Abstract
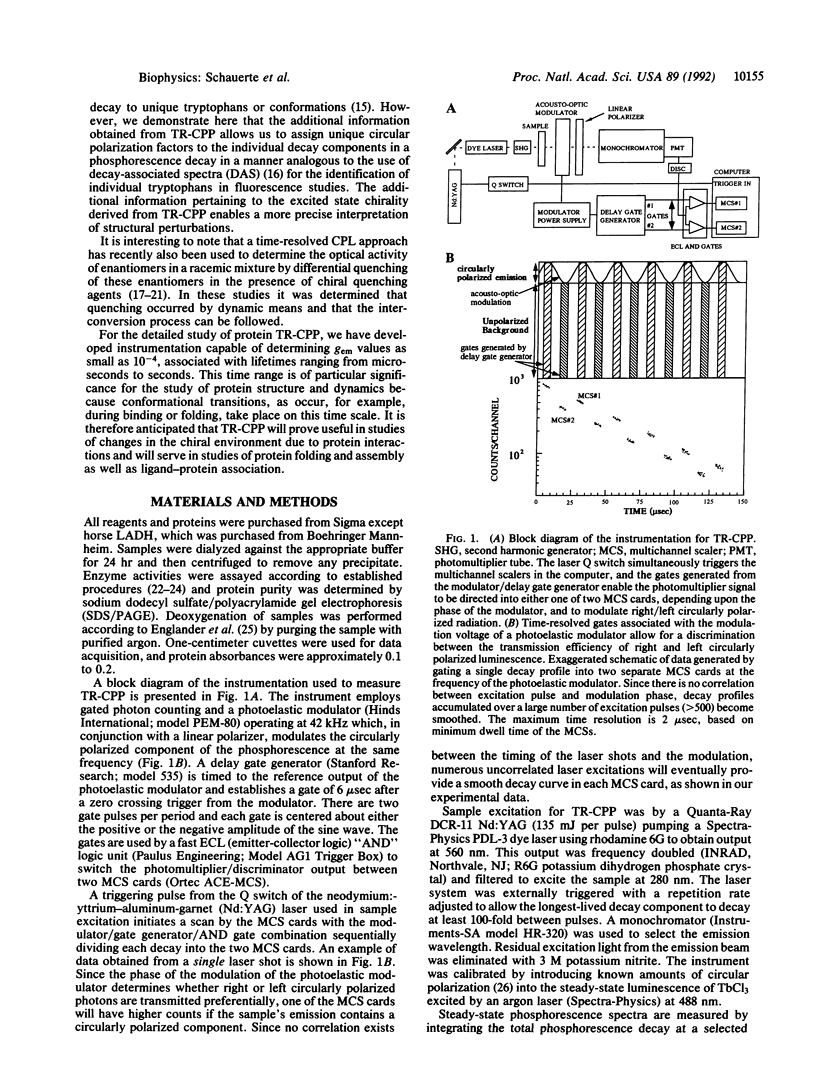
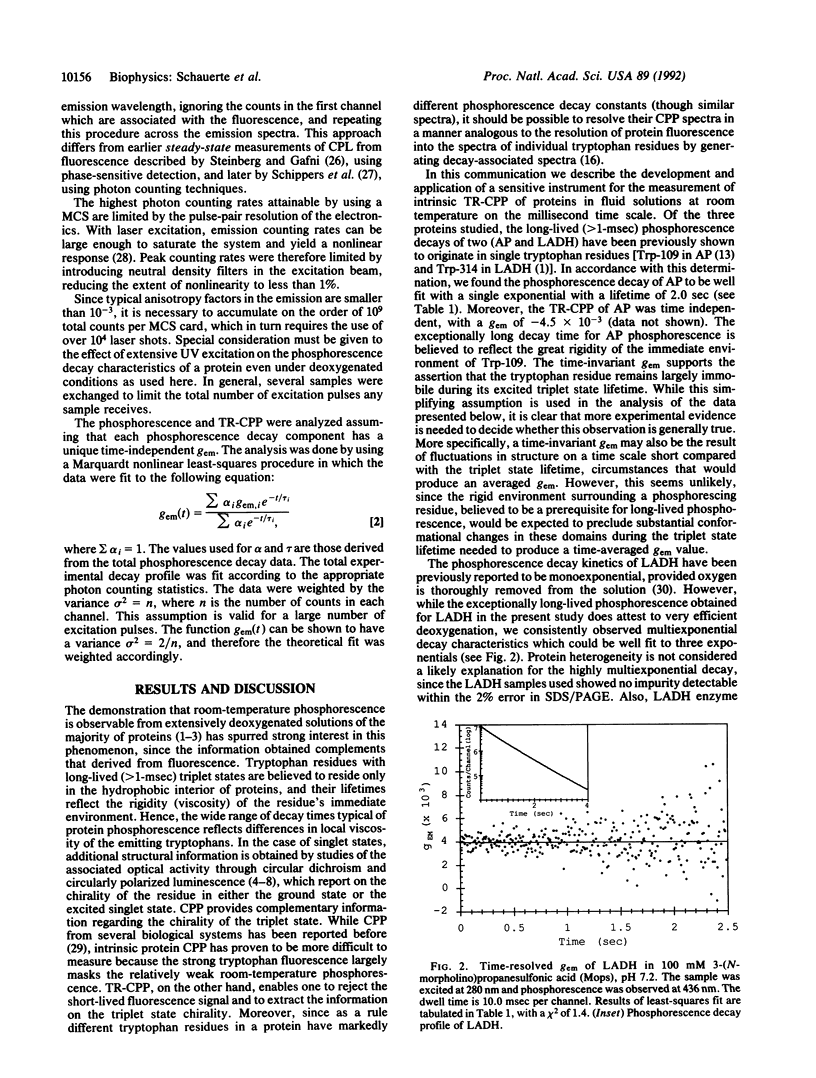
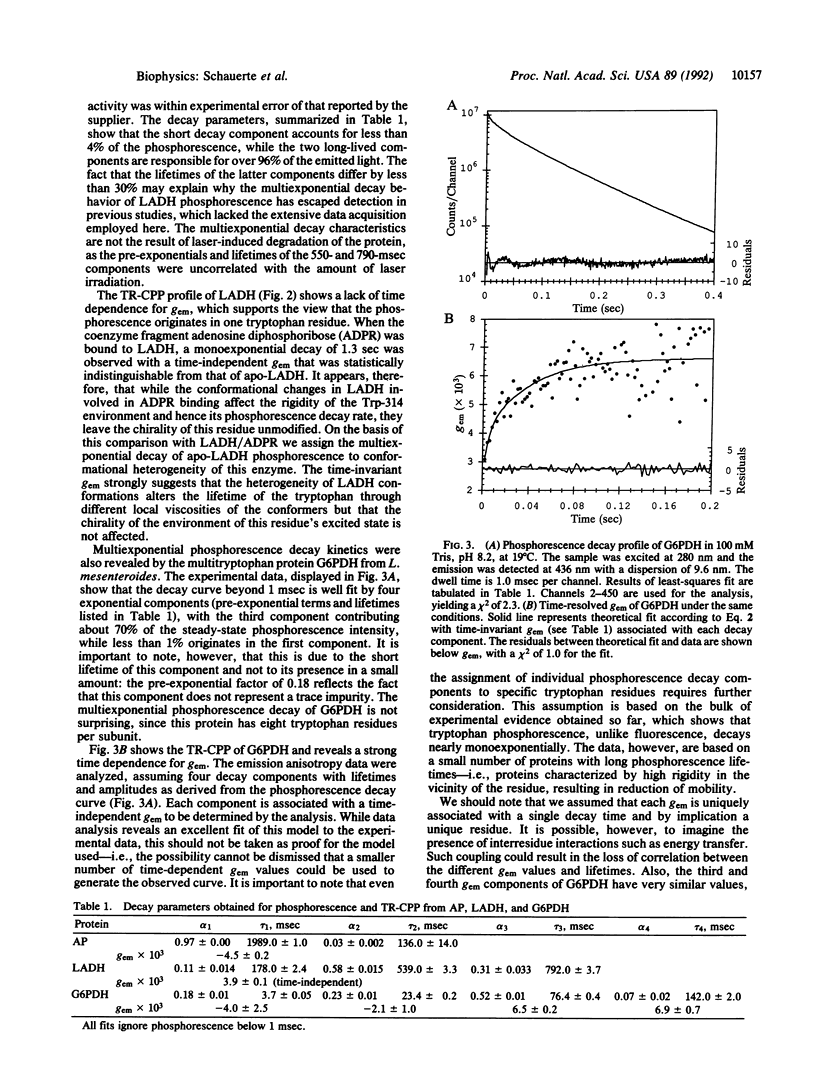
The existence of circular polarization in room-temperature protein phosphorescence is demonstrated, and time-resolved circularly polarized phosphorescence (TR-CPP) is used to characterize unique tryptophan environments in multitryptophan proteins. Circularly polarized luminescence studies provide information regarding the excited state chirality of a lumiphore which can be used to extract sensitive structural information. It is shown by time resolving the circular polarization that it is possible to correlate the excited state chirality with unique decay components in a multiexponential phosphorescence decay profile. The present study presents a concurrent analysis of room-temperature time-resolved phosphorescence and TR-CPP of bacterial glucose-6-phosphate dehydrogenase as well as those of horse liver alcohol dehydrogenase. Only one of the two tryptophan residues per subunit of dimeric alcohol dehydrogenase is believed to phosphorescence, while the dimeric glucose-6-phosphate dehydrogenase has eight tryptophan residues per subunit and shows a corresponding complexity in its phosphorescence decay profile. The anisotropy factor [g(em) = delta I/(Itotal/2); delta I = Ileft circular-Iright circular] for alcohol dehydrogenase is time independent, suggesting a unique excited state chirality. The phosphorescence decay of glucose-6-phosphate dehydrogenase can be well fitted with four exponential terms of 4, 23, 76, and 142 msec, and the TR-CPP of this enzyme shows a strong time dependence that can be resolved into four individual time-independent anisotropy factors of -4.0, -2.1, +6.5, and +6.9 (x10(-3)), each respectively associated with one of the four lifetime components. These results demonstrate how the use of TR-CPP can facilitate the study of proteins with multiple lumiphores.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brittain H. G., Richardson F. S., Martin R. B., Burtnick L. D., Kay C. M. Circularly polarized emission of terbium(III) substituted bovine cardiac troponin-C. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1013–1019. doi: 10.1016/0006-291x(76)91247-x. [DOI] [PubMed] [Google Scholar]
- Brittain H. G., Richardson F. S., Martin R. B. Terbium (III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc. 1976 Dec 8;98(25):8255–8260. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- Burtnick L. D., Schaar P. L. Circular polarization of the fluorescence of actin-bound epsilon ATP Effects of binding DNase I. FEBS Lett. 1979 Jan 1;97(1):166–170. doi: 10.1016/0014-5793(79)80076-9. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J. Biochemistry without oxygen. Anal Biochem. 1987 Mar;161(2):300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Demaille J. G., Gerard D. Terbium as luminescent probe of calmodulin calcium-binding sites; domains I and II contain the high-affinity sites. FEBS Lett. 1980 Jul 28;116(2):269–272. doi: 10.1016/0014-5793(80)80660-0. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Gerard D., Demaille J. G. Terbium binding to octopus calmodulin provides the complete sequence of ion binding. FEBS Lett. 1980 Oct 20;120(1):99–103. doi: 10.1016/0014-5793(80)81055-6. [DOI] [PubMed] [Google Scholar]
- Kim E. E., Wyckoff H. W. Structure of alkaline phosphatases. Clin Chim Acta. 1990 Jan 15;186(2):175–187. doi: 10.1016/0009-8981(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Knutson J. R., Walbridge D. G., Brand L. Decay-associated fluorescence spectra and the heterogeneous emission of alcohol dehydrogenase. Biochemistry. 1982 Sep 14;21(19):4671–4679. doi: 10.1021/bi00262a024. [DOI] [PubMed] [Google Scholar]
- Mersol J. V., Steel D. G., Gafni A. Quenching of tryptophan phosphorescence in Escherichia coli alkaline phosphatase by long-range transfer mechanisms to external agents in the rapid-diffusion limit. Biochemistry. 1991 Jan 22;30(3):668–675. doi: 10.1021/bi00217a012. [DOI] [PubMed] [Google Scholar]
- Olive C., Levy H. R. The preparation and some properties of crystalline glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. Biochemistry. 1967 Mar;6(3):730–736. doi: 10.1021/bi00855a012. [DOI] [PubMed] [Google Scholar]
- Papp S., Vanderkooi J. M. Tryptophan phosphorescence at room temperature as a tool to study protein structure and dynamics. Photochem Photobiol. 1989 Jun;49(6):775–784. doi: 10.1111/j.1751-1097.1989.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Papp S., Vanderkooi J. M. Tryptophan phosphorescence at room temperature as a tool to study protein structure and dynamics. Photochem Photobiol. 1989 Jun;49(6):775–784. doi: 10.1111/j.1751-1097.1989.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowadski J. M., Handschumacher M. D., Murthy H. M., Foster B. A., Wyckoff H. W. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985 Nov 20;186(2):417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- Steinberg I. Z. Circularly polarized luminescence. Methods Enzymol. 1978;49:179–199. doi: 10.1016/s0076-6879(78)49009-3. [DOI] [PubMed] [Google Scholar]
- Steinberg N., Wachtel E. J., Gafni A. Ionic strength dependent conformational changes of transfer ribonucleic acid studied by circular polarization of phosphorescence. Biochemistry. 1982 May 11;21(10):2573–2578. doi: 10.1021/bi00539a043. [DOI] [PubMed] [Google Scholar]
- Strambini G. B. Quenching of alkaline phosphatase phosphorescence by O2 and NO. Evidence for inflexible regions of protein structure. Biophys J. 1987 Jul;52(1):23–28. doi: 10.1016/S0006-3495(87)83184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Calhoun D. B., Englander S. W. On the prevalence of room-temperature protein phosphorescence. Science. 1987 May 1;236(4801):568–569. doi: 10.1126/science.3576185. [DOI] [PMC free article] [PubMed] [Google Scholar]


