Abstract
Pachydermoperiostosis is a rare hereditary disease, which presents with the cutaneous manifestations of pachydermia and cutis verticis gyrata. Histological findings in pachydermia frequently include dermal edema, mucin deposition, elastic fiber degeneration, dermal fibrosis and adnexal hyperplasia. However, the severity of these findings varies between clinical reports, and a systematic multiple‐case clinicopathological correlative analysis has not been performed to date. In the present study, we reviewed the skin biopsy specimens obtained from the pachydermia of six pachydermoperiostosis patients. The severity of the characteristic histological features was semiquantitatively evaluated and correlated with the grade of pachydermia. Dermal edema, mucin deposition and elastic fiber degeneration were observed in all cases. Patients with severe pachydermia had sebaceous gland hyperplasia and fibrosis. These results suggest that the triad of mucin deposition, dermal edema and elastic fiber degeneration are found from very early stage pachydermia, and could be considered diagnostic findings. To ensure an earlier diagnosis of pachydermoperiostosis, a biopsy should be taken when a patient has grade 1 pachydermia to determine the presence of this histological triad.
Keywords: dermal edema, elastic fiber degeneration, fibrosis, mucin deposition, pachydermoperiostosis, sebaceous hyperplasia
Introduction
Pachydermoperiostosis (PDP, Online Mendelian Inheritance in Man no. 614441) is a rare hereditary disease diagnosed by the presence of digital clubbing, periostosis and pachydermia, including cutis verticis gyrata (CVG).1 Recent genetic analysis revealed that homozygous or compound heterozygous mutations in the solute carrier organic anion transporter family member 2A1 (SLCO2A1) gene, which is associated with prostaglandin (PG) metabolism, are significantly associated with PDP.2, 3 Clinically, three distinct forms of this syndrome have been proposed in accordance with the intensity of symptoms: the complete form, characterized by prominent furrowing of the face, CVG, digital clubbing, and primary hypertrophic osteoarthropathy; the incomplete form, in which CVG is absent; and the fruste form, characterized by one or more main skin changes and minimal skeletal involvement.1
Several studies have reported the following histopathological findings of pachydermia: sebaceous gland hyperplasia, dermal edema, mucin deposition in the dermis, elastic fiber loss and dermal fibrosis.4, 5, 6 However, the severity of these findings varies among clinical reports, and a multiple‐case clinicopathological correlative analysis of pachydermia has not been performed to date. To gain insight into the pathogenesis of pachydermia and CVG development, we histologically examined skin biopsy specimens of six PDP patients with known clinical information. We evaluated the degree of each of the histological findings semiquantitatively, and correlated these data with the severity of pachydermia.
Patients and Techniques
This study was approved by the ethics committee of the National Center for Child Health and Development and Keio University School of Medicine, and conformed to the provisions of the Declaration of Helsinki. Six patients who were diagnosed with PDP were included. All samples were collected after obtaining written informed consent. Although these patients' clinical features had been previously reported, none of the histological findings had been evaluated precisely.2, 4, 5, 6, 7 All patients had the complete form of PDP by the end of the study, although two patients had initially been diagnosed with the incomplete form and gradually developed CVG during the observation period (patients 1 and 2). All samples were obtained from the forehead and stained with hematoxylin–eosin, Alcian blue and elastica van Gieson. Normal skin from the forehead of a healthy individual, which was obtained from the area surrounding benign tumors, was used as a control. The slides were independently interpreted by two investigators (K. T. and A. I.) without any knowledge of the clinical data. Any discrepancies in the findings were subsequently reconciled by a third investigator.
The clinical and histological findings in each case are provided in Figures S1 and S2.
Patient 1
This 24‐year‐old male2 initially had no CVG on his scalp and was diagnosed with incomplete PDP. CVG gradually developed, changing the diagnosis to complete PDP.7 The biopsy we studied had been taken at the age of 19 years when he was diagnosed with incomplete PDP, although he already had slight pachydermia on his forehead. Histologically, focal edema and weak but diffuse mucin deposition were observed. Elastic fiber degeneration affected approximately 40% of the entire dermis. However, sebaceous gland hyperplasia and dermal fibrosis were not observed.
Patient 2
This 19‐year‐old male7 initially had no CVG on his scalp and was diagnosed with incomplete PDP. CVG gradually developed, changing the diagnosis to complete PDP. The biopsy we studied was taken when he was diagnosed with complete PDP, and had mild pachydermia on his forehead. Histologically, dermal edema affected approximately 50% of the entire dermis, and diffuse and strong mucin deposition was observed. Elastic fiber degeneration affected approximately 10% of the entire dermis. In the upper dermis, slight fibrosis around the folliculosebaceous unit and mild sebaceous gland hyperplasia were observed.
Patient 3
This 53‐year‐old male4 had CVG on his scalp as well as moderate pachydermia on his forehead. Histologically, focal edema and weak but diffuse mucin deposition were observed. Elastic fiber degeneration affected approximately 10% of the entire dermis. Moderate sebaceous gland hyperplasia and slight fibrosis around the sebaceous glands were also observed.
Patient 4
This 21‐year‐old male7 had CVG on his scalp and moderate pachydermia on his forehead. Histologically, dermal edema affected approximately 50% of the entire dermis, and diffuse and strong mucin deposition was observed. Elastic fiber degeneration affected approximately 10% of the entire dermis. Moderate sebaceous gland hyperplasia and slight fibrosis around the sebaceous glands were also observed.
Patient 5
This 45‐year‐old male5 had severe CVG and pachydermia. Histologically, dermal edema affected the entire dermis, and focal mucin deposition was noted. Elastic fiber degeneration affected the entire dermis. Moderate sebaceous gland hyperplasia was observed, and fibrosis surrounding the folliculosebaceous unit and in parts of the dermis was noted.
Patient 6
This 25‐year‐old male2 had severe CVG and pachydermia. Histologically, focal dermal edema and weak but diffuse mucin deposition were noted. Elastic fiber degeneration affected the entire dermis. Sebaceous gland hyperplasia was prominent, and fibrosis affected the entire dermis.
Results and Discussion
In the present study, we semiquantitatively scored the severity of the pachydermia and characteristic histological findings (dermal edema, mucin deposition, elastic fiber degeneration, sebaceous gland hyperplasia and dermal fibrosis) according to the criteria described in Table 1. Representative pictures of each clinical and histological finding are shown in Figure 1. Histological features of normal skin, which was used as a control, are shown in Figure S3. Thereafter, we correlated the scores with the severity of pachydermia, as summarized in Table 2.
Table 1.
Scoring criteria of the clinical and histological findings used in this study
| Features | Grading | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Clinical features | ||||
| Pachydermia of the forehead | Not furrowed | Not furrowed but shallow ditch between slight swellings is seen | Furrowed but the bottom of furrow is visible | Deeply furrowed so that the bottom of furrow is invisible |
| Histological features | ||||
| Dermal edema | Absent | Limited to part of the upper dermis or lower dermis | Limited in the entire upper dermis or lower dermis | Extended to the entire upper and lower dermis |
| Mucin deposition in the dermis | Absent | Focal deposition | Weak deposition in the entire dermis | Strong deposition in the entire dermis |
| Elastic fiber degeneration | Absent | <20% of entire dermis | 20–50% of entire dermis | >50% of entire dermis |
| Fibrosis | Absent | Restricted around the folliculosebaceous unit | Extended around folliculosebaceous unit and part of the dermis | Extended to entire dermis |
| Sebaceous hyperplasia (sebaceous gland occupation ratea) | <10% | 10–25% | 26–40% | >40% |
For each specimen, the total sebaceous gland area was divided by the area of dermis and the sebaceous gland occupation rate was calculated.
Figure 1.
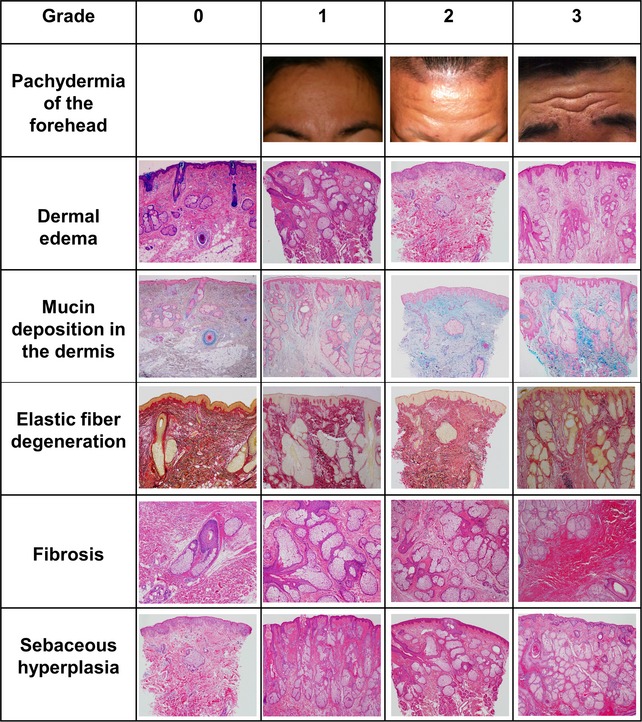
Representative clinical and histological manifestations of pachydermia. Representative pictures of each clinical and histological grading. Pachydermia of the forehead: grade 1 (patient 1), grade 2 (patient 2) and grade 3 (patient 5). Dermal edema (hematoxylin–eosin [HE], whole image): grade 0 (control), grade 1 (patient 3), grade 2 (patient 1) and grade 3 (patient 5). Mucin deposition in the dermis (Alcian blue, whole image): grade 0 (control), grade 1 (patient 5), grade 2 (patient 1) and grade 3 (patient 4). Elastic fiber degeneration (elastica van Gieson, whole image): grade 0 (control), grade 1 (patient 4), grade 2 (patient 1) and grade 3 (patient 5). Fibrosis (HE, original magnification ×20): grade 0 (control), grade 1 (patient 3), grade 2 (patient 5) and grade 3 (patient 6). Sebaceous gland hyperplasia (HE, ×40): grade 0 (patient 1), grade 1 (patient 2), grade 2 (patient 3) and grade 3 (patient 6).
Table 2.
Summary of the clinical and histological findings of pachydermia
| Patient | Current age | Onset age | Sex | SLCO2A1 mutation | Clinical subtype when a biopsy was taken | Pachydermia of the forehead | Histological findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dermal edema | Mucin deposition in the dermis | Elastic fiber degeneration | Fibrosis | Sebaceous hyperplasia | |||||||
| Control | 40 | N/A | M | N/A | N/A | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 24 | 13 | M |
p.R288Gfs*7 p.E427_P430del |
Incomplete | 1 | 2 | 2 | 2 | 0 | 0 (7.50%) |
| 27 | 19 | 15 | M |
p.R288Gfs*7 p.E427_P430del |
Complete | 2 | 2 | 3 | 1 | 1 | 1 (19.64%) |
| 34 | 53 | 20 | M |
p.R288Gfs*7 p.Q556H |
Complete | 2 | 1 | 2 | 1 | 1 | 2 (38.33%) |
| 47 | 21 | 16 | M |
p.R603* p.R252* |
Complete | 2 | 2 | 3 | 1 | 1 | 2 (28.57%) |
| 55 | 45 | 17 | M |
p.R288Gfs*7 p.R288Gfs*7 |
Complete | 3 | 3 | 1 | 3 | 2 | 2 (27.67%) |
| 62 | 25 | 10 | M | Not performed | Complete | 3 | 1 | 2 | 3 | 3 | 3 (71.2%) |
Clinical and histological manifestations shown in Figs S1,S2 are scored according to the grading criteria described in Table 1. A number in each column is a grading score of each manifestation. A percentage in parenthesis in column of sebaceous hyperplasia indicates sebaceous gland occupation rate. M, male; N/A, not available/not applicable.
In the five samples taken from the pachydermia of complete PDP patients (patients 2–6), histological analysis frequently showed fibrosis and sebaceous gland hyperplasia, which tended to become more prominent as the severity of the pachydermia increased. Mucin deposition, dermal edema and elastic fiber degeneration were also noted. In contrast, the sample obtained from the pachydermia of incomplete PDP (patient 1) showed milder histological changes compared with the samples obtained from other patients. Only mucin deposition, focal edema and partial elastic fiber degeneration were observed (Table 2, Figs S1,S2). This sample was obtained from the forehead when slight pachydermia was present, but before CVG became prominent, suggesting that these three findings are the initial histological features of pachydermia. Patients 1 and 2 included in this study had the same SLCO2A1 genotype, and had similar clinical courses with the gradual development of CVG. The biopsy samples of these two patients were taken at different points in the clinical course: one was taken before CVG had developed (patient 1); the other was taken after CVG had developed (patient 2). The sample obtained from patient 2 had mild fibrosis and sebaceous gland hyperplasia in addition to the triad of mucin deposition, dermal edema and elastic fiber degeneration (Table 2, Figs S1,S2). The difference between these two samples implies that fibrosis and sebaceous gland hyperplasia will appear later than the other three findings, in accordance with the development of pachydermia and CVG. Correlation between the severity of pachydermia and SLCO2A1 mutational status needs to be confirmed with a greater number of cases. However, the homozygous or compound heterozygous mutations underlie loss of function or would be degraded by nonsense‐mediated mRNA decay. Such a genetic background may give some insights into severity of the disease as shown in our patients 4 and 5.
We speculate that the incomplete and complete clinical subtypes occur sequentially in the same PDP patient. Pachydermia development is already initiated even in the early incomplete form. The histological triad of mucin deposition, dermal edema and elastic fiber degeneration are found from very early stage pachydermia, and may reflect the pathogenesis of PDP. To ensure an earlier diagnosis of PDP, a biopsy should be taken when a patient has grade 1 pachydermia, and has clubbing and periostosis, to determine the presence of this histological triad.
Supporting information
Figure S1. Clinical and histological manifestations of dermal edema and fibrosis. Left column, clinical manifestations of the forehead. Middle column, hematoxylin–eosin (HE) staining, low‐power magnification. Right column, HE staining, high‐power magnification.
Figure S2. Histological manifestations of mucin deposition and elastic fiber degeneration. Left column, Alcian blue staining. Middle column, elastica van Gieson (EVG) staining, low‐power magnification. Right column, EVG staining, high‐power magnification. The histological features in the biopsy from patient 1 are milder than those of the other patients. Only mucin deposition, focal edema and partial elastic fiber loss were observed. These three histological findings were also present in the samples obtained from all other patients. The histological features in the biopsies of patients 2–6 showed fibrosis and sebaceous gland hyperplasia, which tended to become more prominent as the severity of pachydermia increased.
Figure S3. Representative histological findings of the control used in this study. The sections were stained as indicated. The histological scores were as follows: dermal edema (0), mucin deposition (0), elastic fiber degeneration (0), sebaceous gland hyperplasia (0; occupation rate of 3.33%) and dermal fibrosis (0).
Acknowledgments
We thank the patients and their families for their generous cooperation. This work was supported by a grant from the National Center for Child Health and Development (to H. N.).
Conflict of interest
Nothing to declare.
References
- 1. Castori M, Sinibaldi L, Mingarelli R, Lachman RS, Rimoin DL, Dallapiccola B. Pachydermoperiostosis: an update. Clin Genet 2005; 68: 477–486. [DOI] [PubMed] [Google Scholar]
- 2. Sasaki T, Niizeki H, Shimizu A et al Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype‐genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci 2012; 68: 36–44. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z, Xia W, He J et al Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet 2012; 90: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakahigashi K, Otsuka A, Doi H et al Prostaglandin E2 increase in pachydermoperiostosis without 15‐hydroprostaglandin dehydrogenase mutations. Acta Derm Venereol 2013; 93: 118–119. [DOI] [PubMed] [Google Scholar]
- 5. Tanese K, Wakabayashi A, Yamamoto K, Miyakawa S, Imanishi N. Complete form of pachydermoperiostosis: case report. Rinsho Hifuka 2010; 64: 221–224. [Google Scholar]
- 6. Niitsuma K, Hatoko M, Tada H, Tanaka A, Yurugi S. A case of pachydermoperiostosis treated with plastic surgery using tissue expander. J Jpn Soc Plast Reconstr Surg 2004; 24: 548–553. [Google Scholar]
- 7. Niizeki H, Shiohama A, Sasaki T et al The complete type of pachydermoperiostosis: a novel nonsense mutation p. E141* of the SLCO2A1 gene. J Dermatol Sci 2014; 75: 193–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Clinical and histological manifestations of dermal edema and fibrosis. Left column, clinical manifestations of the forehead. Middle column, hematoxylin–eosin (HE) staining, low‐power magnification. Right column, HE staining, high‐power magnification.
Figure S2. Histological manifestations of mucin deposition and elastic fiber degeneration. Left column, Alcian blue staining. Middle column, elastica van Gieson (EVG) staining, low‐power magnification. Right column, EVG staining, high‐power magnification. The histological features in the biopsy from patient 1 are milder than those of the other patients. Only mucin deposition, focal edema and partial elastic fiber loss were observed. These three histological findings were also present in the samples obtained from all other patients. The histological features in the biopsies of patients 2–6 showed fibrosis and sebaceous gland hyperplasia, which tended to become more prominent as the severity of pachydermia increased.
Figure S3. Representative histological findings of the control used in this study. The sections were stained as indicated. The histological scores were as follows: dermal edema (0), mucin deposition (0), elastic fiber degeneration (0), sebaceous gland hyperplasia (0; occupation rate of 3.33%) and dermal fibrosis (0).


