Abstract
Background
Available literature on psoriasis and psoriatic arthritis (PsA) demonstrates a tremendous burden of disease and suggests underdiagnosis and undertreatment.
Objective
To obtain real‐world physician perspectives on the impact of psoriasis and PsA and its treatment on patients' daily lives, including perceptions of, and satisfaction with, current therapies.
Methods
The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) surveyed dermatologists (n = 391) and rheumatologists (n = 390) in North America (Canada and the United States) and Europe (France, Germany, Italy, Spain and United Kingdom).
Results
Dermatologists classified 20.3% and 25.7% of their patients as having severe psoriasis and severe PsA respectively; rheumatologists indicated that 48.4% of their PsA patients had active disease. Of the psoriasis patients complaining of joint pain, only 33.0% had a diagnosis of PsA. An impact on daily activities or social/emotional well‐being was recognized by 57.2% to 79.3% of physicians. In patients with moderate‐to‐severe psoriasis, dermatologists reported 74.9% were receiving topical therapy, 19.5% conventional oral therapy and 19.6% biologics. Dermatologists and rheumatologists reported similar rates of topical (≈45%) and biologic (≈30%) therapy utilization for their PsA patients; conventional oral therapy was more often prescribed by rheumatologists (63.4%) vs. dermatologists (35.2%). Reasons for not initiating or maintaining systemic therapies were related to concerns about long‐term safety, tolerability, efficacy and costs (biologics).
Conclusion
Physicians in North America and Europe caring for patients with psoriasis and PsA acknowledge unmet treatment needs, largely concerning long‐term safety/tolerability and efficacy of currently available therapies; evidence suggests underdiagnosis of PsA and undertreatment of psoriasis among dermatologists.
Introduction
Current published estimates of the worldwide prevalence of psoriasis range from approximately 1–3%.1, 2, 3, 4, 5, 6 Psoriatic arthritis (PsA) occurs in approximately 10–30% of psoriasis patients. In the vast majority of cases, joint involvement follows skin involvement, often by 10 years.7, 8 Patients with psoriasis and PsA experience a significant burden of disease, including reduced quality of life, decreased physical function and increased comorbidities.7, 9, 10 Available evidence suggests that numerous unmet needs exist in the care of these patients, including underdiagnosis2, 7 and suboptimal treatment.11, 12, 13
Available surveys offer insight into psoriasis and PsA disease burden and treatment. However, most are limited to a specific geographic region, patients currently under the care of a physician and/or being treated in a specific healthcare setting and/or members of a patient association, or lack of both a patient and physician survey component. Moreover, many surveys provide limited information on patient‐ and physician‐related factors influencing treatment decisions.
To better understand the unmet needs from the patient and physician perspective, and to gain deeper insight into the impact of psoriasis and PsA on patients' lives, a population‐based survey of 4400 patients and 800 physicians in North America and Europe was conducted. The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey is the first‐of‐its‐kind, large‐scale, multinational survey conducted. The survey explores the diagnosis and impact of psoriasis and PsA on health‐related quality of life as well as attitudes towards current therapies. The MAPP survey was developed with patient and physician input and aims to provide a better reflection of psoriasis and PsA severity, including the impact of disease and its treatment on patients' daily lives from the physician perspective.
Results from the multinational patient survey, published separately, identified unmet needs related to assessment of disease severity, PsA screening and diagnosis and treatment options.8 The current report includes results from the multinational physician survey.
Materials and methods
Study design
The survey was conducted by Abt SRBI, Inc (New York, NY, USA). Methodology and results of the patient survey have been reported.8 Dermatologists and rheumatologists were identified through national databases for each country. Physicians were contacted from this list through random sampling methods and offered participation in the survey. Screening criteria for dermatologists required that ≥ 50% of their practice be devoted to medical dermatology.
A total of 6530 dermatologists and 5445 rheumatologists were screened; 391 dermatologists and 390 rheumatologists completed interviews. North American results combine findings from Canada and the United States; European results combine findings from France, Germany, Italy, Spain and United Kingdom. For this report, results reflect overall global findings, except when notable differences in responses were observed between North America and Europe. Further analyses of results by geographic regions are planned.
Results
Physician and practice demographics are summarized in Table 1. Dermatologists reported that 86.0% of office visits in a typical month were related to medical dermatology, with 16.1% specifically related to psoriasis and 4.1% to PsA. Rheumatologists reported that 15.0% of office visits were related to PsA. Overall, North American rheumatologists had twice as many PsA patients as European rheumatologists.
Table 1.
Physician and practice demographics
| Dermatologists | Rheumatologists | |||
|---|---|---|---|---|
| North America n = 141 | Europe n = 250 | North America n = 140 | Europe n = 250 | |
| Mean years in practice | 18.8 | 16.2 | 18.4 | 15.1 |
| Physician practice setting, % | ||||
| Urban | 42.6 | 78.4 | 55.7 | 78.0 |
| Suburban | 49.6 | 13.2 | 42.1 | 17.2 |
| Rural | 7.8 | 8.0 | 2.1 | 4.8 |
| Office‐based, % | 96.5 | 45.2 | 90.7 | 35.6 |
| Hospital‐based, % | 3.5 | 54.8 | 9.3 | 64.4 |
| Multispecialty practice, % | 15.6 | 47.2 | 35.7 | 53.2 |
| Mean No. of dermatologists in practice | 3.5 | 4.9 | 1.6 | 1.3 |
| Mean No. of rheumatologists in practice | 0.3 | 1.2 | 3.4 | 3.1 |
| Mean weekly volume of patients | 173.3 | 159.4 | 106.6 | 104.9 |
| Mean proportion of all visits related to medical dermatology, % | 84.1 | 87.1 | – | – |
| Mean proportion of all visits related to psoriasis, % | 13.5 | 17.6 | – | – |
| Mean proportion of all visits related to PsA, % | 3.0 | 5.0 | 14.7 | 15.2 |
PsA, psoriatic arthritis.
Psoriasis patients were most often referred to the participating dermatologist by a primary care physician (63.9%), another specialist (16.2%) or another dermatologist (7.9%); PsA patients were most often referred by a primary care physician (51.2%), another specialist (21.0%) or another dermatologist (7.0%). PsA patients were most often referred to the participating rheumatologist by a primary care physician (51.7%), dermatologist (22.9%) or another specialist (18.4%).
Disease severity
Dermatologists classified 20.3% of their psoriasis patients and 25.7% of their PsA patients with severe disease, 38.1% and 38.3% with moderate and 40.5% and 32.9% with mild respectively. In psoriasis patients, dermatologists most commonly considered the location or size of skin lesions to be the most important factor contributing to disease severity (Fig. 1a). In PsA patients, 63.9% of dermatologists and 74.2% of rheumatologists most commonly considered pain or swelling of the joints to be the most important factor contributing to disease severity (Fig. 1b).
Figure 1.
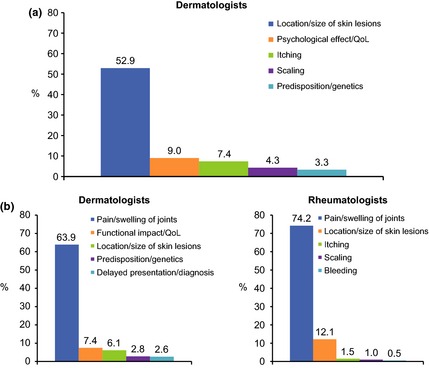
Top five most important factors contributing to psoriasis (a) and psoriatic arthritis (b) disease severity. QoL, quality of life.
Burden of disease
Most dermatologists (62.6%) reported that psoriasis impacted patients' daily activities and/or work, and 79.3% reported it affected their patients socially and/or emotionally; 92.1% agreed the disease burden of psoriasis is frequently underestimated. Dermatologists recognized that a higher proportion of their PsA patients (73.7%) were impacted in their daily activities and/or work, and 76.8% were affected socially and/or emotionally. Fewer rheumatologists recognized the effect of PsA on daily activities and/or work (57.2%) or its social and/or emotional toll (64.9%). However, most proactively discuss the effect of PsA on daily activities (85.3%) or its social and/or emotional toll (67.2%).
Diagnosis and management of PsA
Dermatologists reported discussing the possibility of developing joint disease in all (43.5%) or some (46.5%) of their psoriasis patients. However, most dermatologists and rheumatologists (> 75%) acknowledged that PsA is likely underdiagnosed because of a failure to connect skin and joint symptoms. According to dermatologists, 20.0% of their psoriasis patients complain of joint symptoms, with only 33.0% of these patients having a confirmed diagnosis of PsA, which was most often made by another physician (48.7%) or the participating dermatologist (39.1%).
Collaborative rheumatologist/dermatologist care for PsA patients was reported by 75.6% of dermatologists and 42.9% of rheumatologists. Dermatologists indicated they were solely responsible for prescribing decisions in 23.6% of their PsA patients. In 30.8%, they were primarily responsible for prescribing decisions but patients were monitored by a rheumatologist for joint symptoms, and in 44.8%, rheumatologists made prescribing decisions but the responding dermatologist monitored skin symptoms. While rheumatologists indicated they were solely responsible for prescribing decisions in 66.1% of their PsA patients, 34.2% might also be monitored by a dermatologist for skin symptoms and in 8.7% of patients, the dermatologist made primary prescribing decisions, whereas joint symptoms were monitored by the rheumatologist.
Treatment patterns
Table 2 summarizes physician estimates of the current treatment utilization by patients with moderate‐to‐severe psoriasis or PsA in their practice. Dermatologists reported that 74.9% of their psoriasis patients were receiving topical therapy, 19.5% were receiving conventional oral disease‐modifying antirheumatic drug (DMARD) therapy and 19.6% were receiving biologics. PsA patients were more often receiving oral therapy when treated by rheumatologists (63.4%) vs. dermatologists (35.2%), whereas dermatologists and rheumatologists both reported that approximately 30% of their PsA patients were receiving biologics.
Table 2.
Current treatment utilization in patients with psoriasis and PsA
| Therapies | Psoriasis patients, % | PsA patients, % | |
|---|---|---|---|
| Dermatologists | Dermatologists | Rheumatologists | |
| Topical therapy | 74.9 | 44.5 | 43.1 |
| Systemic steroids | 4.5 | 11.0 | 15.3 |
| UVB/PUVA | 22.3 | 14.9 | 11.6 |
| Conventional oral therapy | 19.5 | 35.2 | 63.4 |
| Biologics | 19.6 | 30.6 | 33.4 |
PsA, psoriatic arthritis; UVB/PUVA, ultraviolet B/psoralen+ultraviolet A.
Physician attitudes: patient management and treatment goals
The top goal for dermatologists treating psoriasis patients was keeping signs/symptoms at bay (52.5%), followed by improving normal functional activities (22.3%) and improving patient self‐esteem (10.7%). Dermatologists reported feeling optimistic (45.2%) and/or hopeful (35.3%) about managing a new patient with moderate‐to‐severe psoriasis. However, approximately one in five felt that managing such a patient was time‐consuming (19.8%) and complicated (18.6%). Approximately, 60% of dermatologists felt that psoriasis patients require more time and support than their other patients and that the current structure of the healthcare system does not allow them adequate time for their care. Dermatologists cited affordability and long‐term safety risks with current medications as the greatest challenges in managing psoriasis patients (Fig. 2a).
Figure 2.
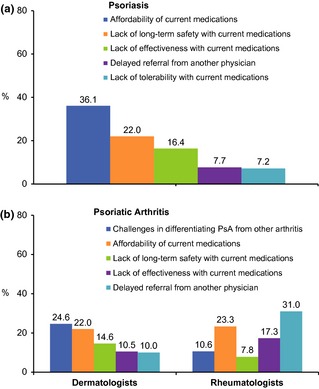
Greatest challenge in managing psoriasis (a) and psoriatic arthritis (b) patients.
When treating PsA patients, dermatologists and rheumatologists reported the top goals were to reduce joint pain and stiffness (33.8% and 36.2% respectively), improve normal functional activities (28.4% and 23.6%) and keep symptoms at bay (25.3% and 23.6%). Dermatologists reported feeling optimistic (30.9%) or hopeful (29.7%) about managing PsA patients with active disease, 26.5% felt it would be complicated and 15.4% said it was time‐consuming; 17.4% thought they would need to refer to or involve other specialists. Rheumatologists reported feeling optimistic (61.3%) or hopeful (37.2%) about managing a PsA patient with active disease; 29.5% agreed that PsA patients require more time and support than their other patients. Dermatologists most often cited differentiating PsA from other arthritic diseases as the greatest challenge (24.6%), whereas rheumatologists most often cited delayed referral (31.0%) (Fig. 2b).
Physician attitudes: treatment options
In all, 53.5% of dermatologists said they would prescribe topical therapies as monotherapy for their moderate‐to‐severe psoriasis patients. Dermatologists most commonly identified the primary challenge with topical therapies as lack of, partial or waning response (25.1%); compliance (18.4%); patient complaints of inconvenience/mess (18.2%); and inability to cover the entire surface area (17.9%).
Dermatologists (47.6%) most commonly felt they were comfortable, but conservative, with the use of conventional oral DMARD therapy, or reported using it sparingly (20.5%), whereas most rheumatologists (68.5%) reported that they felt conventional oral therapy provided benefit and used it broadly and only 9.0% used it sparingly. Reasons for not initiating (Fig. 3a) or continuing (Fig. 3b) oral therapy were often related to long‐term safety and tolerability concerns. In addition, 18.7% of dermatologists and 16.1% of rheumatologists said patient contraindications were a main factor in preventing greater use of conventional oral therapies. Rheumatologists commonly cited lack of response as a limitation to continuing therapy (35.4%). For dermatologists and rheumatologists who held back prescribing oral therapy because of patient refusal or concerns, the most common reasons were related to tolerability, long‐term safety, lifestyle modifications and lack or loss of response (Fig. 3c).
Figure 3.
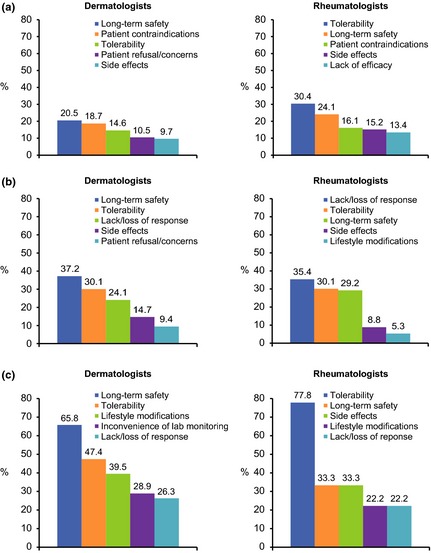
Top five most commonly cited limitations for initiating conventional oral therapy (a), continuing conventional oral therapy (b) and patient concerns regarding the use of conventional oral therapy (c).
Approximately 40% of dermatologists and rheumatologists were comfortable, but conservative, in their use of biologic therapy. More rheumatologists (41.5%) reported aggressive use compared with dermatologists (19.9%), and a greater proportion of dermatologists (10.5%) reported not prescribing biologics compared with rheumatologists (1.8%). Among dermatologists, 65.5% said they would initiate and manage the biologic therapy themselves, compared with 90.3% of rheumatologists. The main reasons for not initiating biologics were related to cost and long‐term safety and tolerability concerns (Fig. 4a). Rheumatologists commonly cited lack or loss of response as a limitation to continuing therapy (Fig. 4b). Long‐term safety was more commonly reported as a limitation to initiating biologics by dermatologists and rheumatologists from North America (32.6% and 54.5% respectively) compared with Europe (13.6% and 17.0% respectively). Biologic therapy‐related tasks most commonly considered burdensome included prior authorization paperwork; ensuring patients comply with laboratory results; handling patient calls regarding questions or possible side‐effects; teaching self‐injection; and discussing with patients the use of and risks associated with injectable biologic therapy (Fig. 4c).
Figure 4.
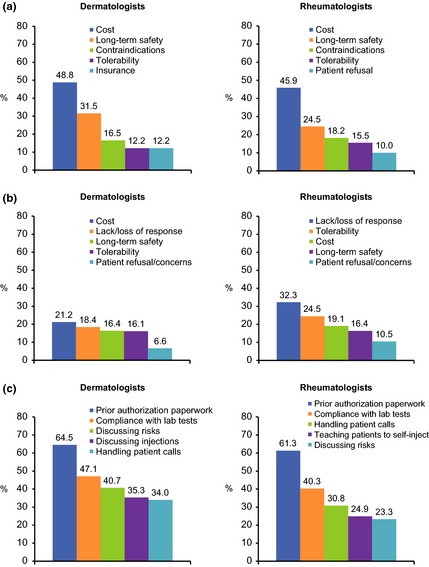
Top five most commonly cited limitations for initiating (a) and continuing (b) patients on biologic therapy and burdensome tasks/steps associated with biologic therapy (c).
While most dermatologists were somewhat or very satisfied with the effectiveness of conventional oral (84.2%) or biologic therapy (95.9%), 27.8% and 25.6% were somewhat/very dissatisfied with their long‐term safety, respectively, and 75.4% and 83.4% expressed concern about the health risks of their long‐term use. Likewise, most rheumatologists were somewhat/very satisfied with the efficacy of currently available therapies (conventional oral therapy: 81.8%; biologics: 97.9%). Concern regarding the long‐term safety of oral and biologic therapies was reported by 11.3% and 15.4%, of rheumatologists respectively. However, 53.6% and 68.5% of rheumatologists reported being somewhat/very concerned with the health risks of long‐term use of oral or biologic therapies respectively. When prescribing topical therapy for psoriasis patients eligible for oral or biologic therapy, 7.9% of rheumatologists stated that their hesitation to prescribe systemic therapy was related to tolerability and long‐term safety concerns.
Among the respondents, 53.0% of dermatologists and 30.5% of rheumatologists agreed that keeping a patient's symptoms at bay was the best the patients could expect from conventional oral therapy; 49.6% and 27.7%, respectively, felt that patients leaving their practice due to frustration or dissatisfaction with currently available therapies is an important issue; and 26.3% and 13.3%, respectively, felt the treatment options could be worse than the condition itself.
The most important attributes of an ideal therapy, identified by the respondents, were largely related to efficacy and safety, which generally mirrored what they considered the greatest unmet therapeutic needs (Table 3). In addition, improved access to therapy and oral administration were among the five most commonly selected attributes; another oral option and new mechanism of action were among the five most commonly noted unmet therapeutic needs.
Table 3.
Top five attributes of an ideal therapy and greatest unmet therapeutic needs
| Ideal therapy | Unmet therapeutic needs | ||
|---|---|---|---|
| Dermatologists psoriasis | Rheumatologists ‒ PsA | Dermatologists ‒ psoriasis | Rheumatologists ‒ PsA |
| No increased risk of serious infection or cancer (36.6%) | Improvement in joint pain (39.7%) | Improved efficacy (35.5%) | Improved efficacy (34.2%) |
| Manageable tolerability profile (17.4%) | Long‐term safety (22.1%) | Improved long‐term safety (33.5%) | A new mechanism of action (23.4%) |
| Provides clearance of at least 50% (18.4%) | Improvement in daily activity (17.2%) | A new mechanism of action (11.8%) | Improved long‐term safety (19.0%) |
| Improved access to therapy (11.0%) | Improved access to therapy (11.5%) | Another oral option (11.5%) | Another oral option (15.4%) |
| Oral administration (12.0%) | Oral administration (7.2%) | Improved tolerability (7.4%) | Improved tolerability (8.0%) |
PsA, psoriatic arthritis.
Discussion
MAPP is a unique large‐scale, multinational survey containing both a patient and physician component aimed at gaining a better understanding of global perspectives on the burden of psoriasis and PsA and their treatment. Results from the multinational patient survey showed a significant burden of disease and unmet treatment needs.8 We report the findings from the physician portion of the survey, which included dermatologists and rheumatologists who care for patients with psoriasis and/or PsA. These findings largely support those of the patient survey, including a large disease burden, underdiagnosis of PsA, differing perceptions of disease severity and challenges with current treatment options.
While dermatologists and rheumatologists recognized the emotional and social toll that psoriasis and PsA may place on patients, most acknowledged that the burden of disease is often underestimated. Dermatologists estimated that 20% of their psoriasis patients and 26% of their PsA patients had severe disease. In the patient portion of the MAPP survey, slightly more psoriasis patients (27%) and twice as many PsA patients (53%) rated their disease as severe.8 Reasons for the disconnect may be related to the use of standardized tools by physicians vs. more subjective assessments by patients. In addition, patients and physicians were not matched, so results cannot be directly compared. However, physicians and patients revealed notable differences in factors contributing to their assessment. While physicians reported that the most important factors contributing to disease severity were location and size of skin lesions in their psoriasis patients and pain and swelling of joints in their PsA patients, itching and location of skin lesions were cited as important factors in patients' assessment of disease severity.8 Current findings show these factors, notably itching, are often not cited by physicians (Fig. 5a,b); only 7% of dermatologists cited itching as the most important factor contributing to disease severity, compared with 38% of patients.8 Itching is not captured in most currently utilized assessment tools, indicating a potential need to examine how disease severity is assessed. Educating healthcare providers (HCPs) regarding the importance of this outcome, as well as increased use of pruritus assessment tools in clinical studies, may help to bridge this disconnect.
Figure 5.
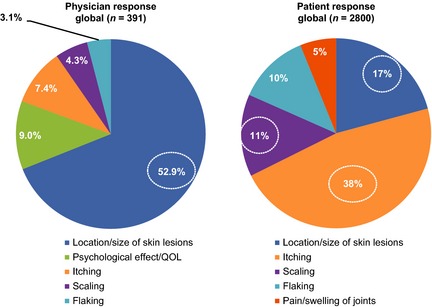
Most important factors contributing to psoriasis disease severity, as reported by physicians and patients.8
Most dermatologists and rheumatologists acknowledged that PsA is likely underdiagnosed because of a failure to connect skin and joint symptoms; however, dermatologists indicated they did not always discuss the possibility of PsA with psoriasis patients. According to dermatologists, 20.0% of their psoriasis patients complained of joint symptoms, which is lower than the 44% of psoriasis patients in the MAPP survey who indicated joint pain.8 In addition, 16% and 26% of patients with psoriasis alone reported symptoms possibly resembling enthesitis and dactylitis respectively.8 According to dermatologists, only 33.0% of psoriasis patients complaining of joint pain had a confirmed diagnosis of PsA. Consistent with previous reports,14 this may indicate that patients are not being fully assessed and diagnosed with PsA despite the presence of joint symptoms, although joint pain may be attributable to a number of conditions other than PsA. Dermatologists most often cited differentiating PsA from other rheumatologic or musculoskeletal diseases as the greatest challenge in managing PsA, whereas rheumatologists most often cited delayed referral. Educating dermatologists on the importance of discussing symptoms of pain, such as joint, back or foot pain, with psoriasis patients and when to refer patients to a rheumatologist may be helpful in increasing PsA identification.
The current findings further indicate that many patients with moderate‐to‐severe disease may be undertreated, especially in the dermatology setting.15 Only 39.1% of dermatologists reported their moderate‐to‐severe psoriasis patients were receiving conventional oral (19.5%) or biologic (19.6%) therapy, and 54% indicated they would prescribe topical therapies as monotherapy for their moderate‐to‐severe psoriasis patients. Treatment rates in PsA patients were higher, with approximately 65% of dermatologists reporting conventional oral (35.2%) or biologic (30.6%) use. The main reasons for not initiating or maintaining these therapies were related to concerns about long‐term safety, tolerability and efficacy, as well as costs (biologics). In addition, physician responses indicate that the most burdensome aspects of biologic therapy were related to the time requirements for patient education and management and prior authorization requirements.
Dermatologists and rheumatologists reported higher treatment rates compared with patients surveyed in MAPP.8 This may be due to the fact that patients could participate in the MAPP survey whether or not they were under the care of a physician, and may not have been treated because they were not seeing a physician for their disease. Challenges related to current therapies also may impact treatment rates. Many dermatologists and rheumatologists felt that the management of psoriasis and PsA was complicated, time‐consuming and challenging. Approximately half of the dermatologists and one quarter of the rheumatologists felt that patients leaving their practice because of frustration or dissatisfaction with current therapies was an important issue. In the patient survey, many patients cited not seeing an HCP in the past 12 months because they did not think the HCP could help, and because of frustration, low expectations, and/or dissatisfaction with current therapies.8 Furthermore, dermatologists and rheumatologists often perceive the management of PsA as challenging. Patients are typically seen by multiple HCPs, and psoriasis and PsA are associated with a number of comorbidities that can make treatment more complex.16
Satisfaction with current therapies may impact patient management as well. Patients generally expressed lower rates of satisfaction with current therapies compared with dermatologists and rheumatologists, particularly for ease of use and long‐term safety.8 Taken together, the findings of the patient and physician surveys suggest that available therapies may not be initiated because of long‐term safety concerns, cost, need for injections and additional time requirements for therapies, and confirm the need for efficacious, less burdensome, cost‐effective therapies with improved safety and tolerability profiles.
Limitations
As with any survey, MAPP was limited by factors such as accurate recall and interpretation of questions. Because the survey was blinded, physicians could not be re‐contacted to clarify answers. The MAPP survey lacked a control group and was not designed to capture differences in use of or attitudes towards various agents within a class of drugs or the impact of healthcare system requirements on decision‐making.
Conclusions
The findings of the physician portion of the MAPP survey support the findings of the patient survey and highlight the importance of screening and assessing psoriasis patients for symptoms of PsA, aligning patient and physician severity assessments and treatment goals, and the ongoing need for safe and effective therapies for psoriasis and PsA. Additional research and education around these findings is warranted.
Acknowledgements
The authors received editorial support in the preparation of this report from Jennifer Schwinn, RPh and Peloton Advantage, funded by Celgene Corporation. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript.
Conflicts of interest
P.C.M. van de Kerkhof has received honoraria as a speaker and/or consultant, and participated in clinical trials sponsored by AbbVie, Allmirall, Amgen, Celgene, Centocor, Eli Lilly, Galderma, Jansen Cilag, LEO Pharma, Mitsubishi, Novartis, Pfizer, Philips and Sandoz. K. Reich has received honoraria as a speaker and consultant for and participated in clinical trials sponsored by Abbott, Biogen‐Idec, Centocor, Janssen‐Cilag, Schering‐Plough and Wyeth. A. Kavanaugh has served as an investigator for AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Janssen and UCB. H. Bachelez has served as a consultant for AbbVie, Almirall, Amgen, Boehringer, Celgene, Centocor, Eli Lilly, Janssen‐Cilag, LEO Pharma, MSD, Merck‐Serono, Novartis, Pfizer, Sandoz and Takeda. J. Barker has acted as consultant for or spoken at sponsored symposia for or received grant funding from AbbVie, Amgen, Celgene, Centocor, Eli Lilly, Janssen, Merck, Novartis and Pfizer. G. Girolomoni has received honoraria as a speaker and/or consultant by AbbVie, Actelion, Boehringer Ingelheim, Celgene, Dompè, Eli Lilly, Galderma, Janssen, LEO Pharma, Maruho, Merck‐Serono, MSD, Mundipharma, Novartis, Otsuka, Pfizer, Rottapharm and Shiseido. R.G. Langley has served as principal investigator for and is on the scientific advisory board of Abbott, Amgen, Centocor and Schering‐Plough. C.F. Paul has served as an investigator or consultant for Abbott, Amgen, Astellas, Celgene, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer and Pierre Fabre. L. Puig has served as a consultant and/or paid speaker for and/or participated in clinical trials for Abbott, Amgen, Celgene, Centocor, Janssen‐Cilag, LEO Pharma, Merck, MSD, Novartis and Pfizer. M.G. Lebwohl has served as an investigator for Amgen, Celgene, Coronado Biosciences, Eli Lilly, Janssen Biotech, LEO Pharma, GlaxoSmithKline and Ranbaxy, and as a consultant for Abbott, Amgen, Anacor, BioLineRx, Celgene, DermiPsor, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Maruho Co., Meda, Novartis, Pfizer and Valeant.
Funding source
The MAPP survey was sponsored by Celgene Corporation.
References
- 1. Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co‐morbidity and age‐related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol 2010; 90: 147–151. [DOI] [PubMed] [Google Scholar]
- 2. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol 2009; 60: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menter A, Gottlieb A, Feldman SR et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58: 826–850. [DOI] [PubMed] [Google Scholar]
- 4. Ferrandiz C, Bordas X, Garcia‐Patos V, Puig S, Pujol R, Smandia A. Prevalence of psoriasis in Spain (Epiderma Project: phase I). J Eur Acad Dermatol Venereol 2001; 15: 20–23. [DOI] [PubMed] [Google Scholar]
- 5. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70: 512–516. [DOI] [PubMed] [Google Scholar]
- 6. Ferrandiz C, Carrascosa JM, Toro M. Prevalence of psoriasis in Spain in the age of biologics. Actas Dermosifiliogr 2014; 105: 504–509. [DOI] [PubMed] [Google Scholar]
- 7. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005; 64(Suppl 2): ii14–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70: 871–881. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology 2012; 225: 121–126. [DOI] [PubMed] [Google Scholar]
- 10. Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health‐related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Are patients with psoriasis undertreated? Results of National Psoriasis Foundation survey. J Am Acad Dermatol 2007; 57: 957–962. [DOI] [PubMed] [Google Scholar]
- 12. Nast A, Reytan N, Rosumeck S, Erdmann R, Rzany B. Low prescription rate for systemic treatments in the management of severe psoriasis vulgaris and psoriatic arthritis in dermatological practices in Berlin and Brandenburg, Germany: results from a patient registry. J Eur Acad Dermatol Venereol 2008; 22: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol 2013; 149: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ, Gladman DD, Papp KA et al Prevalence of rheumatologist‐diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013; 69: 729–735. [DOI] [PubMed] [Google Scholar]
- 15. Maza A, Richard MA, Aubin F et al Significant delay in the introduction of systemic treatment of moderate to severe psoriasis: a prospective multicentre observational study in outpatients from hospital dermatology departments in France. Br J Dermatol 2012; 167: 643–648. [DOI] [PubMed] [Google Scholar]
- 16. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496–509. [DOI] [PubMed] [Google Scholar]


