Abstract
Objective
To assess the cost‐effectiveness and cost‐utility of Spinal Cord Stimulation (SCS) in patients with failed back surgery syndrome (FBSS) refractory to conventional medical management (CMM).
Materials and Methods
We conducted an observational, multicenter, longitudinal ambispective study, where patients with predominant leg pain refractory to CMM expecting to receive SCS+CMM were recruited in 9 Italian centers and followed up to 24 months after SCS. We collected data on clinical status (pain intensity, disability), Health‐Related Quality‐of‐Life (HRQoL) and on direct and indirect costs before (pre‐SCS) and after (post‐SCS) the SCS intervention. Costs were quantified in € 2009, adopting the National Health Service's (NHS), patient and societal perspectives. Benefits and costs pre‐SCS versus post‐SCS were compared to estimate the incremental cost‐effectiveness and cost utility ratios.
Results
80 patients (40% male, mean age 58 years) were recruited. Between baseline and 24 months post‐SCS, clinical outcomes and HRQoL significantly improved. The EQ‐5D utility index increased from 0.421 to 0.630 (p < 0.0001). Statistically significant improvement was first observed six months post‐SCS. Societal costs increased from €6600 (pre‐SCS) to €13,200 (post‐SCS) per patient per year. Accordingly, the cost‐utility acceptability curve suggested that if decision makers' willingness to pay per Quality‐Adjusted‐Life‐Years (QALYs) was €60,000, SCS implantation would be cost‐effective in 80% and 85% of cases, according to the NHS's and societal point of views, respectively.
Conclusions
Our results suggest that in clinical practice, SCS+CMM treatment of FBSS patients refractory to CMM provides good value for money. Further research is encouraged in the form of larger, long‐term studies.
Keywords: Cost‐effectiveness, cost‐utility, failed back surgery syndrome, Spinal Cord Stimulation, quality adjusted life years
Introduction
Failed back surgery syndrome (FBSS) represents one of the main causes of chronic neuropathic or mixed pain. It has been estimated to affect 0.61% of the general population, with an annual incidence of 0.033% 1. In particular, it is estimated that 30% of patients undergoing lumbar spinal surgery will develop FBSS 2.
Compared to the general population, patients with neuropathic pain report lower levels of Health‐Related Quality‐of‐Life (HRQoL). In particular, patients with FBSS have reported the lowest health utility score 3 among the following categories of patients with neuropathic pain: diabetic neuropathy, post‐herpetic neuralgia, phantom limb pain, central neuropathy pain, trigeminal neuralgia, and mixed neuropathy pain.
In patients who experience persistent pain after conventional medical management, Spinal Cord Stimulation (SCS) might be recommended 4. Introduced in the treatment of chronic pain more than 45 years ago 5, SCS has been reported to be effective in relieving pain, improving HRQoL, and reducing disability in FBSS patients 6, 7, 8, 9, 10, 11.
The impact of FBSS and its management on individuals' health and its economic cost to society are considerable 12. Several evaluations have been performed to understand the effectiveness, cost impact, and cost‐effectiveness of SCS treatment in FBSS patients 6, 7, 8, 13, 14, 15, 16.
The Prospective Randomised Controlled Multicentre Trial of the Effectiveness of Spinal Cord Stimulation (PROCESS) 14 estimated the effectiveness of SCS+CMM versus CMM alone in FBSS patients. Important improvements in pain relief, function, and HRQoL were reached six months post‐SCS implant 8, 15 and were sustained at 24 months follow‐up 6, 7. Manca et al. 15 analyzed data from the PROCESS trial and found that 15% of the additional mean cost of SCS is offset within 6‐months by a reduced use of drugs and other non‐drug treatments for pain relief. In 2010, Taylor and colleagues 16 input the results of the PROCESS study into a cost‐effectiveness model, comprised of a short‐term decision tree and long‐term Markov model to estimate the cost‐effectiveness of SCS+CMM versus CMM alone over a 15‐year time period and found an incremental cost per Quality‐Adjusted‐Life‐Years (QALY) ratio of £5624 with an “89% probability that SCS is cost effective at a willingness to pay threshold of £20,000.” In 2008, the National Institute of Health and Care Excellence (NICE) recommended the use of SCS for the treatment of neuropathic pain, including pain caused by FBSS 17. However, NICE specified that it is necessary to conduct observational research able to generate robust evidence regarding the durability of SCS benefits in a real‐world context 17. The present work compares the effects and costs of SCS as an adjunct to CMM treatment with those of CMM alone applied in real‐world clinical practice for patients affected by FBSS.
Materials and Methods
Subjects and Setting
All consecutive patients who, between June 2005 and October 2007, satisfied the eligibility criteria for receiving SCS were recruited from 9 Italian specialized centers (6 pain units and 3 neurosurgery wards). Centers were distributed across the country, and all have at least five years of experience in the management of patients with FBSS with spinal cord stimulation. Inclusion and exclusion criteria used for this study, listed in Table 1, reflect recommendations that have been recently published 18, 19.
Table 1.
Eligibility Criteria
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|---|
|
Eligible patients received information on: 1) the aim of the study; 2) the SCS surgical procedures and potential clinical outcomes; 3) the technical variables (e.g., the self‐regulation parameters), related both to the external neurostimulator (ENS) during the stimulation test period (screening trial) and to the fully implantable neurostimulator (INS); 4) the possible complications; 5) the study data collection procedure. Eligible patients had to sign an informed consent form after receiving all the necessary information on the aim of the study, the type of data and the method of data collection. The study participation of each center was previously approved by the Local Ethics Committee, present in each hospital according to the Italian regulations on clinical research.
Procedure
All the participants, after the lead implantation, were clinically studied during the screening trial. Following clinical practice 2 and according to the study protocol, those who responded positively to the screening trial were implanted with a non‐rechargeable INS and were observed for up to 24 months. A test screening is considered positive when patients experience at least 50% pain relief and at least 80% overlap of pain with stimulation‐induced paresthesia. We did not continue to collect data on patients that did not respond positively to the test and on patients that for different reasons stopped the study before the scheduled 24‐month follow‐up period.
Observational Period
A schematic definition and duration of the observational period is reported in Figure 1. Specifically, the observational period included a pre‐SCS and a post‐SCS period. The pre‐SCS period included the 12‐month period before SCS: 11 months before enrollment and 1 month from enrollment to SCS intervention. The post‐SCS period was intended to be up to 24 months after the SCS intervention and was divided into6‐month periods according to the scheduled follow‐up visits.
Figure 1.
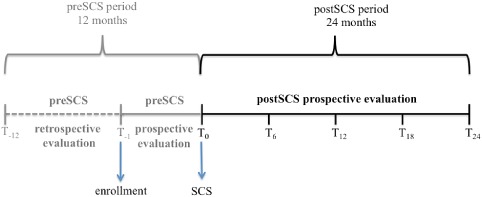
Schematic definition and duration of the observational period.
Data Collection
We collected data on the sociodemographic and clinical characteristics of the patients, their HRQoL, and resource consumption (direct and indirect costs) using a Case Report Form (CRF) and a patient diary.
Data referring to the retrospective pre‐SCS period were collected using a set of questions in the CRF. The physicians answered these questions by asking the patients to recall what happened in the specified pre‐SCS period. Data relating to the prospective pre‐SCS (i.e., from enrollment to SCS) and the post‐SCS period were collected in the CRF at implant and at each scheduled 6‐month follow‐up visit. During the follow‐up period, the patients had a daily diary to be completed in the week before the follow‐up visit, collecting information on pain intensity and drug consumption, which were collected during the follow‐up visit.
Sociodemographic and Clinical Data
Sociodemographic data comprising age, gender, marital status, working status, and education were collected by the physician at enrollment using the CRF. Pain intensity was recorded using the 0–10 NRS 20. The level of disability was measured with the Oswestry Disability Index (ODI), which consists of 10 questions with six possible responses to measure the disability level and functional capability of a person according his/her health status in the last two weeks 21.
HRQoL Data
The patients self‐completed 2 generic questionnaires: the Medical Outcome Study Short Form36 (SF‐36) 22 and the EQ‐5D (now known as the EQ‐5D‐3L) 23. These questionnaires were chosen for their capability to assess both physical and psychological components of health, as they allow comparison within and between different clinical conditions and with the general population. The use of these outcome measures is consistent with other recent studies on FBSS 3, 6, 7.
In the present paper we focus on the results obtained with the EQ‐5D descriptive system (or profile), from which we obtained the QALYs to conduct the economic evaluation. In particular, the EQ‐5D is a generic instrument for describing and valuing individuals' health. The tool consists of two parts: a descriptive system (EQ‐5D profile) and a visual analogue scale (EQ‐5D VAS).With the EQ‐5D the respondents are asked about their HRQoL on the current day. The responses of the EQ‐5D descriptive system can be converted into utility indexes by means of an algorithm that uses population‐based (social) values. The utility index corresponds to the estimate of value of health with a score anchored between 0, corresponding to death, and 1, corresponding to perfect health. Utility indexes are widely used in different disease areas and recommended for the calculation of QALYs to be applied in economic evaluations of health technologies 24.
Resources Consumption and Costs
Data on resource consumption attributable to FBSS and its treatment were collected in the CRF by the physician (e.g., hospitalizations, medical visits, examinations, travels/accommodations, days of productivity lost) and in the diary by the patients (drugs). From these data, we were able to quantify in monetary terms direct medical, nonmedical, and indirect costs attributable to FBSS and its treatment.
Direct medical costs were grouped into two groups: costs related to SCS‐related procedures, and costs incurred for other reasons attributable to FBSS and its treatment. The first group included hospital costs for lead implantation and replacement, implantation and replacement of the INS, and lead removal after screening trial failure. The remaining medical costs included costs related to general practitioner (GP) consultations, specialist consultations and SCS‐related follow‐up consultations, Emergency Room (ER) visits, medical aids, laboratory and instrumental diagnostic examinations, drug therapies, rehabilitation therapies, complementary therapies, and hospitalizations. Direct non‐medical costs included costs of travel and/or accommodation to reach the healthcare providers, and costs of formal care at home. Indirect costs were estimated in terms of productivity loss of the family caregivers (e.g., spouse, other relatives or friends) who helped the patients to manage their condition.
Medical costs were quantified using tariffs and prices applicable in Italy in 2009 and inflated to €2009 25. Diagnostic Related Group (DRG) tariffs 26 were used to value hospitalizations, apart from those related to SCS procedures; the available DRG tariffs were not sufficient to cover all the costs of the SCS procedures and devices, requiring coverage through hospital budget. Therefore, in order to realistically reflect the cost paid by the Italian National Health Service (NHS) for these procedures, we used the hospitalization costs obtained from a previous micro‐costing analysis, where all the cost items attributable to the hospitalization for the SCS procedure were included: trial leads, equipment, personnel, energy consumption, cleaning, housing and food, etc. 27. The cost of the SCS medical devices were provided by Medtronic (data on file, Medtronic Italia) and applied to all the devices used during the study, including devices from other companies. Other medical costs were quantified using the official outpatient tariffs 28 for ER visits, diagnostic examinations, rehabilitation therapies and specialist medical consultations. Specific national tariffs 29 or market prices (data on file, Medtronic Italia) were used for orthopedic aids, depending on whether their cost was reimbursed by the NHS or paid by the patients, respectively. Drug costs were quantified using market prices 30. GP consultations were quantified using tariffs that were estimated and published in literature 31. Complementary therapies, which are not reimbursed by the NHS, were quantified using the information on out‐of‐pocket costs reported by the patients. Direct non‐medical costs were quantified according to the information reported by the patients. Indirect costs were assessed using the human capital approach 32. With this approach, the loss of productivity attributable to the disease and its consequences is quantified in terms of absence from work, and is converted into monetary terms by multiplying the time (days, hours) lost from work by the subjects' remuneration. Because most of the participants in the study were older than 60 years or female, hence likely retired or housewives without a paid job, we decided not to calculate patients' productivity loss, but to focus on the productivity loss of their family caregivers. In particular, we obtained the mean remuneration per day by dividing the caregivers' mean income by 220 working days resulting from the Italian National Institute of Statistics 25. This remuneration per day was then multiplied by the number of days reported as missed from work by the family caregivers with a paid job.
Data Analyses
Baseline patient characteristics were described using absolute and/or relative frequencies, while continuous variables such as time and health status measures were summarized by mean values along with standard deviation (±S.D) and 95% confidence interval (95% CI) as dispersion measures. Costs and effects occurring before and after the SCS intervention were estimated using proportions or means. In particular, direct and indirect costs were calculated as mean €/patient‐year and reported along with the 95% CI. We used the EQ‐5D descriptive system to calculate utility scores by means of an algorithm that uses population‐based (social) values recently estimated in Italy 33. QALYs were then estimated by multiplying the utility scores by the time period (years) referring to the corresponding HRQoL 32.
To obtain the disability rating from the ODI, we applied a specific algorithm 21 grouping patients as follows: 1) 0–20% minimal disability; 2) 21–40% moderate disability; 3) 41–60% severe disability; 4) 61–80% crippled; 5) 81–100% serious.
To avoid possible bias in favor of the SCS treatment (e.g., if only patients that benefited from this procedure remained in the study), missing follow‐up data on costs and benefits of patients who did not continue the study due to screening trial failure or for other reasons during the observational period, were managed using the Last Observation Carried Forward (LOCF) approach 34. In particular, for each patient that did not continue the study until the scheduled end of observational period, we carried forward to 24 months the mean costs (except the upfront SCS related costs, which were applied only at the start of the study), the mean utility indexes and the ODI scores calculated from the last data available.
Comparisons between enrollment and follow‐up were performed using the parametric paired Student's test or the non‐parametric paired Wilcoxon signed rank test for effect measures, depending on the type and distribution of data, assessed using the Shapiro‐Wilk test. To compare utilities and costs between pre‐SCS and post‐SCS we performed a non‐parametric bootstrap with bias corrected and accelerated 35.
The two following techniques were adopted in the economic evaluation: Cost‐Effectiveness Analysis (CEA) and Cost‐Utility Analysis (CUA) 32, which differ in how the benefits, or outcome effects, results are calculated. Both techniques compare the two programs by a ratio, named incremental cost‐effectiveness ratio (ICER) in the CEA, and incremental cost‐utility ratio (ICUR) in the CUA. They correspond to the ratio between the difference in costs (numerator) and the difference in effects (denominator) of the two treatment options, expressing the amount of incremental cost per unit of additional effect. In particular, in the numerator we included the difference between pre‐SCS and post‐SCS periods in the mean of direct plus indirect costs. With regards to the denominator in the CEA, as a measure of clinical effects, we included the incremental NRS given by the difference between pre and post SCS periods in the mean of the Pain Numerical Rating Scale (NRS) 20. In the CUA, the QALY was used as a parameter that estimates individuals' value of health.
The ratio comparison between the above mentioned numerator and denominators resulted respectively in the incremental Cost/NRS ratio (ICER) and the incremental Cost/QALY ratio (ICUR). Uncertainty due to estimation of effects and costs was tested calculating the cost‐effectiveness acceptability curve with the non‐parametric bootstrapping approach 36. The ICER and ICUR analyses were conducted by adopting the NHS, the patient and the societal points of view. NHS in Italy is responsible for providing and paying for most direct medical costs to manage the target patients (e.g., cost of SCS surgery and devices, cost of drug treatment, cost of examinations and medical visits). Patients generally pay for direct non‐medical costs (e.g., formal assistance and travelling/accommodation to reach the healthcare providers) and sometimes also sustain out‐of‐pocket costs for medical resources absorbed in the private sector. The broadest perspective is the societal one, as it also includes the two specified above.
All analyses were conducted using Stata SE 12 (StataCorp, College Station, TX, USA) software.
Results
A total of 80 patients were enrolled in the study. Patients' sociodemographic, clinical and HRQoL description at enrollment are shown in Table 2. After a mean of 46 days from enrollment, the patients were implanted with a lead and observed during the screening trial. Eight patients (10%) had a negative response to the screening trial. After an average of 30 days from lead implantation, patients with a positive test response were implanted with an INS. During follow‐up, the INS was replaced in 8 patients (10%; 1 within 12 months and 7 between 12 and 24 months), while lead dislocation occurred twice in 1 patient, necessitating replacement (1.25% of patients; between 12 and 24 months). During the follow‐up, 17 patients withdrew from the study for the reasons specified in Figure 2.
Table 2.
Patients' Characteristics at Study Enrollment Time
| Mean or frequency | ±S.D. | 95% CI (lower‐upper) | |
|---|---|---|---|
| Total number of patients, N | 80 | – | – |
| Age (years) | 58 | 13 | 54.9–60.7 |
| Male, N (%) | 40 | – | – |
| Education, (%) | |||
| Primary | 49 | – | – |
| Lower secondary | 34 | – | – |
| Upper secondary | 15 | – | – |
| Graduate | 1 | – | – |
| None | 1 | – | – |
| Number of previous surgical interventions, N (%) | |||
| 1 | 23 (33%) | – | – |
| 2 | 31 (44%) | – | – |
| 3 | 13 (19%) | – | – |
| 4 | 3 (4%) | – | – |
| Not available | 10 | – | – |
| Age (years) at pain onset | 48 | 14 | 43.9–51.9 |
| Time (years) between pain onset and recruitment | 11 | 9 | 8.4–13.6 |
| NRS | 7.6 | 1.5 | 7.3–7.9 |
| Oswestry class, N (%) | |||
| Minimal | 0 (0%) | – | – |
| Moderate | 7 (9%) | – | – |
| Severe | 31 (39%) | – | – |
| Crippled | 35 (44%) | – | – |
| Serious | 7 (9%) | – | – |
| EQ‐5D utility | 0.421 | 0.303 | 0.353–0.488 |
Figure 2.

Number of participants observed during follow‐up.
The proportion of patients classified as “severe,” “crippled” or “serious” according to the ODI classes (91% at baseline) decreased significantly (z = 5.754, p < 0.0001) 24 months post‐SCS treatment (47.5%). Accordingly, the mean ODI decreased significantly (t = 7.9845, p < 0.0001) from 61.6 at baseline to 42.4 after 24 months, with statistically significant (t = 6.9333, p < 0.0001) improvement first seen from 61.6 at baseline to 45.6 six months post‐SCS treatment (Table 3). A similar trend was observed with the mean NRS clinical score and with the mean EQ‐5D utility index (Fig. 3). The mean NRS score decreased from 7.56 to 5.11 after 24 months post‐SCS (t = 9.0026, p < 0.0001), which is both clinically and statistically significant. The mean EQ‐5D utility index increased from 0.421 to 0.630 post‐SCS (bootstrap‐t method z = −6.27, p < 0.001). Again, the significant difference in these scores was observed in the first six months post‐SCS. The scores remained stable or increased slightly in the following period.
Table 3.
Oswestry Disability Index
| Oswestry Disability Index | Enrollment | 6m post‐SCS | 12m post‐SCS | 18m post‐SCS | 24m post‐SCS |
|---|---|---|---|---|---|
| Mean ODI (±SD) | 61.6 (±15.0) | 45.6 (±20.1) | 45.5 (±19.6) | 43.0 (±19.2) | 42.4 (±20.1) |
| ODI classes, N patients (%) | |||||
| MINIMAL DISABILITY (0–20) | 0 (0.0) | 9 (11.3) | 8 (10.1) | 7 (8.9) | 10 (12.8) |
| MODERATE DISABILITY (21–40) | 7 (8.8) | 26 (32.5) | 26 (32.9) | 37 (47.5) | 31 (39.7) |
| SEVERE DISABILITY (41–60) | 31 (38.7) | 25 (31.2) | 29 (36.7) | 17 (21.8) | 19 (24.4) |
| CRIPPLED (61–80) | 35 (43.7) | 18 (22.5) | 12 (15.2) | 15 (19.2) | 16 (20.5) |
| SERIOUS (81–100) | 7 (8.8) | 2 (2.5) | 4 (5.1) | 2 (2.6) | 2 (2.6) |
| TOTAL * | 80 (100.0) | 80 (100.0) | 79 (100.0) | 78 (100.0) | 78 (100.0) |
*The total number of patients sum up to the total number of subjects expected to be living at each time period (i.e., excluding the two who died between 6 and 18 months), according to the LOCF approach used to manage missing data from those who dropped out or were lost to follow‐up.
Figure 3.
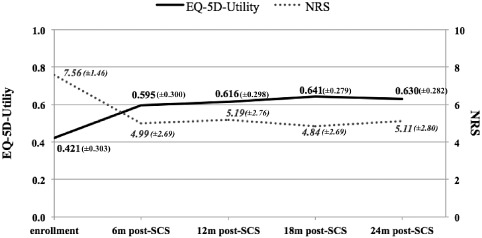
NRS and EQ‐5D‐utility mean (±SD) values during the observational period.
Table 4 shows the mean amount per patient per year of healthcare resources utilized in the pre‐ and post‐SCS periods, while Table 5 focuses on the related mean costs. During the pre‐SCS and post‐SCS periods, the overall cost to society was €6600 and €13,200/patient‐year respectively. In regards to the NHS perspective, while €2600/patient‐year were spent before SCS implantation, costs increased significantly to almost €11,000/patient‐year after SCS (leads and INS). This increase was specifically generated by the high cost of INS devices. Nevertheless, as shown in Figures 3 and 4, while total costs run up in the first year after SCS interventions (around €21,000 per patient in the 0–12 months post 12m post‐SCS period), they decreased in the following months. The costs to society were even lower (€5500 per patient in the 12–24 months post‐SCS period) than those incurred before SCS implant (€6600/patient‐year). In particular, SCS‐related costs were partially offset by savings obtained in non‐SCS‐related medical costs (€1129/patient‐year) for the NHS (Table 5). Furthermore, cost savings were estimated for the patients and their families (€813/patient‐year) and in terms of productivity gain for society as a whole (€682/patient‐year). The utility gained during the observational period corresponds to a QALY increase of 0.173, generating a cost per QALY gained of €38,372 and of €47,000, from the societal and NHS points of view, respectively. The incremental cost per NRS gained corresponds to €2631 and €3222, according to the NHS and societal points of view, respectively (Table 6). Furthermore, when considering the patients' point of view, the SCS+CMM option is dominant, i.e., it is both more beneficial and less costly than CMM alone, with a cost saving of €800/patient‐year.
Table 4.
Mean Number per Patient‐Year of Medical Resources Consumed
| Resource type | Number/patient‐year | |
|---|---|---|
| Pre‐SCS | Post‐SCS | |
| Emergency Room Admissions | 0.5 | 0.3 |
| Medical Aids | 0.8 | 0.3 |
| Diagnostic Exams: | 3.9 | 1.6 |
| X‐ray spine | 0.8 | 0.5 |
| MNR spine | 0.8 | 0.2 |
| CAT spine | 0.6 | 0.3 |
| Bone scintigraphy | 0.1 | 0.0 |
| ECO abdomen | 0.2 | 0.1 |
| EMG | 0.4 | 0.1 |
| Neurophysiological exams | 0.1 | 0.0 |
| Blood tests | 0.8 | 0.4 |
| ECG | 0.0 | 0.0 |
| Urodynamic exams | 0.0 | 0.0 |
| Urine analysis | 0.0 | 0.0 |
| Drugs * | _ | _ |
| SCS‐related Hospital Admissions: | 0.0 | 1.0 |
| INS implantations | 0.0 | 0.5 |
| LEAD implantations | 0.0 | 0.5 |
| INS replacements | 0.0 | 0.1 |
| Other (lead re‐positioning/replacement) | 0.0 | 0.0 |
| Non‐SCS Related Hospital Admissions: | 0.3 | 0.2 |
| Surgical admissions | 0.1 | 0.0 |
| Medical admissions | 0.2 | 0.1 |
| Complementary Therapies * | _ | _ |
| Rehabilitation, Instrumental and Analgesic Therapies: | 23.6 | 14.1 |
| Motor rehabilitation | 6.2 | 2.2 |
| Postural exercises | 2.3 | 4.3 |
| Assisted exercise in water | 2.5 | 4.6 |
| Massotherapy | 2.5 | 0.9 |
| Lymphdrainage | 0.0 | 0.1 |
| Pain electrotherapy | 3.5 | 0.6 |
| Electromagnetic therapy | 2.0 | 0.1 |
| Iontophoresis | 0.5 | 0.2 |
| Therapy with ultrasound | 0.2 | 0.1 |
| Laser pain therapy | 2.2 | 0.1 |
| Injection of therapeutic substances in joints or ligaments | 1.8 | 1.1 |
| Mesotherapy | 0.0 | 0.0 |
| Epidural steroids injections | 0.0 | 0.0 |
| GP Consultations: | 30.5 | 14.2 |
| Ambulatory | 24.3 | 11.5 |
| Home visits | 6.2 | 2.7 |
| Medical Specialist Consultations: | 4.9 | 1.5 |
| Neurologist | 1.8 | 0.6 |
| Orthopedist | 1.5 | 0.5 |
| Neurosurgeon | 0.6 | 0.2 |
| Psychologist | 0.0 | 0.0 |
| Geriatrician | 0.0 | 0.0 |
| Physiatrist | 0.9 | 0.2 |
| Anaesthetist/pain therapist | 0.3 | 0.1 |
| SCS‐related Follow‐up Specialist Consultations: | 0.0 | 1.6 |
*It would be not informative reporting synthetically the mean amount of drugs and complementary therapies used, because the patients used several types and dosages of drug treatments (e.g., opioid, antiepileptic, analgesic, gastroprotective, antibiotic, anxiolytic, antidepressant drugs etc.) and several types of complementary therapies (acupuncture, homeopathic therapy, plantar therapy, pranotherapy).
Table 5.
Comparison of Direct and Indirect Mean Costs/Patient‐Year Between the Pre‐SCS and Post‐SCS Periods
| NHS €/patient‐year | Patient €/patient‐year | Society €/patient‐year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre‐SCS | post‐SCS° | post‐pre | † bsp | pre‐SCS | post‐SCS° | post‐pre | † bsp | pre‐SCS | post‐SCS° | post‐pre | † bsp | |
| Direct costs, thereof | 2,653.6 | 10,797.6 | 8,144.0 | −8.54 ** | 2,128.1 | 1,314.9 | −813.2 | 4.66 ** | 4,781.6 | 12,112.5 | 7,330.9 | −7.46 ** |
| Medical, SCS related procedures | 0.0 | 9,273.1 | 9,273.1 | −8.21 ** | 0.0 | 0.0 | 0.0 | – | 0.0 | 9,273.1 | 9,273.1 | −8.21 ** |
| INS implantation | 0.0 | 5,857.2 | 5,857.2 | −6.78** | 0.0 | 0.0 | 0.0 | – | 0.0 | 2,335.8 | 2,335.8 | −6.78** |
| LEAD impiantation | 0.0 | 2,335.8 | 2,335.8 | −1.74n.s. | 0.0 | 0.0 | 0.0 | – | 0.0 | 5,857.2 | 5,857.2 | −1.74n.s. |
| Explantation | 0.0 | 84.9 | 84.9 | −5.20** | 0.0 | 0.0 | 0.0 | – | 0.0 | 84.9 | 84.9 | −5.20** |
| INS replacement | 0.0 | 938.8 | 938.8 | −5.26** | 0.0 | 0.0 | 0.0 | – | 0.0 | 938.8 | 938.8 | −5.26** |
| LEAD replacement | 0.0 | 56.5 | 56.5 | −3.43* | 0.0 | 0.0 | 0.0 | – | 0.0 | 56.5 | 56.5 | −3.43* |
| Other medical (nonSCS) related procedures | 2,653.6 | 1,524.5 | −1,129.1 | 7.12 ** | 919.7 | 360.8 | −558.9 | 6.82 ** | 3,573.2 | 1,885.4 | −1,687.8 | 9.25 ** |
| Emergency admissions | 9.8 | 6.7 | −3.1 | 1.79n.s. | 0.0 | 0.0 | 0.0 | – | 9.8 | 6.7 | −3.1 | 1.79n.s. |
| Medical aids | 78.9 | 85.3 | 6.4 | −0.27n.s. | 67.3 | 30.3 | −37.0 | 3.60** | 146.2 | 115.6 | −30.6 | 1.04n.s. |
| Diagnostic examinations | 238.1 | 74.2 | −163.9 | 10.08** | 73.1 | 33.0 | −40.1 | 2.32*** | 311.2 | 107.2 | −204.0 | 8.98** |
| Drugs | 726.5 | 482.9 | −243.6 | 2.75* | 131.0 | 35.4 | −95.6 | 4.77** | 857.5 | 518.4 | −339.1 | 3.50** |
| Hospitalizations | 743.4 | 395.8 | −347.6 | 3.98** | 0.0 | 0.0 | 0.0 | n.s. | 743.4 | 395.8 | −347.6 | 3.98** |
| Complementary therapies | 0.0 | 0.0 | 0.0 | – | 268.6 | 74.0 | −194.6 | 4.37** | 268.6 | 74.0 | −194.6 | 4.37** |
| Rehabilitation therapies | 194.0 | 108.9 | −85.1 | 1.40n.s. | 0.0 | 0.0 | 0.0 | – | 194.0 | 108.9 | −85.1 | 1.40n.s. |
| GP consultations | 614.3 | 329.8 | −284.5 | 5.68** | 0.0 | 0.0 | 0.0 | – | 614.3 | 329.8 | −284.5 | 5.68** |
| Medical specialist consultations | 48.6 | 12.4 | −36.2 | 7.37** | 379.7 | 188.1 | −191.6 | 6.74** | 428.2 | 200.5 | −227.7 | 7.32** |
| SCS‐related follow‐up specialist consultations | 0.0 | 28.6 | 28.6 | – | 0.0 | 0.0 | 0.0 | – | 0.0 | 28.6 | 28.6 | – |
| Non‐medical | 0.0 | 0.0 | 0.0 | – | 1,208.4 | 954.1 | −254.3 | 1.54n.s. | 1,208.4 | 954.1 | −254.3 | 1.54n.s. |
| Accommodation | 0.0 | 0.0 | 0.0 | – | 68.1 | 29.3 | −38.8 | 1.77n.s. | 68.1 | 29.3 | −38.8 | 1.77n.s. |
| Formal assistance | 0.0 | 0.0 | 0.0 | – | 578.4 | 681.9 | 103.5 | −0.52n.s. | 578.4 | 681.9 | 103.5 | −0.52n.s. |
| Travel | 0.0 | 0.0 | 0.0 | – | 561.8 | 242.9 | −318.9 | 5.06** | 561.8 | 242.9 | −318.9 | 5.06** |
| Indirect costs | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | 0.0 | – | 1,785.2 | 1,103.3 | −681.9 | 2.65* |
| Family caregiver's loss of productivity | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | 0.0 | – | 1,785.2 | 1,103.3 | −681.9 | 2.65* |
| Total | 2,653.6 | 10,797.6 | 8,144.0 | −8.54 ** | 2,128.1 | 1,314.9 | −813.2 | 4.66 ** | 6,566.8 | 13,215.8 | 6,649.0 | −4.39 ** |
°Mean costs between 12 and 24 months post‐SCS.
†Bootstrap‐t test and corresponding p‐value: *p‐value < 0.01; **p‐value < 0.001; ***p‐value < 0.05.
n.s., p ≥ 0.05; –, not applicable.
Figure 4.
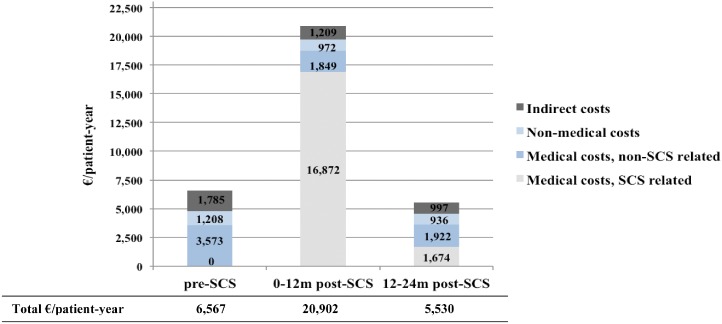
Trend of direct and indirect (societal point of view) costs during the observational period.
Table 6.
Incremental Cost‐Effectiveness and Cost‐Utility ratios
| Perspective | Cost (€) difference | QALY difference | ICUR | NRS difference | ICER |
|---|---|---|---|---|---|
| (post*—pre SCS) | (post*—pre SCS) | (€/QALY) | (post*—pre SCS) | (€/NRS) | |
| NHS | 8,144 | 0.173 | 47,000 | 2.528 | 3,222 |
| Society | 6,649 | 0.173 | 38,372 | 2.528 | 2,631 |
*Mean cost between 12 and 24 months post‐SCS.
The cost utility acceptability curve results (Fig. 5) suggest that SCS implantation is cost‐effective in 80% of cases from the NHS point of view, and 85% of cases from the societal point of view, at the willingness‐to‐pay threshold of €60,000/QALY, which was proposed for Italy some years ago 37.
Figure 5.
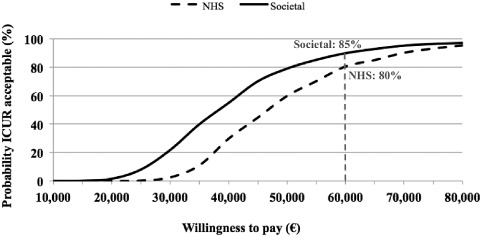
Cost‐utility acceptability curve for the SCS implantation according to the societal and NHS's point of view.
Discussion
This real‐world study compares from different perspectives including the societal one, the effects and costs of SCS implant added to CMM (SCS+CMM) in the treatment of patients with FBSS with predominant leg pain and refractory to CMM to those of CMM alone, using the patient, NHS, and societal points of view. Accordingly, this observational study contributes insights on SCS based on real‐world data, as recommended by NICE in 2008 17. This design differs from the approach used in the PROCESS study 6, 7, 8, 14, 15, in which two parallel groups of patients were randomized to SCS+CMM or CMM alone and followed up in a controlled context. The approach of this study can provide insights into clinical practice and the benefits patients can actually achieve in a real‐world uncontrolled setting, and has been demonstrated to provide insight where the clinical superiority of one option over the alternative is already established 38. Similarly, SCS treatment has been proven to be effective in pain relief, improvement of HRQoL and disability in patients that are refractory to CMM 6, 7, 8, 9, 11, 39. Hence, all the eligible patients enrolled during the present study were offered the more effective option. With our approach, we could obtain comparative data attributable to a longer period than those available from previous research. While we collected data on our patients for one year before and up to 24 months after SCS, in the PROCESS trial only six months of follow‐up data were available for both groups of treatment, since for ethical reasons the patients had the opportunity to crossover from CMM to SCS beyond that time period.
A further strength of this study is the relatively high number of participants, which is particularly notable for being the highest enrollment so far from a single country.
Our results show that at baseline, patients had low levels of health, while 24 months after SCS implantation, both clinical outcomes and HRQoL demonstrated a clinically and statistically significant increase. The most important changes were found within the first six months of SCS treatment, and then maintained or slightly improved during the following period.
Our results on benefits also are quite consistent with those observed in previous clinical studies. In particular, patients who received SCS implantation in the PROCESS study reported a significant increase in the mean EQ‐5D utility score from baseline (0.13) to 24 months (0.41), with the highest mean value (0.49) estimated three months from SCS 7, 15, as calculated using the UK tariffs. Actually, these results would be similar to ours (0.07 at baseline and 0.40 at 24 months) if we applied, like the authors of the PROCESS study, the UK social tariffs 40 to convert the responses from the EQ‐5D descriptive system into utilities. However, because our data refer to Italian patients, the new and more accurate Italian‐specific social tariffs, which are higher than the UK social tariffs 33, were applied to calculate utilities in our study, generating higher mean scores (0.42 at baseline and 0.63 at 24 months) and a consequently reduced gain between the pre‐SCS and the post‐SCS period. This differential gain attributable to the different social tariffs depends on the maximum limit of the utility index, which is bounded to 1. Actually, the difference between two higher utilities attributable to the two alternatives could be influenced by this limit and could be lower than the difference generated by two lower utilities for the same health states.
In regards to the ODI in the PROCESS trial, this score decreased significantly from 60 at baseline to 42 after three months of SCS therapy, while no further significant changes were found until the end of the observational period 7. It is worth mentioning that even though we did not observe outcomes at three months after the intervention, it cannot be excluded that similar results in our study were already reached at that time.
The costs for SCS‐related procedures in this study, which correspond to €9300 per patient per year paid by the NHS, is partially offset by the decrease in other costs, resulting in a cost saving for the patients of €800 per patient per year. Our results are not directly comparable with those from previous research, since in the PROCESS trial they were spread out on a much shorter time period (6 months instead of 24 months). However, similarities in costs estimated in different health care systems (i.e., with different unit costs, different payers and payment procedures) generally cannot be expected. Furthermore, unlike in the PROCESS study, in which only medical costs from the third party payer were considered, we estimated also direct ‐non‐medical costs, adopting the patients' perspective, and indirect costs, from the patient, NHS, and societal perspectives. In particular, the importance of including indirect costs or benefits in economic evaluations has been recently underlined by Budd 41 and by Taylor and Taylor 12.
A cost/NRS gain of €3200 and €2600, and a cost/QALY gain of €47,000 and €38,372 were estimated for the NHS and the societal perspectives, respectively. Cost‐effectiveness or cost‐utility ratios do not themselves provide information on the efficiency of treatments. This actually depends on the payer's willingness to consider those ratios as acceptable or not. In the United Kingdom, the NICE recommends that interventions are normally considered not cost‐effective if the ICER is higher than 30,000£/QALY (about €45,000/QALY gained) 17. With this threshold, SCS+CMM would be cost effective in around 40% (NHS perspective) and 70% (societal perspective) of cases. However, according to the threshold of €60,000/QALY gained, which was specifically proposed for Italy some years ago 37, SCS+CMM would be cost effective in 80% (NHS perspective) and 85% (societal perspective) of cases. With these results, budget holders and practitioners can be more aware of the positive balance between benefits and costs of SCS compared to standard care, and can be guided towards better decisions aimed to optimize the management of the target patients.
This study has some potential limitations. First, although we included indirect costs in the analyses, we did not consider patients' loss of productivity because many of them were already retired or housewives. However, even patients without a paid job could lose productivity in terms of everyday activities they generally do, but the available methods (such as the human capital approach) make it very difficult to correctly quantify them. However, we can consider our approach as conservative, assuming that similar to their family caregivers, a gain in productivity also was achieved, although this was not reflected in the analyses. Our approach is in line with the results published a number of years ago on this topic 38, when the extreme difficulties in quantifying productivity loss or gains in these patients was shown.
A second limitation can be acknowledged because the pre‐SCS data were collected retrospectively, potentially causing some recall bias, e.g., some cost items or amounts were forgotten or not precisely remembered by the patients. However, unlike a randomized clinical trial, the collection of retrospective data in this observational study was necessary to make comparisons between a pre‐ and a post‐SCS period. However, we do not expect relevant biases from this approach, and chose 12 months as a retrospective time period, since it was considered as reasonable for this category of chronic and intensively treated patients, to obtain data reliable enough to be compared with those collected during the prospective period.
A third limitation could be ascribed to the LOCF approach adopted to manage the missing data of the 25 patients (31%) that did not continue the study until the 24th month of follow‐up. Actually, from our analyses applied using the LOCF approach we do not expect results that are biased in favor of SCS, rather we consider our approach conservative. From the data that were collected during the follow‐up, we estimated costs that were on average decreasing, and utility index and ODI results that were on average increasing after the SCS implantation. Accordingly, mean costs carried forward to fill missing data were generally higher, and mean utility indexes and ODI estimates carried forward were generally lower than those estimated from the data available. In particular for the patients that failed the SCS test, we assumed that the mean costs and health indexes of these patients remained equal to those registered before the test.
Fourth, although we observed the patients for up to 24 months, we cannot consider this time horizon as sufficient to capture all costs and consequences as a result of SCS. There are costs that are not reflected, including those related to routine INS replacement for battery depletion and possible hardware complications (e.g., revisions for lead migration or fracture). The results obtained with a decision analytic model built on the PROCESS findings and simulating costs and QALYs over a 15‐year time horizon are promising, and reflect the QALY gained in addition to costs over the full life of a device 16. In our study, the SCS option is cost‐effective already after 24 months, which contributes to support decision processes based on relatively short‐term budget constraints.
Conclusion
In conclusion, the results of this study, which is novel in providing real‐world data on both outcomes and costs of the treatment options compared suggest that in clinical practice SCS+CMM treatment of FBSS patients refractory to CMM provides good value for money, from the NHS, the patient, and society perspectives. These results can help clinical practitioners, together with budget holders, to arrive at more informed and appropriate decisions aimed to optimize the management of FBSS patients responding to the selection criteria used in this study. However, additional research conducted for longer observational periods, using high quality routine healthcare data sources such as administrative databases or registries, can provide more insights on the overall benefits and costs for the patients and their families, for the third party payer and for the society as a whole.
Acknowledgements
The authors would like to thank G. Beccagutti, M. Grifi, and D. D'Ostilio from Medtronic Italy and T. De Santo from Medtronic EMEA Regional Clinical Center for their technical assistance. For the support provided during data collection, the authors would like to thank: C. Bonezzi, MD—Unità di Medicina del Dolore IRCCS Fondazione Salvatore Maugeri, Pavia; B. CIoni, MD—U.O. Neurochirurgia Funzionale e Spinale Policlinico Gemelli, Roma; G. De Carolis, MD—U.O. Terapia del Dolore Azienda Ospedaliero Universitaria Pisana, Presidio Opsedaliero “S. Chiara,” Pisa; G. De Falco, MD—U.O. Fisiopatologia del dolore e Cure Palliative A.S.O. “S. Croce e Carle,” Cuneo; E. Obertino, MD—U.O. Algologia e Cure palliative A.S.O. “S. Croce e Carle,” Cuneo; MG Rusconi, MD—Dipartimento Anestesia, Rianimazione, Cure Palliative e Terapia del Dolore Azienda Ospedaliera “ G. Salvini.” The affiliations are valid at the time of data collection.
Authorship Statements
All the authors contributed to the study design. Dr. Zucco coordinated the study supported by Drs. Lavano and Costantini. Drs. Zucco, Lavano, De Rose, Poli, Fortini, De Simone, Menardo, Demartini, Cisotto, and Meglio recruited the patients and collected the data. Drs. Zucco, Lavano, and Constantini interpreted the results. Drs. Mantovani, Scalone, and Ciampichini assisted with data analysis and results interpretation and prepared the manuscript with important intellectual input and review from Drs. Zucco, Lavano, Constantini, De Rose, Poli, Fortini, De Simone, Menardo, Demartini, Cisotto, and Meglio. All authors approved the submitted version of the manuscript.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/bw/submit.asp?ref=1094‐7159&site=1
Source(s) of financial support: The study was sponsored by Medtronic Italy.
Conflict of Interest: The authors reported no conflict of interest.
Dr. Piero Cisotto has passed since the writing of this manuscript.
References
- 1. Thomson S, Jacques L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract 2009;9:206–215. [DOI] [PubMed] [Google Scholar]
- 2. Van. Buyten JP, Linderoth B. The failed back surgery syndrome: definition and therapeutic algorithms—An update. Eur J Pain Supplements 2010;4:273–286. [Google Scholar]
- 3. Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta‐analysis of health utilities. Pain 2010;149:338–344. [DOI] [PubMed] [Google Scholar]
- 4. Cruccu G, Aziz TZ, Garcia‐Larrea L et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol 2007;14:952–970. [DOI] [PubMed] [Google Scholar]
- 5. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg 1967;46:489–491. [PubMed] [Google Scholar]
- 6. Eldabe S, Kumar K, Buchser E, Taylor RS. An analysis of the components of pain, function, and health‐related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation 2010;13:201–209. [DOI] [PubMed] [Google Scholar]
- 7. Kumar K, Taylor RS, Jacques L et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24‐month follow‐up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008;63:762–770. [DOI] [PubMed] [Google Scholar]
- 8. Kumar K, Taylor RS, Jacques L et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179–188. [DOI] [PubMed] [Google Scholar]
- 9. Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta‐analysis. J Pain Symptom Manage 2006;31 (4 Suppl.):S13–S19. [DOI] [PubMed] [Google Scholar]
- 10. North RB, Kidd D, Shipley J, Taylor RS. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery 2007;61:361–368. [DOI] [PubMed] [Google Scholar]
- 11. North RB, Ewend MG, Lawton MT, Kidd DH, Piantadosi S. Failed back surgery syndrome: 5‐year follow‐up after spinal cord stimulator implantation. Neurosurgery 1991;28:692–699. [PubMed] [Google Scholar]
- 12. Taylor RS, Taylor RJ. The economic impact of failed back surgery syndrome. British Journal of Pain 2012;6:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost‐effectiveness analysis. Neurosurgery 2002;51:106–115. [DOI] [PubMed] [Google Scholar]
- 14. Kumar K, North RB, Taylor RS et al. Spinal cord stimulation versus conventional medical management: a prospective, randomized, controlled,multicenter study of patients with Failed Back Surgery Syndrome (PROCESS study). Neuromodulation 2005;8:213–218. [DOI] [PubMed] [Google Scholar]
- 15. Manca A, Kumar K, Taylor RS et al. Quality of life, resource consumption and costs of spinal cord simulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain 2008;12:1047–1058. [DOI] [PubMed] [Google Scholar]
- 16. Taylor RS, Ryan J, O'Donnell R, Eldabe S, Kumar K, North RB. The cost‐effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain 2010;26:463–469. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Care Excellence . Spinal Cord Stimulation for Chronic Pain of Neuropathic or Ischaemic Origin. London: National Institute for Health and Clinical Excellence, 2008. http://guidance.nice.org.uk/TA159/Guidance/pdf/English
- 18. Atkinson L, Sundaraj SR, Brooker C et al. Recommendations for patient selection in spinal cord stimulation. J Clin Neurosci 2011;18:1295–1302. [DOI] [PubMed] [Google Scholar]
- 19. North R, Shipley J, Prager J et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med 2007;8 (Suppl. 4):S200–S275. [DOI] [PubMed] [Google Scholar]
- 20. Dworkin RH, Turk DC, Farrar JT et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 21. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940–2952. [DOI] [PubMed] [Google Scholar]
- 22. Ware J Jr, Kosinski M, Keller SD. SF‐36 physical and mental health summary scales: a user's manual. Boston (MA): The Health Institute; 1994.
- 23. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 24. National. Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisals, 2008. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf
- 25. Italian. National Institute of Statistics (ISTAT) . AnnuarioStatisticoItaliano. Roma: Istituto Nazionale di Statistica. Coefficienti per tradurre valori monetari dei periodi sotto indicati in valori del 2009. http://rivaluta.istat.it/Rivaluta/
- 26. Conferenza. delle Regioni e Province Autonome . Compensazione Interregionale della mobilità sanitaria. TestoUnico. Versione in vigore per le attività dell'anno 2008. Roma, 29 luglio 2009.
- 27. Demartini L, Mearini M, Beccagutti G, Grifi M, Grabbi E. Neurostimolazione spinale: analisi dei costi di ospedalizzazione in due centri Lombardi. Poster presented at the 4th National Congress of the Italian Society of HTA (SIHTA), Udine (Italy), November 17–19, 2011.
- 28. Italian Agency for Regional Healthcare Services (Agenzia Nazionale per i Servizi Sanitari Regionali, Age.na.s) . Prestazioni specialistiche ambulatoriali. Confronto tra le tariffe nazionali ex DM 1996 e le tariffe regionali relative all'anno 2009. http://www.agenas.it/monitoraggio_costi_tariffe/2009_SPECIALISTICA_ex%20DM%2096per%20sito.pdf
- 29. Italian. Ministry of Health . Regolamento recante norme per le prestazioni di assistenza protesica erogabili nell'ambito del Servizio sanitario nazionale: modalità di erogazione e tariffe. Decreto Ministeriale 27 Agosto 1999. Italian Official Gazette n. 227, 27th September 1999.
- 30. L'informatore farmaceutico—Medicinali 2008. Milano: Elsevier Masson, 2008. [Google Scholar]
- 31. Lucioni C, Mangrella M, Mazzi S, Negrini C, Vaghi A. Impiego di un'associazione fissa di formoterolo e budesonide nel trattamento del paziente asmatico. Una valutazione farmacoeconomica rispetto ad alcune alternative terapeutiche. Pharmacoeconomics—Italian Research Articles 2002;4:15–23. [Google Scholar]
- 32. Drummond M, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes, 3rd ed. Oxford, UK: Oxford University Press, 2005. [Google Scholar]
- 33. Scalone L, Cortesi PA, Ciampichini R et al. Italian population‐based values of EQ‐5D health states. Value Health 2013;16:814–822. [DOI] [PubMed] [Google Scholar]
- 34. Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry 2002;47:68–75. [PubMed] [Google Scholar]
- 35. Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non‐parametric bootstrap. Stat Med 2000;19:3219–3236. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien BJ, Briggs AH. Analysis of uncertainty in healthcare cost‐effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455–468. [DOI] [PubMed] [Google Scholar]
- 37. Messori A, Santarlasci B, Trippoli S, Vaiani M. Drug economic equivalent and clinical benefit: state of the art on methodology and application of a pharmacoeconomic algorithm. Pharmacoeconomics—Italian Research Articles 2003;5:53–67. [Google Scholar]
- 38. Olivieri I, de Portu S, Salvarani C et al. The psoriatic arthritis cost evaluation study: a cost‐of‐illness study on tumor necrosis factor inhibitors in psoriatic arthritis patients with inadequate response to conventional therapy. Rheumatology 2008;47:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. North R, Shipley J, Prager J et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med 2007;8 (Suppl. 4):S200–S275. [DOI] [PubMed] [Google Scholar]
- 40. Badia X, Roset M, Herdman M, Kind P. A comparison of United Kingdom and Spanish general population time‐trade‐off values for EQ‐5D health states. Med Decis Making 2001;21:7–16. [DOI] [PubMed] [Google Scholar]
- 41. Budd K. Spinal cord stimulation: cost‐benefit study. Neuromodulation 2002;5:75–78. [DOI] [PubMed] [Google Scholar]


