study visits. Clinician reported the site investigator completed programming information and assessments. Either the site investigator or a nurse functioning as a study coordinator supervised self‐reported subject assessments.
Study Initiation
While continuing to use tonic SCS, the following assessments were conducted: VAS, demographics, pain history, PCS, and paresthesia mapping. After the tonic stimulation assessment, the subject's device was reprogrammed for burst stimulation. Each subject's burst program was defined as described above in the Devices and Stimulation Parameters section.
After Seven Days of Burst
Subjects returned to the clinic after seven days (±2 days) of burst stimulation. VAS, paresthesia mapping (only if their programming was modified), and programming information were recorded. If subjects requested changes to their stimulation, adjustments were made to the burst programming parameters to optimize pain relief, but subjects were not switched back to tonic stimulation at any point during the study.
After 14 Days of Burst
At a minimum of 14 days, but no more than 21 days, after the initial burst stimulation programming, subjects attended the final follow‐up and were exited from the study. Subjects reported VAS, PCS, programming information, paresthesia mapping (all subjects), satisfaction ratings, and subject preference of tonic or burst stimulation. Subjects were then exited from the study and returned to their original tonic stimulation mode with approved programming devices.
Safety
AEs and adverse device effects (collectively termed AEs) were solicited at every study visit, including any unscheduled visits. All AEs volunteered by the subject or elicited by the investigator were recorded from the time of consent through to the end of study whether or not considered device or procedure‐related. In the event of a reported AE, an appropriate intervention was initiated to ensure subject safety.
Data Analysis
Data from 22 subjects were included in analyses. The analyses were conducted with Microsoft Excel and SAS v9.3.using the available data; missing data were excluded. Significant effects were those with a probability lower than α = 0.05. The primary endpoint of this study was VAS scores during burst stimulation compared with baseline tonic stimulation. For each VAS score (trunk, limb, and overall daily), repeated measures analysis of variance (RMANOVA) determined differences in pain scores across study visits. For significant global F tests, Bonferroni‐corrected t‐tests provided pairwise comparisons between tonic stimulation and the 14‐day burst stimulation follow‐up. Effect sizes to estimate the magnitude of statistically significant effects are reported as Cohen's d for pairwise comparisons.
Secondary measures of paresthesia, change in paresthesia, satisfaction, and stimulation mode preference were examined using relevant descriptive statistics. Changes in thoughts about pain were inspected by computing a change in the PCS total score and subscale scores between tonic and burst stimulation. The distribution of subject's reported overall daily VAS while using tonic stimulation was examined, using metrics defined statistically by Aicher 14, as an exploratory inspection of possible influences on reported differences in pain intensity between burst and tonic stimulation.
Results
Subjects
A total of 44 patients were screened for enrollment; 23 subjects were enrolled. The most cited reason for a screen failure was lack of interest in the study. One subject was ineligible for inclusion because of an incompatible device and was withdrawn from the study before undergoing any study procedures. A total of 22 subjects were included in the study and received burst stimulation for 14 days. One subject failed to report VAS scores during tonic stimulation and was excluded from the primary analyses; this subject's data is included for descriptive measures not dependent on tonic‐burst comparison (e.g., preference, demographics, satisfaction). Subjects were, on average, 58 years old (±13.9), 13 (60%) were female, and 100% were Caucasian. The primary indications for these subjects were failed back surgery syndrome (N = 7, 32%), radiculopathies (N = 8, 36%), complex regional pain syndrome type I (N = 1, 4%), and 6 (26%) subjects reported chronic pain conditions of other origins, including disc injury/disease, nerve injury and neuropathic pain. Subjects reported an average of 8.9 years (±4.0) since the onset of their pain symptoms. The majority of subjects in the study were unemployed (N = 16, 73%), for the following reasons: disability (N = 3, 14%), retired (N = 6, 27%), or no reason provided (N = 7, 32%). Four (18%) subjects reported working full time, and 2 (9%) subjects reported working part time.
Devices and Stimulation Parameters
All subjects had percutaneous leads and a rechargeable implantable pulse generator (IPG); 21 (95.5%) subjects were implanted with 2 leads and 1 (4.5%) subject was implanted with 1 lead. Seventeen subjects (77%) had 8‐contact percutaneous leads; 4 subjects had Lamitrode S8 percutaneous paddle leads, and 1 subject did not report the lead model number. Twenty‐one of the 22 subjects had thoracic leads (19 with T8—T12 placement, 2 with T4—T7 placement). One subject had cervical (C6) lead placement.
The majority (14/22; 63.6%) of subjects used tonic stimulation for 18–24 h/d; 4 (18.2%) used tonic for 12–18 h/d, and the remaining 4 subjects (18.1%) used the stimulator for less than 12 h/d. Most subjects (15/22; 68.2%) reported recharging their IPG either two to three times per week or weekly and reported that recharging required less than two hours (18/22; 81.8%).
After 14 days of burst stimulation, the majority (20/22; 90.9%) of subjects used their device 18–24 h/d; 2 subjects reported using burst for 12–18 h/d. Most subjects (16/22; 72.7%) reported IPG recharging at a frequency of two to three times per week. Three subjects (13.6%) reported charging daily and three (13.6%) subjects reported recharging weekly or every other week while using burst stimulation. While using burst stimulation, the majority of subjects (11/22; 50.0%) reported needing less than 30 min to recharge the IPG.
During the subject's favorite tonic program, mean comfort amplitude was 6.5 mA (±3.4). Mean comfort amplitude during burst programs was 1.4 mA (±1.0). See Table 1 for perception and max tolerable amplitudes for both tonic and burst programs.
Table 1.
Summary of Programming Parameters for Both Tonic and Burst Stimulation
| Tonic | Burst | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Perception amplitude | 4.2 | 2.3 | 1.0 | 9.0 | 0.3 | 0.5 | 0.05 | 2.0 |
| Comfort amplitude | 6.5 | 3.4 | 1.0 | 14.0 | 1.4 | 1.0 | 0.05 | 4.2 |
| Max tolerable amplitude | 8.9 | 5.0 | 1.0 | 21.0 | 2.5 | 0.8 | 0.03 | 5.0 |
| Frequency | 54.8 | 18.8 | 30.0 | 96.0 | ** | ** | ** | ** |
| Pulse width (μs) | 319.5 | 78.0 | 200.0 | 500.0 | ** | ** | ** | ** |
**For all patients, burst was set to 40 Hz with an intraburst rate of 500 Hz, 5 pulses per burst, and a pulse width of 1000 μs.
Efficacy
The average overall daily VAS reported during tonic stimulation was, on average (±SD), 54.0 mm (±19.8), compared with 28.3 mm (±23.0) after seven days of burst stimulation, and 28.3 mm (±17.3) after 14 days of burst stimulation. RMANOVA revealed a significant difference across time (F[2,21] = 20.08, p < 0.001). Pairwise comparisons indicated that overall daily VAS was significantly reduced from tonic after both seven days [t(21) = −5.08, p = 0.001, d = 1.12] and 14 days [t(21) = −5.89, p < 0.001, d = 1.18] of burst stimulation (Fig. 1). The mean percentage change from tonic stimulation for overall daily VAS scores was 50% after seven days of burst stimulation and 46% after 14 days of burst stimulation. Of the 21 subjects with overall daily VAS scores at the start of the study, 16 (76%) reported a reduction in pain intensity after 14 days of burst stimulation. Five (24%) reported an increase in overall daily VAS scores after 14 days of burst. The increase for these subjects was 5%, on average, and no subjects had an increase in overall daily VAS greater than 10 mm on the scale.
Figure 1.
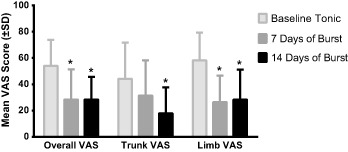
VAS Scores at Baseline, 7 days, and 14 days. Mean VAS scores (±SD) during baseline tonic stimulation, after 7 days of burst stimulation, and after 14 days of burst stimulation. * indicates statistically significant difference compared with baseline tonic stimulation (p < 0.05).
When subjects were stratified by overall VAS reported during tonic stimulation, 8/21 subjects (38%) reported overall daily VAS ≤ 47 mm with a mean (±SD) of 31.5 mm (±9.6); mean reported overall daily VAS while using burst stimulation for this group was 20.6 mm (±10.9). Five of the 8 subjects (63%) with a VAS ≤ 47 reported a reduction in VAS of any magnitude‐using burst. Thirteen of 21 subjects (62%) reported VAS > 47 mm using tonic stimulation with a group mean (±SD) of 67.9 mm (±7.4). Mean (±SD) overall daily VAS for subjects with a VAS > 47 was 32.7 mm (±25.6) after using burst stimulation for 14 days. Eleven of the 13 subjects (85%) with a VAS > 47 while using tonic reported a reduction in VAS of any magnitude while using burst stimulation.
At the start of the study, 16 subjects provided trunk VAS. Average trunk VAS was 44.2 mm (±27.5), 31.3 mm (±27.0) and 17.9 mm (±19.8) for tonic stimulation, day 7, and day 14 of burst, respectively. RMANOVA of trunk VAS scores indicated a significant difference across time (F[2,18] = 8.55, p = 0.002). Bonferroni comparisons revealed a significant reduction in trunk VAS scores after 14 days of burst [t(18) = −4.10, p = 0.002, d = 0.78] compared to tonic stimulation. Trunk VAS scores after seven days of burst were not significantly different from tonic stimulation [t(18) = −2.16, p = 0.13] (Fig. 1). The mean percentage of change in trunk VAS scores from tonic to burst stimulation was 31% at 7 days and 33% at 14 days.
For the 21 subjects reporting limb pain, mean (±SD) VAS was 58.2 mm (±21.2) during tonic stimulation, 26.4 mm (±20.2) after 7 days of burst stimulation, and 28.3 mm (±22.8) after 14 days of burst stimulation. A significant difference across time was revealed for the limb pain VAS (F[2,21] = 25.22, p < 0.001). Bonferonni comparisons indicated that limb VAS scores were significantly reduced after both 7 days [t(21) = −6.63, p < 0.001, d = 1.28] and 14 days [t(21) = −6.17, p < 0.001, d = 1.20] of burst compared to tonic stimulation (Fig. 1). Limb VAS scores changed from tonic, on average, by 51% after both 7 and 14 days of burst stimulation.
A reduction in overall daily VAS scores, of any magnitude, was reported by 76% (16/21) of subjects. Fourteen of 21 (67%) subjects reported a 30% or greater change in pain intensity; 10 of 21 (48%) subjects reported a 50% or greater change in overall daily VAS scores. Ten of the 16 subjects (63%) reporting trunk VAS scores at the start of the study reported both a 30% and 50% or greater reduction in pain. For limb VAS scores, 15 of 21 (71%) subjects reported at least a 30% pain reduction, and 10 (48%) reported at least a 50% reduction in pain scores.
Safety
A total of 4 AEs were reported by 3 subjects. Two events for the same subject were classified as device‐related. On 1 occasion, a subject reported dizziness along with a sensation of warm feet; at a later date, the same subject reported a persistent warm sensation of the foot at a moderate level of discomfort. The remaining 2 AEs were unrelated to the study procedures or device.
Paresthesia
All 22 subjects reported at least one area of paresthesia during tonic stimulation on the body map. After 14 days of burst stimulation, 16 subjects (73%) reported no paresthesia, 3 subjects (14%) reported between a 50% and 99% reduction in the number of paresthesia areas, 2 subjects (9%) reported between a 1% and 49% reduction in the number of paresthesia areas, and 1 subject (5%) reported an increase in the number of paresthesia areas.
Pain Catastrophizing Scale
During tonic stimulation, the mean total PCS score was 17.9 (±12.9). After 14 days of burst stimulation, mean total PCS score was 10.3 (±9.9), representing a mean difference of 7.6 points in PCS scores. While the decrease in scores was small for most subjects, the pattern of results was consistent, as indicated by a statistically significant difference (CI = −10.59, −4.61; p < 0.001).
The significant decrease in total PCS score reflects a decrease in all three subscales. Rumination scores decreased from 6.0 (±4.4) during tonic stimulation to 3.6 (±3.7) after 14 days of burst stimulation (CI = −3.52, −1.39; p < 0.001). The magnification subscale was 3.6 (±3.2) during tonic stimulation and decreased to 1.7 (±1.8) at the end of 14 days of burst stimulation (CI = −2.96, −0.95; p < 0.001). The average helplessness subscale score was 8.2 (±6.1) during tonic stimulation and 5.1 (±5.4) after 14 days of burst stimulation (CI = −5.02, −1.34; p = 0.002).
Satisfaction With Burst Stimulation and Preference
As shown in Table 2, 20 subjects (91%) reported being either very satisfied or satisfied with burst stimulation after seven days. After 14 days, 21 subjects (95%) reported being very satisfied or satisfied with the stimulation mode. The remaining subjects, 2 at 7 days and 1 at 14 days, reported being dissatisfied with burst stimulation.
Table 2.
Satisfaction Ratings at Each Follow‐Up Visit
| n(%) Out of 22 total patients | 7 days | 14 days |
|---|---|---|
| Very satisfied | 16 (73%) | 18 (82%) |
| Satisfied | 4 (18%) | 3 (14%) |
| Neither satisfied or dissatisfied | 0 (0%) | 0 (0%) |
| Dissatisfied | 2 (9%) | 1 (5%) |
| Very dissatisfied | 0 (0%) | 0 (0%) |
After 14 days of burst stimulation, burst mode was preferred by 20 subjects (91%), tonic was preferred by 1 subject (5%), and 1 subject (5%) reported no preference. The most common reason cited for preference was better pain relief with the preferred stimulation type. See Table 3 for more details.
Table 3.
Stimulation Preference and Reasons for Preference
| n(%) Out of 22 total patients | 7 days | 14 days |
|---|---|---|
| Preferred burst | 19 (86%) | 20 (91%) |
| Better pain relief | 16 | 18 |
| Lack of paresthesia | 3 | 2 |
| Preferred tonic | 2 (9%) | 1 (5%) |
| Better pain relief | 1 | 1 |
| Prefer paresthesia | 1* | 0 |
| No preference ** | 1 (5%) | 1 (5%) |
| No reason provided | 1 | 0 |
| No significant difference between therapies | 0 | 1 |
*Patient preferring tonic for paresthesia at day 7, changed preference to burst for better pain relief at day 14.
**The same patient rated no preference at both assessments.
Discussion
The results of our study suggest that burst stimulation, in the short term, is better than or equivalent to tonic stimulation for reducing pain intensity in subjects with chronic, intractable pain who have at least 90 days of experience using tonic stimulation. Most subjects (76%) reported a reduction in overall daily pain intensity while using burst stimulation compared to tonic. Significant decreases in rumination about pain, magnification of pain, and feelings of helplessness attributed to pain were also noted at the end of the two‐week study period as evidenced by reduced PCS total scores and reduced scores for each subscale. A majority of subjects reported reductions in paresthesia sensations and preferred burst to tonic stimulation citing better pain relief. Safety‐related events were mild and were similar to those seen during tonic stimulation.
IMMPACT recommendations suggest that a 15–20% change in VAS is clinically minimal 15. A VAS change of 30% or more is associated with a clinically meaningful change in pain intensity, and VAS improvements of 50% or more are linked to substantial changes in pain. After 14 days of burst stimulation, a majority of subjects in this study achieved a clinically meaningful reduction from tonic stimulation for average overall daily (63.3% of patients), average daily trunk (62.5% of patients), and average daily limb (68.2% of patients) pain, as indicated by a 30% or greater change in recalled VAS scores. Additionally, about half of the subjects (45–50%) reported a 50% or more reduction in pain intensity, achieving substantial changes in pain for each of the three VAS scores.
A proportion of the subjects (24%) reported an increase in average overall daily VAS. The increases for these subjects were less than 10 mm on the 100 mm scale, which according to IMMPACT recommendations is not a clinically meaningful change 15. For these subjects not reporting a decrease in pain intensity, pain while using burst stimulation was similar to that reported while the subjects used tonic stimulation indicating that, over the short‐term, burst stimulation is at least equivalent to tonic stimulation at reducing pain.
The differences between the mean pain intensity for tonic and burst stimulation revealed by this analysis may be driven by subjects whose pain scores were high at the start of the study while using tonic stimulation. In our sample, a larger proportion of subjects (85%) with higher pain intensities while using tonic reported reduced pain intensity while using burst; 63% of subjects with lower pain intensities using tonic reported a reduction using burst. By Aicher's metric defined statistically in headache subjects, VAS > 47 is considered severe pain 14. Jenson and colleagues, using means from postoperative pain subjects, defined a VAS from 45 to 74 as moderate pain and VAS > 75 as severe pain 16. By either metric, subjects in our study with a VAS over 47 continued to experience moderate to severe pain despite the use of tonic stimulation. While most subjects reported a reduction in pain intensity using burst stimulation, the magnitude of change appears to be larger for the subjects with a VAS > 47 while using tonic. These subjects may be driving the statistically significant differences revealed by our analysis. Larger studies are needed to properly investigate the ability of burst stimulation to provide rescue pain relief for subjects who are not achieving ideal pain relief using tonic stimulation.
The inferences drawn from this study are limited by the small cohort size, lack of a control group, and the short treatment duration. While most subjects experienced an improvement in pain relief using burst compared to tonic in the short term, this study precludes any conclusions about the long‐term safety and efficacy of burst stimulation. Moreover, limitations in study design do not rule out possible confounding influences such as additional attention to the subject by study personnel, biases inherent to convenience sampling, and lack of comparison to pre‐SCS baseline pain. The potential measurement error introduced by using recall for pain assessment in this study is likely minimized by the short duration of the study.
While providing additional safety information regarding burst stimulation, the results of this study show that burst stimulation may provide additional pain relief to some patients who have experience with tonic stimulation. The cohort for this study contained a variety of chronic pain conditions, increasing the representative value of our patient sample to heterogeneous chronic pain populations receiving treatment with SCS. Within the limitations of our study design, these findings supplement the growing body of literature supporting the use of burst stimulation for the treatment of chronic pain.
Acknowledgement
This was a sponsored clinical research study for St. Jude Medical.
Authorship Statements
Drs. Espinet, Courtney, Russo, Mitchell, Muir, and Verrils were investigators for this sponsored clinical research study and were responsible for patient recruitment and data collection. Kristina Davis assisted with protocol development, data analysis, and manuscript writing. All authors provided intellectual contributions to the manuscript and reviewed the final manuscript prior to submission. All authors contributed to the manuscript in accordance with ICMJE guidelines for authorship.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/bw/submit.asp?ref=1094‐7159&site=1
Source(s) of financial support: St. Jude Medical funded this study.
Conflict of Interest: Drs. Espinet, Courtney, Mitchell, Russo, and Verrills are paid consultants for St. Jude Medical. Kristina Davis is an employee of St. Jude Medical. Drs. Courtney and Mitchell serve on advisory boards for St. Jude Medical.
References
- 1. Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: Results of a systematic review and meta‐analysis. J Pain Symptom Manage 2006;31 (4 Suppl.):S13–S19. [DOI] [PubMed] [Google Scholar]
- 2. Kumar K, Taylor RS, Jacques L et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179–188. [DOI] [PubMed] [Google Scholar]
- 3. Kumar K, Taylor RS, Jacques L et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24‐month follow‐up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008;63:762–770. [DOI] [PubMed] [Google Scholar]
- 4. Oakley JC, Weiner RL. Spinal cord stimulation for complex regional pain syndrome: a prospective study of 19 patients at two centers. Neuromodulation 1999;2:47–50. [DOI] [PubMed] [Google Scholar]
- 5. DeRidder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia‐free pain suppression. Neurosurgery 2010;66:986–990. [DOI] [PubMed] [Google Scholar]
- 6. Schu S, Slotty PJ, Bara G, von Knop M, Edgar D, Vesper J. A prospective, randomised, double‐blind, placebo‐controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;17:443–450. doi: 10.1111/ner.12197 [DOI] [PubMed] [Google Scholar]
- 7. Lopez‐Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur J Neurosci 1994;6:998–1007. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson MB, Sjölund BH, Nielzén S. Long term results of peripheral conditioning stimulation as an analgesic measure in chronic pain. Pain 1979;6:335–347. [DOI] [PubMed] [Google Scholar]
- 9. Mannheimer C, Carlsson CA. The analgesic effect of transcutaneous electrical nerve stimulation (TNS) in patients with rheumatoid arthritis. A comparative study of different pulse patterns. Pain 1979;6:329–334. [DOI] [PubMed] [Google Scholar]
- 10. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;80:642–649. [DOI] [PubMed] [Google Scholar]
- 11. de Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2013;17:152–159. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7:524–532. [Google Scholar]
- 13. Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing—a prognostic factor for outcome in patients with low back pain: A systematic review. Spine J 2014;14:2639–2657. doi: 10.1016/j.spinee.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Aicher B, Peil H, Peil B, Diener HC. Pain measurement: Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgia 2012;32:185–197. [DOI] [PubMed] [Google Scholar]
- 15. Dworkin RH, Turk DC, Wyrwich KW et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 16. Jenson MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J Pain 2003;4:407–414. [DOI] [PubMed] [Google Scholar]


