Abstract
The risk factors for childhood overweight and obesity are known and can be identified antenatally or during infancy, however, the majority of effective interventions are designed for older children. This review identified interventions designed to reduce the risk of overweight/obesity that were delivered antenatally or during the first 2 years of life, with outcomes reported from birth to 7 years of age. Six electronic databases were searched for papers reporting randomised controlled trials of interventions published from January 1990 to September 2013. A total of 35 eligible studies were identified, describing 27 unique trials of which 24 were behavioural and three were non‐behavioural. The 24 behavioural trials were categorised by type of intervention: (1) nutritional and/or responsive feeding interventions targeted at parents of infants, which improved feeding practices and had some impact on child weight (n = 12); (2) breastfeeding promotion and lactation support for mothers, which had a positive effect on breastfeeding but not child weight (n = 5); (3) parenting and family lifestyle (n = 4); and (4) maternal health (n = 3) interventions that had some impact on feeding practices but not child weight. The non‐behavioural trials comprised interventions manipulating formula milk composition (n = 3). Of these, lower/hydrolysed protein formula milk had a positive effect on weight outcomes. Interventions that aim to improve diet and parental responsiveness to infant cues showed most promise in terms of self‐reported behavioural change. Despite the known risk factors, there were very few intervention studies for pregnant women that continue during infancy which should be a priority for future research.
Keywords: infancy, prevention, obesity, overweight, intervention
Introduction
Worldwide, more than 40 million children under the age of 5 were overweight or obese in 2011 (World Health Organization 2013). Over a fifth (22.6%) of UK children aged 4–5 years who were measured in 2012/2013 were either overweight or obese (Health and Social Care Information Centre 2013), with the highest rates found in children living in economically deprived areas and children from Black or Black British, Asian or Asian British ethnic groups (Health and Social Care Information Centre 2013). As a child's weight at 5 years of age is a good indicator of future health (Gardner et al. 2009) and risk of obesity in adulthood (Dietz 1998), there is a strong case for early intervention that prevents or reduces the risk (National Institute for Health and Clinical Excellence 2006; Darzi 2008). The risk factors for childhood overweight and obesity are known and can be identified antenatally or during infancy. A systematic review identified significant associations between childhood overweight and maternal pre‐pregnancy overweight, smoking during pregnancy, high infant birthweight and rapid weight gain (Weng et al. 2012). Estimates vary, but between 25% (Ekelund et al. 2006) and 33% (Hui et al. 2008) of infants gain weight more rapidly than is desirable during the first 6 months of life and this is a strong risk factor for the development of childhood obesity (Baird et al. 2005; Monteiro & Victora 2005; Ong & Loos 2006). From the infant perspective, rapid weight gain may be modifiable with interventions targeting parental feeding practices. For example, a meta‐analysis found breastfeeding decreased the odds of childhood overweight by 15% (Weng et al. 2012). There is no systematic review evidence regarding the protective effects of later introduction of solid foods and longer durations of breastfeeding on childhood overweight (Weng et al. 2012). However, single studies have found significant associations between early introduction of solid foods (Sloan et al. 2008; Hawkins et al. 2009; Huh et al. 2011), shorter breastfeeding duration (Weyermann et al. 2006), higher energy intake (Ong et al. 2006), shorter sleep duration (Taveras et al. 2008), high maternal control over feeding (Farrow & Blissett 2006), sedentary lifestyle (Brophy et al. 2009) and the risk of childhood obesity. Responsive feeding (DiSantis et al. 2011) may be protective against childhood obesity.
Despite the epidemiological evidence and calls for prevention strategies targeting multiple risk factors that begin at birth (Dattilo et al. 2012), the majority of effective interventions are designed for older children (Waters et al. 2011). Hesketh & Campbell (2010) conducted a systematic review of obesity prevention interventions for children under 5 years of age. They identified five interventions for children under 2 years, all of which reported limited positive impact on feeding practices but not weight outcomes (Hesketh & Campbell 2010). This finding may be at least partially attributed to the focus of the review, which excluded some interventions that potentially modify rapid weight gain such as breastfeeding. It is clinically important to explore whether interventions exist that directly or indirectly address known risk factors (Weng et al. 2012) and to examine the impact of these interventions on the development of childhood overweight or obesity. This systematic review includes studies describing randomised controlled trials (RCTs) of interventions that aim to address the risk factors for childhood overweight and obesity identified in an earlier review by the authors (Weng et al. 2012). Interventions that commenced antenatally or during the first 2 years of life with outcomes reported from birth to 7 years of age are the subject of this review.
Key messages
The most promising obesity prevention interventions for children under 2 years of age are those that focus on diet and responsive feeding.
Although the number of published studies on obesity prevention interventions for children under 2 years of age has risen exponentially since 2010, interventions for pregnant women with follow‐up during early life are rare. This should be a priority for future research.
Future interventions for obesity prevention in children under 2 years of age should consider the option of advising some families to offer lower protein formula milk together with behavioural change components.
Future intervention development should explore the most appropriate behaviour change theory to use with parents of young children.
Methods
Inclusion/exclusion criteria
The inclusion criteria were:
Study design
Prospective studies that identified themselves as RCTs were considered for inclusion. No restriction was placed on geographical location or setting of the intervention programme.
Participants
The participants were pregnant mothers, parents (carers, guardians) of infants <2 years old and healthy infants <2 years old.
Intervention
Behavioural and non‐behavioural interventions were included in this review.
Comparison
Studies with any type of comparison group were eligible for inclusion.
Primary outcomes
The primary outcomes were infant/child body mass index (BMI), weight, weight gain velocity, weight‐for‐length and weight‐for‐age from birth to 7 years of age.
Secondary outcomes
Secondary outcomes were breastfeeding uptake and duration, timing of introduction of solid food, diet intake and quality, responsive feeding practices and physical activity from birth to 7 years of age.
Studies were excluded if the intervention commenced when the infant was 2+ years of age. Studies of interventions which aimed to increase the weight of infants (e.g. in malnourished infants or low‐birthweight infants) were excluded. Studies that selectively recruited infants with specific conditions or diseases at the time of the study (e.g. chronic diarrhoea, diabetes, sustained high‐blood pressure) were also excluded.
Search strategy
A full electronic search was carried out in August 2012 and updated in September 2013. Six electronic databases (PubMed, Medline, CINAHL, PsychINFO, Cochrane, EMBASE and the Cochrane Library) were identified and searched for articles published from 1990 onwards. This was chosen as a cut‐off because a scoping search could not identify trials of obesity prevention interventions in early childhood prior to this date. Full search terms are provided in Box 1.
Box 1. Search terms for review.
child OR children OR infant OR newborn OR pediatric OR pre‐school OR nursery OR nurseries or parent OR caregivers/education
‘body mass index’ OR BMI OR ‘weight gain’ OR overweight OR obesity OR ‘body fat’ OR adiposity OR ponderal OR anthropometric OR growth OR ‘child development’ OR ‘body height’ OR ‘body weight’ OR weight‐for‐age OR weight‐for‐length
nutrition OR ‘complementary feeding’ OR baby‐led OR ‘feeding interaction’ OR ‘formula feeding’ OR ‘formula fed’ OR ‘infant food’ OR ‘nutritional requirements’ OR ‘energy intake’ OR diet OR ‘diet therapy’ OR ‘feeding behavior’ OR ‘food preferences’ OR ‘breast feeding’ OR weaning OR parenting OR ‘health education’ OR ‘health facilities’ OR ‘health promotion’ OR ‘physical activity’ OR exercise OR sedentary OR ‘tummy time’ OR sleep
prevention OR intervention
‘randomized controlled trial’ OR RCT OR random OR ‘control group’
[1 AND 2 AND 3 AND 4 AND 5]
Reference lists of articles identified using this strategy and of currently published systematic reviews were scanned to identify potential studies for inclusion in the review that may have otherwise been missed.
Two databases for the registration of clinical trials (http://www.clinicaltrials.gov/ and http://www.controlled-trials.com/) were searched to identify any ongoing or unpublished research trials. An advertisement was distributed to members of the Association for the Study of Obesity (UK) to identify ongoing and/or unpublished studies or those published in the grey literature.
Data extraction and synthesis
Two reviewers extracted the data from the included studies (SR and BE). Details collected included study characteristics, participants, intervention details, outcomes and quality assessment. The data extraction sheet is available as Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12184/suppinfo.
Quality assessment
The Jadad scale (Jadad et al. 1996) was used to assess the quality of published clinical trials based on the description and appropriateness of random assignment, blinding and the flow of patients.
Random assignment
Two points were awarded if assignment was explicitly stated as randomised (including the use of words such as randomly, random and randomisation) and the method to generate the sequence of randomisation was described and appropriate (table of random numbers, computer generated, concealed allocation, etc.). Otherwise, the trial scored zero points.
Blinding
Non‐behavioural trials were awarded two points if they were double‐blinded where neither the person doing the assessments nor the study participant could identify the intervention being assessed, or if in the absence of such a statement the use of ‘active placebos’, ‘identical placebos’ or ‘dummies’ were mentioned. Behavioural trials were awarded one point if single‐blinded (as behavioural trials are not possible to double‐blind) where the person(s) collecting and/or assessing outcome data were blind to participants’ group allocation, and the study assessed whether blinding had been a success. In all other cases, the trial scored zero points.
Flow of participants
One point was awarded if the number and reasons for withdrawal in each group were stated. If there was no statement on withdrawals or the description of withdrawals was incomplete, the trial was awarded zero points.
In addition, internal validity (i.e. was the intervention delivered as planned) and external validity (i.e. how generalisable is the delivery of the intervention to other settings) were examined in accordance with the evidence‐based behavioural medicine (EBBM) guidelines (Davidson et al. 2003) for studies on behavioural interventions. Data were collected on whether trials on behavioural interventions reported (1) training of treatment providers; (2) supervision of treatment providers; (3) preferred treatment of choice of those investigating, providing and receiving the intervention; (4) treatment integrity; and (5) assessment of participants' adherence to study treatment. Studies scored one point for each of these criteria.
Results
Figure 1 shows the flow diagram of the review process. Electronic searches identified 1784 titles and a further 27 were identified through other searches (see Fig. 1). Of these, 605 duplicate studies were removed. Two reviewers (BE and SR) screened 1206 titles and abstracts; of which, 1064 did not meet the eligibility criteria. The remaining 142 abstracts were eligible for full‐text review. One full text study was translated from German to English (Jungmann et al. 2010). The 142 full‐text studies were examined by at least two authors; of these, 107 did not meet inclusion criteria. The most common reason for exclusion was that the intervention was designed and delivered to a child older than 2 years of age. There were also a number of studies that met the inclusion criteria but on closer inspection it was revealed that the focus was on malnourished, underweight or low‐birthweight infants rather than those with the potential to be overweight or obese. These studies were also excluded from the review. A total of 35 eligible studies were identified, describing 27 unique trials of interventions (24 behavioural and 3 non‐behavioural).
Figure 1.
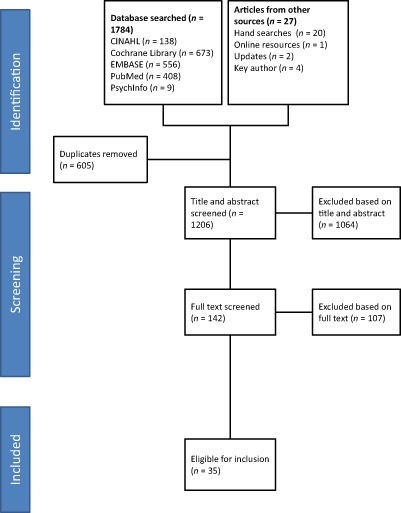
Flow diagram of review process.
Details of the main findings in relation to feeding and weight outcomes can be found in Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12184/suppinfo.
The interventions identified were heterogeneous and did not all directly target obesity risk during infancy. Interventions that met the inclusion criteria included those that tackled known risk factors, such as breastfeeding, but did not specifically focus on obesity prevention. Specific obesity prevention interventions and parenting interventions were identified and included in the review. The two reviewers proposed categorising the studies to reflect their intended outcomes. The proposed categories were discussed during a full team meeting, amended and agreed by all authors.
Trials of interventions that specifically addressed breastfeeding and lactation support were grouped separately from the diet and/or responsive feeding interventions. The reason for this was that these interventions emphasised improving uptake and duration of breastfeeding not obesity prevention. The diet and/or responsive feeding interventions included breastfeeding as a component but additionally addressed other aspects of infant feeding with outcomes focused on aspects of obesity prevention such as weight and parental feeding practices. The diet and/or responsive feeding interventions were considered distinct from the interventions that addressed general parenting and lifestyle. These interventions had some outcomes associated with obesity prevention, such as infant weight, but mainly measured aspects of parenting. Specific interventions addressing maternal health with outcomes reported during infancy were grouped separately. The non‐behavioural studies reported weight but not feeding outcomes and were therefore assigned to a separate category.
Nutritional and/or responsive feeding interventions
Sixteen studies reported the findings of 12 trials of diet, nutrition and/or feeding behaviour interventions. These interventions were heterogeneous consisting of multiple components designed to improve energy intake and output and diet quality and/or feeding responsiveness. Four trials reported that the intervention had a significant impact on weight outcomes in the desired direction (Paul et al. 2011; Daniels et al. 2012; Wen et al. 2012; Verbestel et al. 2013). The most effective trials were either driven by behavioural change theory (Verbestel et al. 2013) or included diet, nutrition and parental responsiveness components (Paul et al. 2011; Daniels et al. 2012; Wen et al. 2012).
Seven interventions focused on parent education around diet and feeding practices (Lapinleimu et al. 1994, 1995; Watt et al. 2009; Scheiwe et al. 2010a; Wen et al. 2011, 2012; French et al. 2012; Jonsdottir et al. 2012; Campbell et al. 2013; Verbestel et al. 2013). In addition, these interventions included components to improve early physical activity (Wen et al. 2011, 2012) and reduce sedentary behaviours (Campbell et al. 2013; Verbestel et al. 2013). Only one of these interventions (Healthy Beginnings) was successful at improving the duration of breastfeeding (Wen et al. 2011, 2012), whereas most of the specific breastfeeding interventions reported this as a successful outcome (Morrow et al. 1999; Kramer et al. 2001, 2002, 2007; Bhandari et al. 2003; Agrasada et al. 2005; Bonuck et al. 2005; Agrasada & Kylberg 2009). The Healthy Beginnings home‐visiting trial reported positive intervention effects on children's fruit and vegetable intake, along with others (Watt et al. 2009; Scheiwe et al. 2010a; French et al. 2012), and an increase in an aspect of physical activity known as tummy time (supervised infant laid prone on mat) (Wen et al. 2011, 2012). The Healthy Beginnings trial also reported that parents delayed the introduction to solid food following their intervention (Wen et al. 2011, 2012). The Melbourne Infant trial reported reductions in television watching (Campbell et al. 2013) and a trial by Verbestel reported reductions in screen time following their intervention (Verbestel et al. 2013). The Melbourne Infant trial reported a reduction in sweet snack intake (Campbell et al. 2013) and a trial by French reported a reduction in juice intake (French et al. 2012). Healthy Beginnings included an educational component around parent–child interaction and the authors report a significant reduction in parents giving food as a reward (Wen et al. 2011, 2012).
Three of these studies report outcomes in contrast to the desired direction. Watt et al. (Watt et al. 2009; Scheiwe et al. 2010a) reported that the intervention group infants were heavier when compared with controls at follow‐up. Although Verbestel et al. (2013) reported that their intervention had a positive impact on weight outcomes, dietary‐related behaviours became less healthy in both groups over the study period.
Three interventions included components to help parents understand about responsiveness to infant cues as well as teaching them about diet and feeding. Infants receiving the NOURISH intervention (Daniels et al. 2012, 2013) had a significantly lower BMI‐for‐age z‐score than those in the control group (intervention, 0.23 ± 0.93; control, 0.42 ± 0.85) at 9 months of age. The control group was also more likely to show rapid weight gain from birth to 9 months of age [odds ratio (OR) = 1.6, 95% confidence interval (CI) = 1.1 to 2.4]. Mothers in the control group were more likely to use non‐responsive feeding practices that overrode child satiety signals (P < 0.001). Although there were no significant differences in anthropometric measures between the groups at aged 2, the intervention group mothers had significantly fewer controlling feeding practices and exhibited more instrumental feeding and parental encouragement to eat (Daniels et al. 2013).
Black et al. (2001) also provided education around (1) recognition of infants' cues; (2) non‐food strategies for managing infant behaviour; and (3) mother–grandmother negotiation strategies. Mothers in the intervention group were nearly four times more likely to adhere to the American Academy of Paediatrics guidelines on infant feeding (OR 3.8: 1.6–9.1) compared with mothers who did not receive the intervention. Kavanagh et al. (2008) delivered a behavioural intervention to formula‐feeding caregivers in the Special Supplemental Nutrition Programme for Women, Infants and Children. The intervention, comprised information about infant satiety cues and formula milk preparation, only had a positive impact on parents' knowledge about feeding. Infants' growth in the intervention group was greater than in the control group.
The SLIMTIME intervention (Paul et al. 2011) was delivered to 160 first‐time mothers who intended to breastfeed. This intervention focused on responsive feeding rather than diet. A significantly slower rate of weight gain was reported for infants receiving ‘soothe/sleep’ intervention suggesting that educating parents of infants about responsive feeding may be more beneficial than dietary advice alone.
Finally, a unique study by Fewtrell et al. (2012) compared the weights of infants receiving one of two different types of bottle design with a breastfed reference group and found no significant differences in anthropometry at 2, 3 and 4 months post‐partum.
Breastfeeding promotion and lactation support interventions
Seven studies reported the findings of five trials of breastfeeding promotion and lactation support interventions (Morrow et al. 1999; Kramer et al. 2001, 2002, 2007; Albernaz et al. 2003; Bonuck et al. 2005; Chapman et al. 2013). The majority of interventions demonstrated highly significant improvements in the outcomes assessed, which included uptake, duration and exclusivity of breastfeeding (Morrow et al. 1999; Kramer et al. 2001, 2002, 2007; Bhandari et al. 2003; Agrasada et al. 2005; Bonuck et al. 2005; Agrasada & Kylberg 2009), with two studies reporting significant improvements only in some of the outcomes assessed (Albernaz et al. 2003; Chapman et al. 2013). The PROBIT (Kramer et al. 2001, 2002, 2007) trial reported that infants in the intervention sites weighed significantly more at 1 month of age but this difference was not significant by the age of 12 months.
Parenting and lifestyle interventions
Four trials were identified that delivered broad parenting and health interventions, with infant feeding components, via home visiting. These interventions had a significant impact on feeding behaviours but overall, this type of intervention reported fewer improvements than those focusing exclusively on diet feeding. Johnson et al. (1993) provided peer mentoring for first‐time mothers via home visiting. Cow's milk was introduced significantly later to infants in the intervention group and they consumed significantly fewer inappropriate foods. The Miller Early Childhood Sustained Home‐Visiting (MECSH) intervention also provided sustained and structured nurse home‐visiting to improve parenting and family health (Kemp et al. 2011). Intervention infants were breastfed for significantly longer duration than controls. The MOMENTS trial (Cupples et al. 2011) delivered a home‐visit intervention which aimed to reduce health inequalities for women living in socio‐economically deprived communities of Northern Ireland. The intervention did not have an impact on breastfeeding or weight outcomes. PROKIND (Jungmann et al. 2010) found no effect of a home‐visiting intervention to improve maternal mental health and child health on infant weight at 12 months of age.
Maternal health interventions
Three trials were identified where an intervention was delivered to a woman either antenatally or post‐natally with outcomes that potentially had an impact on the infant. However, none of these interventions led to significant improvements in the infant's weight in the desired direction. Dewey et al. (1994) delivered an aerobic exercise intervention to breastfeeding women which had no impact on the volume or content of breast milk or on infant weight at 12 weeks. The INFAT trial (Hauner et al. 2012) found that the intervention resulted in prolonged gestation and that infants in the intervention group had higher birthweight (P = 0.019), weight‐for‐length (P < 0.01) and BMI (P < 0.01) than infants in the control group. However, no differences in body weight were found at 6 weeks of age. Laitinen et al. (2009) evaluated an intervention starting in the first trimester of pregnancy which focused on counselling around a balanced healthy diet containing plant stanol ester products (e.g. soft margarine). There was no effect on infant weight at birth or over the first 12 months of life.
Formula milk interventions (non‐behavioural)
Three studies (one trial; Koletzko et al. 2009; Socha et al. 2011; Escribano et al. 2012) investigated the effects of providing infants follow‐on cow's milk formula with lower or higher protein contents on weight gain. Weight gain velocity was significantly greater in the infants fed higher protein milk from birth to 6 months. Group differences were greatest at 12 months of age; infants fed with lower protein content formula had a significantly lower mean weight‐for‐age z‐score, mean weight‐for‐length z‐score and mean BMI z‐score than infants who received higher protein content formula. At 24 months of age, infants who received formula with lower protein content had a weight‐for‐length z‐score 0.2 lower (95% CI 0.06 to 0.34) than infants who received formula with higher protein content.
Two further trials investigated the impact of infant formula containing hydrolysed protein on growth (Rzehak et al. 2009; Mennella et al. 2011). Both found infants grew more slowly when fed hydrolysed protein formula. Infants who received the extensively hydrolysed casein formula in the GINI trial had significantly slower sex‐adjusted BMI gains from 8 to 48 weeks of age, but not beyond (Rzehak et al. 2009). Mennella reported that infants fed with hydrolysate formula had significantly lower weight‐for‐age z scores from 3.5 to 7.5 months and significantly lower weight‐for‐length z scores from 2.5 to 7.5 months. The weight‐gain velocity of infants fed with hydrolysed protein formula conformed to the World Health Organization (WHO) norms, whereas the weight gain velocity of infants consuming the cow's milk formula exceeded the WHO growth norms over the study period. This study was predominantly non‐behavioural but some behavioural aspects were reported. In particular, infants fed with protein hydrolysate formula were satiated with less formula that those fed cow's milk (Mennella et al. 2011).
Quality assessment
Quality assessment was undertaken by two reviewers (SR and BE). Each reviewer initially scored the papers separately and then they met to agree the ratings. Where there was disagreement, the two reviewers re‐read the paper and justified their rating to each other. The final ratings were agreed through further discussion. Full details about the quality assessment ratings can be found in Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12184/suppinfo.
Unsurprisingly, non‐behavioural interventions scored higher on the Jadad scale than behavioural studies; but clearly, it is not possible to conduct double‐blind behavioural studies. Thirteen trials were considered to have used an appropriate method of randomisation for the allocation of participants to experimental groups. Of the remaining trials, the method of randomisation was not described in enough detail in the study protocol or associated research articles to determine whether the method of randomisation was appropriate. Five trials were considered to have used an appropriate method of blinding and, where appropriate, had evaluated the success of blinding. All of these trials were of non‐behavioural interventions. Behavioural interventions trials usually did not report if the blinding process had been evaluated, nor if it had been successful. Thirteen trials reported participants flow adequately. The remaining studies failed to provide adequate descriptions for study attrition. Quality problems were inconsistent between papers and the majority of trials had some limitations.
Internal and external validity of 24 behavioural intervention trials was assessed using the EBBM criteria. Descriptions of the training received by intervention providers in the delivery of the intervention varied considerably in the trials reviewed. Five trials (Kramer et al. 2001, 2002, 2007; Albernaz et al. 2003; Jungmann et al. 2010; French et al. 2012; Campbell et al. 2013) reported that the intervention was delivered by health care professionals (HCPs) (e.g. nurses, dieticians, consultants) who received additional training in intervention delivery. Two trials (Watt et al. 2009; Scheiwe et al. 2010b; Cupples et al. 2011) stated that the intervention was led by peer supporters or other voluntary workers after receiving training in the intervention. The intervention was delivered by HCPs without further training in four trials (Lapinleimu et al. 1994, 1995; Wen et al. 2011, 2012; Daniels et al. 2012, 2013; Jonsdottir et al. 2012). Two trials reported intervention delivery by trained personnel, but offered no details of their training or professional status (Kavanagh et al. 2008; Hauner et al. 2012). Three trials did not state if treatment providers had been trained (Dewey et al. 1994; Laitinen et al. 2009; Paul et al. 2011).
Two trials (Albernaz et al. 2003; Cupples et al. 2011) reported on the level of supervision received throughout the study period by staff delivering the interventions. Intervention provider or participant preference for allocation to the intervention or control group was not specified explicitly in any of the trials reviewed. Dewey et al. (1994), however, reported the number of women who declined participation to avoid being assigned to the control group.
Adherence (i.e. whether or not the intervention was received by participants) was reported in 11 trials (Dewey et al. 1994; Lapinleimu et al. 1994, 1995; Kramer et al. 2001, 2002, 2007; Albernaz et al. 2003; Watt et al. 2009; Jungmann et al. 2010; Scheiwe et al. 2010b; Cupples et al. 2011; Paul et al. 2011; Wen et al. 2011; Daniels et al. 2012, 2013; Campbell et al. 2013). Adherence was typically monitored by recording the number of classes attended or home visits received. Five trials (Kavanagh et al. 2008; Watt et al. 2009; Scheiwe et al. 2010b; Cupples et al. 2011; Daniels et al. 2012, 2013; Campbell et al. 2013) indicated that attempts had been made to monitor whether the intervention had been delivered as planned. The majority of behavioural interventions lacked theoretical underpinnings and where theory was used this tended to focus on individual behaviour change rather than change at the environmental or system level. One trial used social learning theory (Black et al. 2001); one used social support (Watt et al. 2009; Scheiwe et al. 2010a); one used the health belief model (Wen et al. 2011, 2012); one used Kolb's experiential learning cycle (Kavanagh et al. 2008) and one used an information‐processing, elaboration‐likelihood, precaution‐adoption process model (Verbestel et al. 2013).
Discussion
Main findings
This review identified interventions designed to reduce the risk of overweight and obesity from birth to 7 years of age which included those that commenced antenatally and/or during the first 2 years of life. A wide range of heterogeneous interventions were identified, the majority of which were multi‐component. Behavioural (n = 24) and non‐behavioural (n = 4) interventions were tested in 27 trials and reported in 35 articles dating from 1994 to 2013. Twenty of these articles were published since Hesketh and Campbell's review in 2010 (Hesketh & Campbell 2010).
The finding that specific interventions that promote breastfeeding and lactation support were effective in improving the uptake and duration of breastfeeding has been previously established. There is strong evidence from systematic reviews that any antenatal breastfeeding education (peer counselling, lactation counselling and formal breastfeeding education) can increase uptake of breastfeeding and duration (Morrow et al. 1999; Lumbiganon et al. 2011). Epidemiological evidence suggests that any breastfeeding may be protective against the risk of childhood obesity (Weng et al. 2012) but none of the breastfeeding trials identified in this review reported a positive effect of the intervention on infant weight in the first 2 years of life. Nevertheless, breastfeeding‐support interventions should be available to all women, particularly those whose infants may be at greater risk, e.g. those mothers who are overweight/obese.
The majority of multi‐component interventions showed positive effects in relation to an aspect of infant diet or responsive feeding behaviours (Black et al. 2001; Paul et al. 2011; Wen et al. 2011; French et al. 2012; Campbell et al. 2013; Daniels et al. 2013). However, the NOURISH (Daniels et al. 2012, 2013) and Healthy Beginnings trials (Wen et al. 2011, 2012) appear to be more effective than the Melbourne Infant Trial (Campbell et al. 2013) and other trials that focused on diet and dietary practices (Lapinleimu et al. 1994, 1995; Watt et al. 2009; Scheiwe et al. 2010a; French et al. 2012; Jonsdottir et al. 2012; Verbestel et al. 2013). It is possible that the inclusion of components around parent interaction and responsiveness and adherence to behavioural change theory enhanced the positive impact for these trials. Future intervention developers may wish to place more emphasis on these aspects particularly because the experimental psychology literature reports strong associations between maternal responsiveness and control over infant feeding in the development of childhood obesity (Farrow & Blissett 2006). Interventions specifically designed to build maternal self‐efficacy around infant feeding, which combine behavioural components, such as responsive feeding and soothing strategies with specific dietary advice and anticipatory guidance around feeding behaviours, may lead to more sustainable changes. Further work is needed around the theory of behavioural change that might underpin the development of educational interventions for parents in this area.
Four trials reported that the intervention had a significant impact on weight outcomes in the desired direction (Paul et al. 2011; Daniels et al. 2012; Wen et al. 2012; Verbestel et al. 2013) but this effect was not present at later follow‐up for one trial (Daniels et al. 2013). These small intervention effects may be due to sampling strategy rather than sample size. All the trials randomised groups of parents or prospective parents, whose infants may or may not have been at risk of developing childhood obesity, to preventative interventions. The consequence of this is that intervention effects may not translate to demonstrable changes in anthropometry in samples where many infants have a low risk of obesity.
Three non‐behavioural interventions were identified which tested different types of formula milk. One trial reported that infants given higher protein cow's milk formula feed grew more rapidly, when compared with infants fed lower protein formula (Koletzko et al. 2009; Socha et al. 2011; Escribano et al. 2012). The authors have subsequently published a further paper which, although outside the remit of this review, demonstrates that infants fed with lower protein formula maintain their lower BMIs at school age (Weber et al. 2014). Although the formulas used in this trial were all within the normal prescribed formulation range, the higher protein formula milk was at the upper end of an infant's requirements. Two further trials reported slower infant weight gain in infants fed hydrolysed formula (Rzehak et al. 2009; Mennella et al. 2011). One trial reported infants had better satiation with less formula when fed hydrolysed protein (Mennella et al. 2011).
All of the non‐behavioural trials were all high quality and blinded both researchers and participants (parents) to intervention group. However, the issue of ‘blinding’ parents to different types of infant formula, where there is evidence that consumption could lead to higher or lower risk of obesity, is ethically questionable. Parents, as health care consumers, have a right to information about risk, however small, to enable them to make an informed choice about their infant's diet (Have et al. 2013).
Clinical implications
There have been a number of calls for early prevention of childhood overweight and obesity (Institute of Medicine 2011; Laws et al. 2014). The findings of this review could potentially support the development of an early childhood obesity prevention strategy. The review identified a number of interventions some of which contained successful components. These components could form the basis of a multifaceted approach to obesity risk which has the potential to be individually tailored by the health professional and parent.
The findings of the non‐behavioural trials suggest that there is merit in further exploring the option of recommending lower protein formula milk. However, the formula milks used for these trials are not the standard milks that are available for parents to purchase over the counter. Of those that are available to parents, ‘follow‐on’ formula milks tend to have higher protein content than first‐stage milks. Raising parents' awareness of the different constituents of formula milk and advising them to refrain from upgrading their infants onto follow‐on or toddler milks may be prudent interventions in terms of reducing obesity risk. Although the evidence suggests that recommending hydrolysed protein may be beneficial for infants at risk of rapid weight gain, caution is needed. The authors of this review suggest that using formula derived from hydrolysed protein in the absence of medical need may have adverse implications, i.e. allergies. Furthermore, hydrolysed protein formula has a different taste from normal formula and unless there is a medical need, parents may not wish to expose their infant to this.
Focusing support for obesity prevention on vulnerable families at greatest risk is another option. However, health professionals may find this difficult as they may perceive it to be stigmatising and fear it may jeopardise their relationship with parents (Redsell et al. 2011, 2013c). A debate is needed about the feasibility and acceptability of targeted vs. generic parental behaviour change interventions. Several tools are available that can identify infant obesity risk (Santorelli et al. 2013; Weng et al.; Weng et al. 2013), although these require feasibility testing prior to implementation in clinical practice. The team has developed the Infant Risk of Obesity Checklist (IROC) (Weng et al. 2013a) which, together with the findings from this review, has been incorporated into clinical guidelines for Public Health Nurses (UK health visitors) (Redsell et al. 2013a, 2013b). The IROC tool and intervention strategies have been developed into an interactive, education programme for UK health visitors to use to facilitate discussions about obesity prevention with parents during routine home visits. The Proactive Assessment of Obesity Risk during Infancy (ProAsk) programme is delivered via digital technology and is currently being subjected to a feasibility trial, the results of which will be delivered in late 2016. Funding for this has been awarded by the UK Medical Research Council Public Health Intervention Development Scheme (MRC‐PHIND PH01/14‐15).
Limitations
The majority of behavioural interventions were not underpinned by a theory of change and where a theory was applied, this tended to be social‐cognitive in nature targeting the individual and/or family rather than the environment or health system. This approach is appropriate where individuals might be at higher risk but this was not the case for the majority of studies which recruited general samples. Trial quality (Jadad et al. 1996) was an issue for the behavioural interventions and whilst lack of blinding is inevitable for this type of intervention, it was disappointing that many studies failed to adequately describe their randomisation method or attrition rates. Davidson et al. described five categories (training, supervision, preference, adherence and integrity) for assessing internal and external validity of trials of behavioural interventions (Davidson et al. 2003). Furthermore, although the interventions have been delivered by both trained volunteers and health practitioners, the majority of studies lacked sufficient detail to make valid conclusions about which might be most effective (Davidson et al. 2003). Reporting of participant adherence and fidelity of intervention delivery was variable and problems in these areas might explain the small effect sizes. Although not part of the EBBM criteria, this review also examined the application of behaviour change theory in the trials identified; for most interventions, this was underexplored and/or underreported. Intervention development for behavioural change in this area could benefit from greater attention to theory, supported by a robust trial design that includes the use of a checklist to ensure internal and external validity.
Two high‐quality, systematic reviews of obesity prevention interventions delivered during early years have been published (Hesketh & Campbell 2010; Laws et al. 2014), one fairly recently (Laws et al. 2014). Both reviews broadly concluded that further research in this area is urgently needed. Although our review can only report the findings of completed intervention studies, there were a number of protocols of interest, e.g. Brambilla et al. (2010). Thirty‐two ongoing RCTs that appear to have fitted the inclusion criteria were identified via the clinical trials registers (see Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12184/suppinfo). The findings of these studies may better inform strategies to prevent childhood obesity during infancy.
Conclusion
This systematic review was broad in range in order to identify any important interventions which have the potential to prevent childhood obesity. Interventions that aim to improve parental feeding practices, including infant diet and parental responsiveness to infant cues, showed most promise in relation to behaviour change but not weight. The option of advising some families to offer lower protein formula milk is worthy of further exploration if imbedded into a multi‐component intervention together with behavioural change components. Despite the known risk factors for child obesity, there were very few intervention studies for pregnant women that continued during infancy. Further research by the team will explore the feasibility of intervention to prevent obesity during infancy.
Source of funding
This work was funded by a grant from the Burdett Trust for Nursing.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
SR (Reviewer 2) led the study, drafted the original protocol, screened 1206 abstracts, read the full‐text papers, checked the Jadad and EBBM scores, identified the ongoing trials and drafted the paper. BE (Reviewer 1) refined the original protocol, undertook the searches, screened 1206 abstracts, read the full‐text papers, undertook data entry, checked the Jadad and EBBM scores, drafted the Supporting Information tables and drafted the results. SW advised the team about review procedures and helped with data presentation. JAS, CG, DN and ANS refined the protocol, screened abstracts where there was disagreement between the two reviewers, read the full‐text papers and co‐wrote the paper.
Supporting information
Table S1. Data extraction sheet.
Table S2. Main findings in relation to weight and feeding outcomes.
Table S3. Quality assessment and process evaluation.
Table S4. Ongoing registered trials.
Acknowledgements
We would like to thank Pippa Atkinson and Vicki Watson (Nottingham City Care Partnership) for their contributions.
Redsell, S. A. , Edmonds, B. , Swift, J. A. , Siriwardena, A. N. , Weng, S. , Nathan, D. , and Glazebrook, C. (2016) Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern Child Nutr, 12: 24–38. doi: 10.1111/mcn.12184.
References
- Agrasada G.V. & Kylberg E. (2009) When and why Filipino mothers of term low birth weight infants interrupted breastfeeding exclusively. Breastfeeding Review: Professional Publication of the Nursing Mothers' Association of Australia 17(3), 5–10. [PubMed] [Google Scholar]
- Agrasada G.V., Gustafsson J., Kylberg E. & Ewald U. (2005) Postnatal peer counselling on exclusive breastfeeding of low‐birthweight infants: a randomized, controlled trial. Acta Paediatrica 94(8), 1109–1115. [DOI] [PubMed] [Google Scholar]
- Albernaz E., Victora C.G., Haisma H., Wright A. & Coward W.A. (2003) Lactation counseling increases breast‐feeding duration but not breast milk intake as measured by isotopic methods. The Journal of Nutrition 133, 205–210. [DOI] [PubMed] [Google Scholar]
- Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H. & Law C. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ (Clinical Research Ed.) 331, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari N., Bahl R., Mazumdar S., Martines J., Black R.E. & Bhan M.K. (2003) Effect of community‐based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 361, 1418–1423. [DOI] [PubMed] [Google Scholar]
- Black M.M., Siegel E.H., Abel Y. & Bentley M.E. (2001) Home and videotape intervention delays early complementary feeding among adolescent mothers. Pediatrics 107, E67. [DOI] [PubMed] [Google Scholar]
- Bonuck K.A., Trombley M., Freeman K. & McKee D. (2005) Randomized, controlled trial of a prenatal and postnatal lactation consultant intervention on duration and intensity of breastfeeding up to 12 months. Pediatrics 116, 1413–1426. [DOI] [PubMed] [Google Scholar]
- Brambilla P., Bedogni G., Buongiovanni C., Brusoni G., Di Mauro G., Di Pietro M. et al (2010) ‘Mi voglio bene’: a pediatrician‐based randomized controlled trial for the prevention of obesity in Italian preschool children. Italian Journal of Pediatrics 36, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy S., Cooksey R., Gravenor M.B., Mistry R., Thomas N., Lyons R.A. et al (2009) Risk factors for childhood obesity at age 5: analysis of the millennium cohort study. BMC Public Health 9, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K., Lioret S., McNaughton S.A., Crawford D.A., Salmon J., Ball K. et al (2013) A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics 131, 652–660. [DOI] [PubMed] [Google Scholar]
- Chapman D.J., Morel K., Bermudez‐Millan A., Young S., Damio G. & Perez‐Escamilla R. (2013) Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics 131, e162–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples M.E., Stewart M.C., Percy A., Hepper P., Murphy C. & Halliday H.L. (2011) A RCT of peer‐mentoring for first‐time mothers in socially disadvantaged areas (the MOMENTS Study). Archives of Disease in Childhood 96, 252–258. [DOI] [PubMed] [Google Scholar]
- Daniels L., Mallan K.M., Battistutta D., Nicholson J.M., Perry R. & Magarey A. (2012) Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. International Journal of Obesity 36, 1292–1298. [DOI] [PubMed] [Google Scholar]
- Daniels L., Mallan K.M., Nicholson J.M., Battistutta D. & Magarey A. (2013) Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics 132, e109–e118. [DOI] [PubMed] [Google Scholar]
- Darzi A.D. (2008) High Quality Care for All: NHS Next Stage Review Final Report – Summary. Available at: http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_085825
- Dattilo A.M., Birch L., Krebs N.F., Lake A., Taveras E.M. & Saavedra J.M. (2012) Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. Journal of Obesity 2012, 123023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K.W., Goldstein M., Kaplan R.M., Kaufmann P.G., Knatterud G.L., Orleans C.T. et al (2003) Evidence‐based behavioral medicine: what is it and how do we achieve it? Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine 26, 161–171. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Lovelady C.A., Nommsen‐Rivers L.A., McCrory M.A. & Lonnerdal B. (1994) A randomized study of the effects of aerobic exercise by lactating women on breast‐milk volume and composition. The New England Journal of Medicine 330, 449–453. [DOI] [PubMed] [Google Scholar]
- Dietz W.H. (1998) Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 101, 518–525. [PubMed] [Google Scholar]
- DiSantis K.I., Hodges E.A., Johnson S.L. & Fisher J.O. (2011) The role of responsive feeding in overweight during infancy and toddlerhood: a systematic review. International Journal of Obesity 35, 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U., Ong K., Linne Y., Neovius M., Brage S., Dunger D.B. et al (2006) Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). The American Journal of Clinical Nutrition 83, 324–330. [DOI] [PubMed] [Google Scholar]
- Escribano J., Luque V., Ferre N., Mendez‐Riera G., Koletzko B., Grote V. et al (2012) Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. International Journal of Obesity (2005) 36, 548–553. [DOI] [PubMed] [Google Scholar]
- Farrow C. & Blissett J. (2006) Does maternal control during feeding moderate early infant weight gain? Pediatrics 118, e293–e298. [DOI] [PubMed] [Google Scholar]
- Fewtrell M.S., Kennedy K., Nicholl R., Khakoo A. & Lucas A. (2012) Infant feeding bottle design, growth and behaviour: results from a randomised trial. BMC Research Notes 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G.M., Nicholson L., Skybo T., Klein E.G., Schwirian P.M., Murray‐Johnson L. et al (2012) An evaluation of mother‐centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics 130, e507–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Hosking J., Metcalf B.S., Jeffery A.N., Voss L.D. & Wilkin T.J. (2009) Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36). Pediatrics 123, e67–e73. [DOI] [PubMed] [Google Scholar]
- Hauner H., Much D., Vollhardt C., Brunner S., Schmid D., Sedlmeier E.‐M. et al (2012) Effect of reducing the n‐6:n‐3 long‐chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open‐label randomized controlled trial. The American Journal of Clinical Nutrition 95, 383–394. [DOI] [PubMed] [Google Scholar]
- Have M.T., van der Heide A., Mackenbach J.P. & De Beaufort I.D. (2013) An ethical framework for the prevention of overweight and obesity: a tool for thinking through a programme's ethical aspects. European Journal of Public Health 23, 299–305. [DOI] [PubMed] [Google Scholar]
- Hawkins S., Cole T.J., Law C. & Millennium Cohort Study Child Health Group (2009) An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. Journal of Epidemiology & Community Health 63, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Social Care Information Centre (2013) National Child Measurement Programme: England, 2012/2013. Available at: http://www.hscic.gov.uk/catalogue/PUB13115/nati-chil-meas-prog-eng-2012-2013-rep.pdf
- Hesketh K. & Campbell K. (2010) Interventions to prevent obesity in 0–5 year olds: an updated systematic review of the literature. Obesity (Silver Spring) 18, S27–S35. [DOI] [PubMed] [Google Scholar]
- Huh S., Rifas‐Shiman S.L., Taveras E.M., Oken E. & Gillman M.W. (2011) Timing of solid food introduction and risk of obesity in preschool‐aged children. Pediatrics 127, E544–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L., Schooling C.M., Leung S.S., Mak K.H., Ho L.M., Lam T.H. et al (2008) Birth weight, infant growth, and childhood body mass index: Hong Kong's children of 1997 birth cohort. Archives of Pediatrics & Adolescent Medicine 162, 212–218. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2011) Early Childhood Obesity Prevention Policies. Institute of Medicine (IoM): Washington, DC: Available at: http://www.iom.edu/~/media/Files/Report%20Files/2011/Early-Childhood-Obesity-Prevention-Policies/Young%20Child%20Obesity%202011%20Recommendations.pdf [Online]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A., Moore A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J. et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 17, 1–12. [DOI] [PubMed] [Google Scholar]
- Johnson Z., Howell F. & Molloy B. (1993) Community mothers' programme: randomised controlled trial of non‐professional intervention in parenting. BMJ (Clinical Research Ed.) 306, 1449–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir O.H., Thorsdottir I., Hibberd P.L., Fewtrell M.S., Wells J.C., Palsson G.I. et al (2012) Timing of the introduction of complementary foods in infancy: a randomized controlled trial. Pediatrics 130, 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann T., Kurtz V., Brand T., Sierau S. & von Klitzing K. (2010) [Child health as a prevention objective in the context of the pilot project ‘Pro Kind’. Preliminary results of a randomized controlled trial]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 53, 1180–1187. [DOI] [PubMed] [Google Scholar]
- Kavanagh K.F., Cohen R.J., Heinig M.J. & Dewey K.G. (2008) Educational intervention to modify bottle‐feeding behaviors among formula‐feeding mothers in the WIC program: impact on infant formula intake and weight gain. Journal of Nutrition Education and Behavior 40(4), 244–250. [DOI] [PubMed] [Google Scholar]
- Kemp L., Harris E., McMahon C., Matthey S., Vimpani G., Anderson T. et al (2011) Child and family outcomes of a long‐term nurse home visitation programme: a randomised controlled trial. Archives of Disease in Childhood 96, 533–540. [DOI] [PubMed] [Google Scholar]
- Koletzko B., von Kries R., Closa R., Escribano J., Scaglioni S., Giovannini M. et al (2009) Lower protein in infant formula is associated with lower weight up to age 2 years: a randomized clinical trial. The American Journal of Clinical Nutrition 89, 1836–1845. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Chalmers B., Hodnett E.D., Sevkovskaya Z., Dzikovich I., Shapiro S. et al (2001) Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA: The Journal of the American Medical Association 285, 413–420. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Guo T., Platt R.W., Shapiro S., Collet J., Chalmers B. et al (2002) Breastfeeding and infant growth: biology or bias? Pediatrics 110, 343–347. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Matush L., Vanilovich I., Platt R.W., Bogdanovich N., Sevkovskaya Z. et al (2007) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 years: evidence from a large randomized trial. The American Journal of Clinical Nutrition 86(6), 1717–1721. [DOI] [PubMed] [Google Scholar]
- Laitinen K., Isolauri E., Kaipiainen L., Gylling H. & Miettinen T.A. (2009) Plant stanol ester spreads as components of a balanced diet for pregnant and breast‐feeding women: evaluation of clinical safety. The British Journal of Nutrition 101(12), 1797–1804. [DOI] [PubMed] [Google Scholar]
- Lapinleimu H., Jokinen E., Myyrinmaa A., Viikari J., Rönnemaa T., Välimäki I. et al (1994) Individualized dietary counselling of families: serum cholesterol concentration and growth of children aged 7–13 months. Acta Paediatrica 83(4), 383–388. [DOI] [PubMed] [Google Scholar]
- Lapinleimu H., Viikari J., Jokinen E., Salo P., Routi T., Leino A. et al (1995) Prospective randomised trial in 1062 infants of diet low in saturated fat and cholesterol. Lancet 345(8948), 471–476. [DOI] [PubMed] [Google Scholar]
- Laws R., Campbell K.J., van der Pligt P., Russell G., Ball K., Lynch J. et al (2014) The impact of interventions to prevent obesity or improve obesity related behaviours in children (0–5 years) from socioeconomically disadvantaged and/or indigenous families: a systematic review. BMC Public Health 14, 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbiganon P., Martis R., Laopaiboon M., Festin M.R., Ho J.J. & Hakimi M. (2011) Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database of Systematic Reviews 12(9), CD006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J.A., Ventura A.K. & Beauchamp G.K. (2011) Differential growth patterns among healthy infants fed protein hydrolysate or cow‐milk formulas. Pediatrics 127, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P. & Victora C.G. (2005) Rapid growth in infancy and childhood and obesity in later life – a systematic review. Obesity Reviews 6, 143–154. [DOI] [PubMed] [Google Scholar]
- Morrow A.L., Guerrero M.L., Shults J., Calva J.J., Lutter C., Bravo J. et al (1999) Efficacy of home‐based peer counselling to promote exclusive breastfeeding: a randomised controlled trial. The Lancet 353, 1226–1231. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2006) Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. Clinical Guideline 43 [Online]. Available at: http://www.nice.org.uk/guidance/CG43 [PubMed]
- Ong K. & Loos R. (2006) Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatrica 95, 904–908. [DOI] [PubMed] [Google Scholar]
- Ong K.K., Emmett P.M., Noble S., Ness A., Dunger D.B. & Team A.S. (2006) Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 117, e503–e508. [DOI] [PubMed] [Google Scholar]
- Paul I.M., Savage J.S., Anzman S.L., Beiler J.S., Marini M.E., Stokes J.L. et al (2011) Preventing obesity during infancy: a pilot study. Obesity (Silver Spring) 19, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redsell S.A., Atkinson P., Nathan D., Siriwardena A.N., Swift J. & Glazebrook C. (2011) Preventing childhood obesity during infancy in UK primary care: a mixed‐methods study of HCPs' knowledge, beliefs and practice. BMC Family Practice 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redsell S., Edmonds B., Nathan D., Siriwardena A.N., Swift J., Weng S.F. et al (2013a) Guideline for UK Midwives/Health Visitors to Use with Parents of Infants at Risk of Developing Childhood Overweight/Obesity. Edited by Institute of Health Visiting. Available at: http://WWW.IHV.ORG.UK/NEWS_EVENTS/NEWS/66/GUIDELINE%2520FOR%2520OBESITY%2520PREVENTION%2520DURING%2520INFANCY
- Redsell S., Edmonds B.E., Glazebrook C., Swift J., Nathan D., Siriwardena A.N. et al (2013b) Development of an evidence‐based practice guideline for UK public health nurses (health visitors) to use with parents of infants at risk of obesity. European Childhood Obesity Conference, Liverpool, UK. Appetite, 204.
- Redsell S., Swift J.A., Nathan D., Siriwardena A.N., Atkinson P. & Glazebrook C. (2013c) UK health visitors' role in identifying and intervening with infants at risk of developing obesity. Maternal and Child Nutrition 9, 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzehak P., Sausenthaler S., Koletzko S., Reinhardt D., von Berg A., Kramer U. et al (2009) Short‐ and long‐term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 years of age: results from the German Infant Nutritional Intervention Study. The American Journal of Clinical Nutrition 89, 1846–1856. [DOI] [PubMed] [Google Scholar]
- Santorelli G., Petherick E.S., Wright J., Wilson B., Samiei H., Cameron N. et al (2013) Developing prediction equations and a mobile phone application to identify infants at risk of obesity. PLoS ONE 8, e71183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiwe A., Hardy R. & Watt R.G. (2010a) Four‐year follow‐up of a randomized controlled trial of a social support intervention on infant feeding practices. Maternal & Child Nutrition 6, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiwe A., Hardy R. & Watt R.G. (2010b) Four‐year follow‐up of a randomized controlled trial of a social support intervention on infant feeding practices. Maternal and Child Nutrition 6, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S., Gildea A., Stewart M., Sneddon H. & Iwaniec D. (2008) Early weaning is related to weight and rate of weight gain in infancy. Child: Care, Health and Development 34, 59–64. [DOI] [PubMed] [Google Scholar]
- Socha P., Grote V., Gruszfeld D., Janas R., Demmelmair H., Closa‐Monasterolo R. et al (2011) Milk protein intake, the metabolic‐endocrine response, and growth in infancy: data from a randomized clinical trial. The American Journal of Clinical Nutrition 94, 1776S–1784S. [DOI] [PubMed] [Google Scholar]
- Taveras E.M., Rifas‐Shiman S.L., Oken E., Gunderson E.P., Gillman M.W., Taveras E.M. et al (2008) Short sleep duration in infancy and risk of childhood overweight. Archives of Pediatrics & Adolescent Medicine 162, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbestel V., De Coen V., Van Winckel M., Huybrechts I., Maes L. & De Bourdeaudhuij I. (2013) Prevention of overweight in children younger than 2 years old: a pilot cluster‐randomized controlled trial. Public Health Nutrition 17(6), 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E., de Silva‐Sanigorski A., Hall B.J., Brown T., Campbell K.J., Gao Y. et al (2011) Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews 7(12), CD001871. [DOI] [PubMed] [Google Scholar]
- Watt R.G., Tull K.I., Hardy R., Wiggins M., Kelly Y., Molloy B. et al (2009) Effectiveness of a social support intervention on infant feeding practices: randomised controlled trial. Journal of Epidemiology and Community Health 63, 156–162. [DOI] [PubMed] [Google Scholar]
- Weber M., Grote V., Closa‐Monasterolo R., Escribano J., Langhendries J.P., Dain E. et al (2014) Lower protein content in infant formula reduces BMI and obesity risk at school age: follow‐up of a randomized trial. The American Journal of Clinical Nutrition 99, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Wen L.M., Baur L.A., Simpson J.M., Rissel C. & Flood V.M. (2011) Effectiveness of an early intervention on infant feeding practices and ‘tummy time’: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 165, 701–707. [DOI] [PubMed] [Google Scholar]
- Wen L.M., Baur L.A., Simpson J.M., Rissel C., Wardle K. & Flood V.M. (2012) Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ (Clinical Research Ed.) 344, e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S., Redsell S.A., Nathan D., Swift J.A., Qureshi N. & Glazebrook C. External validation of Infant Risk of Obesity Checklist (IROC) tool using the Avon Longitudinal Study of Parents and Children (ALSPAC). In preparation.
- Weng S., Redsell S.A., Nathan D., Swift J.A., Yang M. & Glazebrook C. (2013) Developing an algorithm to estimate overweight risk in childhood from predictors during infancy. Pediatrics 132, e414–e421. [DOI] [PubMed] [Google Scholar]
- Weng S.F., Redsell S.A., Swift J.A., Yang M. & Glazebrook C.P. (2012) Systematic review and meta‐analyses of risk factors for childhood overweight identifiable during infancy. Archives of Disease in Childhood 97, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyermann M., Rothenbacher D. & Brenner H. (2006) Duration of breastfeeding and risk of overweight in childhood: a prospective birth cohort study from Germany. International Journal of Obesity (2005) 30, 1281–1287. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2013) Obesity and Overweight. Factsheet No. 311 [Online]. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data extraction sheet.
Table S2. Main findings in relation to weight and feeding outcomes.
Table S3. Quality assessment and process evaluation.
Table S4. Ongoing registered trials.


