Abstract
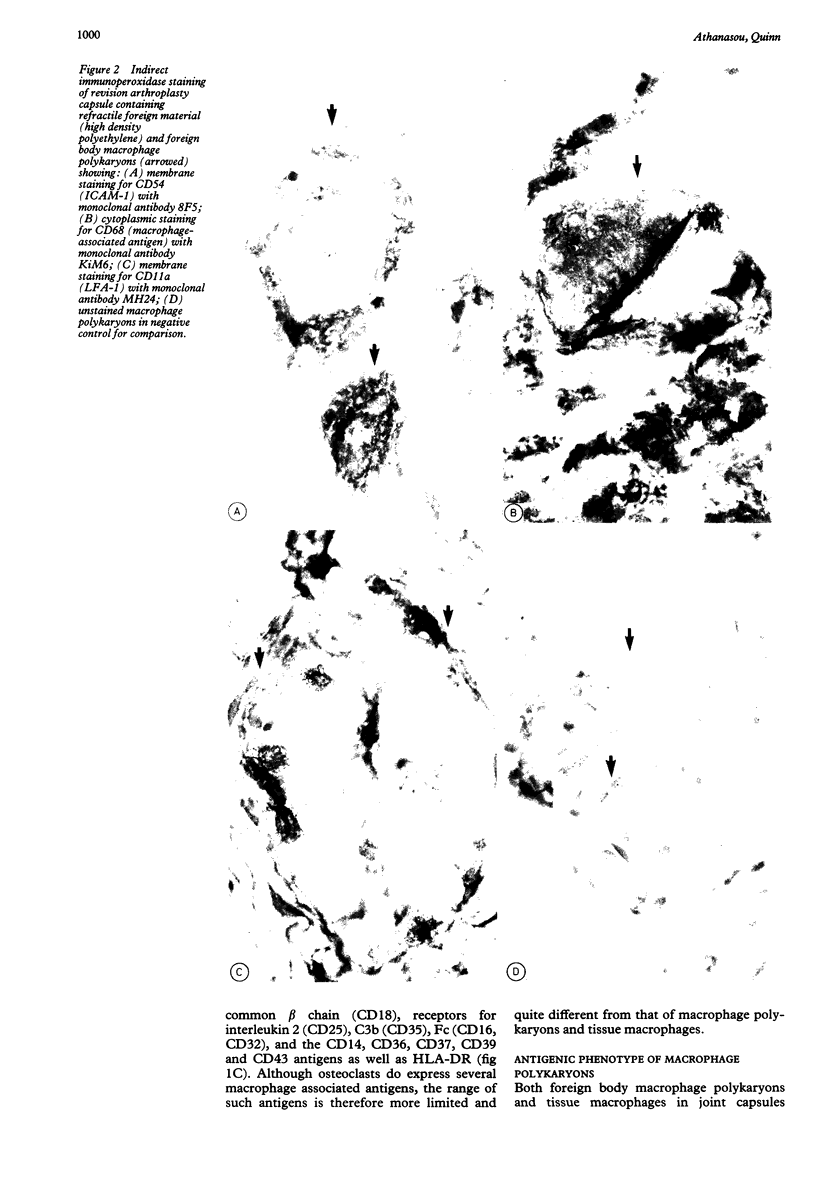
The antigenic phenotype of human fetal osteoclasts was compared with that of human tissue macrophages and macrophage polykaryons in foreign body lesions using a large number of monoclonal antibodies directed against myeloid (granulocyte/mononuclear phagocyte) antigens. Osteoclasts expressed a restricted range of macrophage-associated antigens including CD13, CD15A, CD44, CD45, CD54, (ICAM-1), CD71 (transferrin receptor), and CD68. These antigens were also present on macrophages and macrophage polykaryons both of which also strongly expressed CD11a,b,c, CD18, (LFA family), CD14, CD31, CD36, CD37, CD39 and CD43 antigens. There was also weak and occasional expression of CD16 (FcRIII), CD25 (interleukin 2 receptor), CD32 (FcRII), CD35 (C3b receptor) and HLA-DR by macrophage polykaryons. The presence of some macrophage associated antigens on osteoclasts is consistent with their originating from cells of the mononuclear phagocyte system. The numerous differences in antigenic phenotype between osteoclasts and macrophage polykaryons, however, suggest that their pathways of development and differentiation are not identical. The differences discerned in antigenic phenotype should also permit distinction between these polykaryons (and possibly their mononuclear precursors) in normal and diseased tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanasou N. A., Heryet A., Quinn J., Gatter K. C., Mason D. Y., McGee J. O. Osteoclasts contain macrophage and megakaryocyte antigens. J Pathol. 1986 Dec;150(4):239–246. doi: 10.1002/path.1711500403. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Heryet A., McGee J. O. Localization of platelet antigens and fibrinogen on osteoclasts. J Cell Sci. 1988 Jan;89(Pt 1):115–122. doi: 10.1242/jcs.89.1.115. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Horton M. A., McGee J. O. New sites of cellular vitronectin receptor immunoreactivity detected with osteoclast-reacting monoclonal antibodies 13C2 and 23C6. Bone Miner. 1990 Jan;8(1):7–22. doi: 10.1016/0169-6009(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., McGee J. O. Immunocytochemical analysis of the human osteoclast: phenotypic relationship to other marrow-derived cells. Bone Miner. 1988 Mar;3(4):317–333. [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., McGee J. O. Leucocyte common antigen is present on osteoclasts. J Pathol. 1987 Oct;153(2):121–126. doi: 10.1002/path.1711530205. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Wells C. A., Quinn J., Ferguson D. P., Heryet A., McGee J. O. The origin and nature of stromal osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis and macrophage biology. Br J Cancer. 1989 Apr;59(4):491–498. doi: 10.1038/bjc.1989.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher M. E., Franklin W. A., Simon M. A. Immunohistochemical study of mononuclear phagocyte antigens in giant cell tumor of bone. Am J Pathol. 1986 Nov;125(2):252–257. [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Magnus C. J. Calcitonin alters behaviour of isolated osteoclasts. J Pathol. 1982 Jan;136(1):27–39. doi: 10.1002/path.1711360104. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Multinucleate giant cells. J Pathol. 1978 Nov;126(3):125–148. doi: 10.1002/path.1711260302. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. The cellular basis of bone resorption. Clin Orthop Relat Res. 1980 Sep;(151):283–293. [PubMed] [Google Scholar]
- Chambers T. J. The pathobiology of the osteoclast. J Clin Pathol. 1985 Mar;38(3):241–252. doi: 10.1136/jcp.38.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia P. F., Krivit W., Cervenka J., Clawson C., Kersey J. H., Kim T. H., Nesbit M. E., Ramsay N. K., Warkentin P. I., Teitelbaum S. L. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med. 1980 Mar 27;302(13):701–708. doi: 10.1056/NEJM198003273021301. [DOI] [PubMed] [Google Scholar]
- Davey F. R., Cordell J. L., Erber W. N., Pulford K. A., Gatter K. C., Mason D. Y. Monoclonal antibody (Y1/82A) with specificity towards peripheral blood monocytes and tissue macrophages. J Clin Pathol. 1988 Jul;41(7):753–758. doi: 10.1136/jcp.41.7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey C. K., Bick K. L. Ultrastructural analysis of glycosaminoglycan hydrolysis in the rat periodontal ligament. I. Evidence for macrophage involvement in bone remodelling. Calcif Tissue Res. 1977 Dec 28;24(2):135–141. doi: 10.1007/BF02223307. [DOI] [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. The osteoclast: review of ultrastructure, origin, and structure-function relationship. Clin Orthop Relat Res. 1976 Oct;(120):201–231. [PubMed] [Google Scholar]
- Hanaoka H., Friedman B., Mack R. P. Ultrastructure and histogenesis of giant-cell tumor of bone. Cancer. 1970 Jun;25(6):1408–1423. doi: 10.1002/1097-0142(197006)25:6<1408::aid-cncr2820250622>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- JEE W. S., NOLAN P. D. ORIGIN OF OSTEOCLASTS FROM THE FUSION OF PHAGOCYTES. Nature. 1963 Oct 19;200:225–226. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukita T., McManus L. M., Miller M., Civin C., Roodman G. D. Osteoclast-like cells formed in long-term human bone marrow cultures express a similar surface phenotype as authentic osteoclasts. Lab Invest. 1989 Apr;60(4):532–538. [PubMed] [Google Scholar]
- Lasser A. The mononuclear phagocytic system: a review. Hum Pathol. 1983 Feb;14(2):108–126. doi: 10.1016/s0046-8177(83)80239-1. [DOI] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Congenital osteopetrotic mutations as probes of the origin, structure, and function of osteoclasts. Clin Orthop Relat Res. 1984 Oct;(189):239–263. [PubMed] [Google Scholar]
- Marks S. C., Jr, Popoff S. N. Bone cell biology: the regulation of development, structure, and function in the skeleton. Am J Anat. 1988 Sep;183(1):1–44. doi: 10.1002/aja.1001830102. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr The origin of osteoclasts: evidence, clinical implications and investigative challenges of an extra-skeletal source. J Oral Pathol. 1983 Aug;12(4):226–256. doi: 10.1111/j.1600-0714.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Vrijheid-Lammers T., Mulder R. J., Blok J. Cell surface antigens on osteoclasts and related cells in the quail studied with monoclonal antibodies. Histochemistry. 1985;83(4):315–324. doi: 10.1007/BF00684377. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Bell L. V., Clevinger B., Osdoby P. Identification of osteoclast-specific monoclonal antibodies. J Cell Biol. 1985 May;100(5):1592–1600. doi: 10.1083/jcb.100.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Walters M. N. Macrophage polykarya. CRC Crit Rev Toxicol. 1979 Aug;6(3):211–255. doi: 10.3109/10408447909037484. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Kreipe H., Hansmann M. L., Barth J. Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol. 1986 Oct;125(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- Regezi J. A., Zarbo R. J., Lloyd R. V. HLA-DR antigen detection in giant cell lesions. J Oral Pathol. 1986 Sep;15(8):434–438. doi: 10.1111/j.1600-0714.1986.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Revell P. A. Tissue reactions to joint prostheses and the products of wear and corrosion. Curr Top Pathol. 1982;71:73–101. doi: 10.1007/978-3-642-68382-4_3. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sminia T., Dijkstra C. D. The origin of osteoclasts: an immunohistochemical study on macrophages and osteoclasts in embryonic rat bone. Calcif Tissue Int. 1986 Oct;39(4):263–266. doi: 10.1007/BF02555216. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkler S. M., Williams D. M., Johnson N. W. Osteoclast formation in response to intraperitoneal injection of 1 alpha-hydroxycholecalciferol in mice. J Anat. 1981 Aug;133(Pt 1):91–97. [PMC free article] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Primavera M. V. Monocytes from circulating blood fuse in vitro with purified osteoclasts in primary culture. J Cell Sci. 1984 Mar;66:335–342. doi: 10.1242/jcs.66.1.335. [DOI] [PubMed] [Google Scholar]