Summary
Patients with mantle cell lymphoma (MCL) generally respond to first‐line immunochemotherapy, but often show chemoresistance upon subsequent relapses, with poor outcome. Several studies of the immunomodulator, lenalidomide, have demonstrated its activity in MCL including the MCL‐001 study in relapsed/refractory patients who had failed defined prior therapies of anthracyclines or mitoxantrone, cyclophosphamide, rituximab and also bortezomib. We present here the long‐term efficacy follow‐up of the prospective phase II MCL‐001 study (N = 134), including new exploratory analyses with baseline Ki‐67 (MIB1), a biological marker of tumour proliferation. With longer follow‐up, lenalidomide showed a 28% overall response rate [ORR; 8% complete response (CR)/CR unconfirmed (CRu)]. Median duration of response (DOR), progression‐free survival and overall survival were 16·6, 4·0 and 20·9 months, respectively. Myelosuppression continued to be the most common grade 3/4 toxicity. Several studies of MCL patients treated with chemotherapy, rituximab and bortezomib have shown an inverse association between survival and Ki‐67. Ki‐67 data in 81/134 MCL‐001 patients showed similar ORRs in both low (<30% or <50%) versus high (≥30% or ≥50%) Ki‐67–expressing groups, yet lower Ki‐67 levels demonstrated superior CR/CRu, DOR and survival outcomes. Overall, lenalidomide showed durable efficacy with a consistent safety profile in heavily pretreated, relapsed/refractory MCL post‐bortezomib.
Keywords: efficacy, Ki‐67, lenalidomide, mantle cell lymphoma, safety
Mantle cell lymphoma (MCL) constitutes 3–6% of all non‐Hodgkin lymphoma (NHL) cases (The NHL Classification Project, 1997; Turner et al, 2005; Zhou et al, 2008), is generally found in older males (i.e. ≥60 years) and presents with advanced‐stage, disseminated disease (The NHL Classification Project, 1997; Goy & Kahl, 2011). Initial treatment for MCL relies on chemoimmunotherapy combinations; however, subsequent lines of treatment are less clearly defined. Relapsed/refractory disease is characterized by frequent chemoresistance and remains challenging to manage; patients historically demonstrated a median overall survival (OS) of 1–2 years in the pre‐rituximab and pre‐bortezomib era (Goy & Kahl, 2011; McKay et al, 2012; Vose, 2012). At present, relapsed patients have already experienced rituximab and bortezomib treatment, thus presenting with a more resilient form of disease upon relapse.
Lenalidomide is an oral immunomodulator with established anti‐neoplastic and antiproliferative effects in NHL tumour models (Wu et al, 2008; Zhang et al, 2009; Qian et al, 2011). The clinical activity and tolerable safety profile of lenalidomide have been established in 2 phase II studies (NHL‐002 and NHL‐003) of heavily pretreated patients with relapsed/refractory aggressive NHL, including MCL (Wiernik et al, 2008; Habermann et al, 2009; Witzig et al, 2011; Zinzani et al, 2013). Responses were notable in patients with relapsed/refractory MCL; those from the NHL‐002 study demonstrated a 53% overall response rate (ORR) and 20% complete response (CR), along with durable activity shown by a median duration of response (DOR) of 13·7 months (Wiernik et al, 2008; Witzig et al, 2011). MCL patients from the NHL‐003 study showed a 35% ORR, 12% CR/CR unconfirmed (CRu), and a median DOR of 16·3 months, as assessed by an independent central review committee (Zinzani et al, 2013). Median progression‐free survival (PFS) in the NHL‐002 and NHL‐003 studies was 5·6 and 8·8 months, respectively (Wiernik et al, 2008; Witzig et al, 2011; Zinzani et al, 2013). The safety profile of single‐agent lenalidomide in both studies (NHL‐002 and NHL‐003, respectively) showed that neutropenia (40% and 46%) and thrombocytopenia (33% and 30%) were the most common grade 3/4 adverse events (AEs) in patients with MCL (Habermann et al, 2009; Zinzani et al, 2013). These studies laid the foundation for the MCL‐001 (EMERGE™) phase II study of efficacy and safety of single‐agent lenalidomide in heavily pretreated patients [median 4 prior therapies (range, 2–10) including required prior anthracycline or mitoxantrone, cyclophosphamide, rituximab and relapsed, progressed or refractory to bortezomib] (Goy et al, 2013). Results provided by the MCL‐001 study led to the approval of lenalidomide in 2013 by the US Food and Drug Administration for MCL patients whose disease has relapsed or progressed after 2 prior therapies, including bortezomib (https://www.celgene.com/content/uploads/revlimid_full_prescribing_info.pdf, Goy et al, 2013).
For MCL patients, the MCL International Prognostic Index (MIPI) provides an independent approach to characterize MCL patients into low‐, intermediate‐, and high‐risk groups based on age, Eastern Cooperative Oncology Group performance status (ECOG PS), white blood cell counts and lactate dehydrogenase values (Hoster et al, 2008). In relation to MIPI endpoints, the prognostic relevance of the biological marker of cell proliferation Ki‐67 (MIB1) was initially explored in patients with a 10% elevation, although the standard cut‐off is 30%, and has also been examined in several retrospective analyses as a surrogate measure of malignant cell proliferation (Tiemann et al, 2005; Hoster et al, 2008). Ki‐67 as a surrogate marker of proliferation can be measured in several ways and defined by gene expression profiling, in which Ki‐67 is measured using immunohistochemistry (IHC), thus the term Ki‐67 is used rather than MIB1. The small dataset available for Ki‐67 detection, and challenges associated with standardization and reproducibility of results, have limited the use of Ki67 as a valid biological index along with MIPI scoring (Hoster et al, 2008). However, most studies have confirmed high Ki‐67 levels associated with shorter OS in MCL patients in the setting of chemoimmunotherapy (anti‐CD20 monoclonal antibodies) and bortezomib (Tiemann et al, 2005; Goy et al, 2010; Salek et al, 2014). We explore here the impact of Ki‐67 expression level on the outcome of patients treated with lenalidomide in the pivotal trial (after failing a minimum of four agents) along with longer‐term follow‐up of efficacy and safety data for the MCL‐001 study.
Patients and methods
Study design
MCL‐001 (EMERGE™) was an open‐label, global, multicentre phase II study (NCT00737529) that examined the efficacy and safety of lenalidomide in relapsed/refractory patients with MCL following bortezomib. Detailed study design and eligibility criteria have been previously described (Goy et al, 2013). Lenalidomide was self‐administered orally at 25 mg/d on days 1–21 of each 28‐d cycle until progressive disease, intolerability or voluntary withdrawal. Longer‐term efficacy and safety results are reported here, with a data cut‐off of 20 March 2013, which was an additional 8 months of follow‐up from the initial report. In addition, a new retrospective analysis of lenalidomide activity and its potential relationship with Ki‐67 (by IHC) is also reported.
Patients
Patients were ≥18 years old with an ECOG PS of 0–2 and at least one measurable lesion ≥2 cm by computerized tomography. Diagnosis of MCL was reviewed by a central pathology laboratory for cyclin D1 overexpression by IHC or for t[11;14][q13;q32] by fluorescence in situ hybridization. Patients were required to have failed prior regimens containing anthracycline (or mitoxantrone), cyclophosphamide, rituximab and bortezomib. Bortezomib failure was defined as a relapse or progression ≤12 months from the last dose of bortezomib following a CR or partial response (PR) or refractoriness with less than a PR after ≥2 cycles of bortezomib. There were no limitations to the number of prior therapies.
Assessments and statistical analyses
This study was approved at each institution by the Institutional Review Board/Independent Ethics Committee and in accordance with local rules and regulations. Each study site reviewed the study protocol and patient‐provided informed consent prior to initiation. Ethical requirements per International Conference on Harmonization, Title 21 of the US Code of Federal Regulations and Declaration of Helsinki were met.
Primary endpoints were ORR and DOR. Secondary endpoints included CR/CRu, time to response (TTR), PFS, OS, and safety. Correlation analysis of Ki‐67 levels and efficacy outcomes was an exploratory endpoint. Longer‐term response rates and time‐to‐event data were analysed by independent central reviewers according to the modified International Workshop Lymphoma Response Criteria (Cheson et al, 1999; Fisher et al, 2006; Kane et al, 2007) and Kaplan–Meier estimates (Kaplan & Meier, 1958), respectively. AEs were categorized by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Exploratory Ki‐67 baseline data were examined retrospectively from 31 of 42 institutions (50% academic‐based). Only baseline Ki‐67 values were captured (no biopsies were examined post‐baseline at subsequent relapses). As a result, baseline Ki‐67 expression data were available in 81 of 134 patients (60%) based on IHC performed by a central pathology laboratory using tumour biopsy samples (n = 24) or based on Ki‐67 scores reported in local pathology reports (n = 57). Cut‐off values of 30% and 50% were used for efficacy analyses; these values were chosen based on prior evaluation of Ki‐67 expression in patients with newly diagnosed and relapsed/refractory MCL (Determann et al, 2008; Goy et al, 2010) and the univariate significance (but not bivariate) for OS with Ki‐67 elevated by 10% when evaluated as part of the MIPI scoring system (Hoster et al, 2008). Fisher's exact test was used for comparing binary variables. Kaplan–Meier method and log‐rank test were used for comparing time‐to‐event variables. A P‐value less than 0·05 was considered statistically significant.
Results
Patient characteristics
Baseline characteristics for these 134 patients were previously reported (Goy et al, 2013). In brief, the median age of patients was 67 years (range, 43–83); 63% of patients were ≥65 years of age. At baseline, 93% of patients had stage III/IV disease, 67% had intermediate‐high MIPI score, 57% showed high tumour burden (≥1 lesion with a diameter of ≥5 cm or ≥3 lesions with a diameter of ≥3 cm), and 33% showed bulky disease (≥1 lesion with a diameter of ≥7 cm). Patients had received a median of 4 prior therapies (range, 2–10); all patients had received prior bortezomib (60% were refractory) and 55% were refractory to their last therapy.
Efficacy
With an additional 8 months of follow‐up, longer‐term efficacy analyses showed an ORR of 28% and CR/CRu of 8% (Table 1), with an additional patient achieving a CR/CRu compared with the initial efficacy analysis. Median TTR was 2·3 months for all patients and median TTR to achieve a CR/CRu was 4·1 months. Median DOR was 16·6 months [95% confidence interval (CI), 9·1–26·7] by central review (Fig 1A), which was not reached (NR) for CR/CRu patients [95% CI, 10·4–NR (ongoing at 33·2 months)]. Median PFS was 4·0 months (95% CI, 3·6–6·9; Fig 1B), and median OS was 20·9 months (95% CI, 13·7–24·4; Fig 1C) at a median follow‐up of 13·2 months.
Table 1.
MCL‐001 longer‐term efficacy of lenalidomide (central review; data cut‐off 20 March 2013)
| Efficacy parameter (N = 134) | Longer‐term efficacy results |
|---|---|
| ORR, n (%) | 38 (28) |
| CR/CRu, n (%) | 11 (8) |
| PR, n (%) | 27 (20) |
| SD, n (%) | 39 (29) |
| Median TTR (range) | 2·3 months (1·7–13·1) |
| Median time to CR/CRu (range) | 4·1 months (1·9–13·2) |
| Median DOR (95% CI) | 16·6 months (9·1–26·7) |
| Median PFS (95% CI) | 4·0 months (3·6–6·9) |
| Median OS (95% CI) | 20·9 months (13·7–24·4) |
CI, confidence interval; CR, complete response; CRu, CR unconfirmed; DOR, duration of response; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; PR, partial response; SD, stable disease; TTR, time to response.
Figure 1.
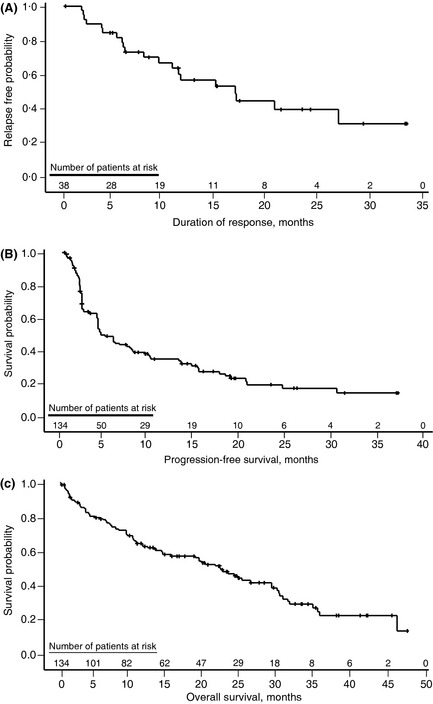
MCL‐001 updated follow‐up for (A) duration of response (DOR), (B) progression‐free survival (PFS) and (C) overall survival (OS) (following lenalidomide in patients with relapsed or refractory mantle cell lymphoma.
Safety
At an average 20 mg/d dose of lenalidomide, the most common grade ≥3 AEs of neutropenia (44%) and thrombocytopenia (28%) were consistent with the known safety profile for lenalidomide in NHL, previous safety evaluations for the MCL‐001 study and other studies of lenalidomide in MCL (Wiernik et al, 2008; Witzig et al, 2011; Goy et al, 2013). At the longer follow‐up time, invasive second primary malignancies (SPM) were reported in four patients. The type of SPM, time to onset and best response by central review included metastatic colon cancer (9·7 months; PR), metastatic squamous cell carcinoma (7·3 months; PR), myelodysplastic syndrome (3·1 months; SD) and bladder cancer metastasis to the liver (40·8 months; CRu). The fourth SPM was newly identified in one patient at this later follow‐up time.
Exploratory analysis of Ki‐67 and efficacy
Baseline Ki‐67 levels were available in 81 of 134 patients and were correlated with efficacy endpoints by using 30% and 50% cut‐off thresholds for Ki‐67 expression. Both thresholds are shown to provide a more thorough view of potential variations in outcomes in MCL depending on the selected threshold level, which varies with the literature (Determann et al, 2008; Hoster et al, 2008; Goy et al, 2010). This exploratory assessment showed similar ORR outcomes in both the lower and higher Ki‐67 groups (Fig 2). In the 50% cut‐off group, it is clear that these patients do not respond as well to lenalidomide because no patients with ≥50% Ki‐67 expression had a CR/CRu or stable disease (SD). For both cut‐off values (30% and 50%), the percentage of patients with SD was inversely proportional to the percentage of patients with lower Ki‐67 expression (i.e. higher percentage of patients with SD in those with lower Ki‐67 levels).
Figure 2.
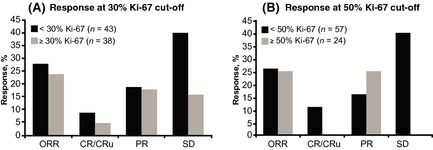
MCL‐001 exploratory analysis of Ki‐67 expression and response following lenalidomide in patients with relapsed/refractory MCL: response rates at (A) 30% and (B) 50% cut‐off thresholds. CR/CRu, complete response/CR unconfirmed; ORR, overall response rate; PR, partial response; and SD, stable disease.
For DOR survival curves with the 30% Ki‐67 cut‐off, the median DOR was not yet reached for either </≥30% or <50% groups, although the median DOR for the ≥50% Ki‐67 group was 5·7 months (95% CI, 1·8–NR). The hazard ratio (HR) for DOR groups ≥30% vs. <30% Ki‐67 was 1·50 (95% CI, 0·37–6·03). For the DOR of the ≥50% vs. <50% Ki‐67 groups, HR was 4·13 (95% CI, 0·92–18·57).
Median PFS for ≥30% vs. <30% Ki‐67 patients was 1·9 months (95% CI, 1·7–5·6) versus 4·5 months (95% CI, 3·7–7·6), respectively, with a HR of 1·54 (95% CI, 0·91–2·62). In the ≥50% vs. <50% Ki‐67 patient groups, median PFS was 1·8 months (95% CI, 1·6–2·8) versus 4·5 months (95% CI, 3·7–7·2), respectively, with a HR of 2·10 (95% CI, 1·19–3·68).
OS was shorter for the group with ≥30% Ki‐67 compared with the group with <30% Ki‐67 [9·7 months (95% CI, 3·4–18·6) versus 28·4 months (95% CI, 19·5–34·7), P = 0·0197; HR = 1·91 (95% CI, 1·10–3·33); Fig 3]. A similar trend was observed for OS using the 50% cut‐off for Ki‐67 expression, including 7·4 months (95% CI, 2·7–19·0) for ≥50% Ki‐67 vs 23·9 months (95% CI, 13·7–34·7) for <50% Ki‐67 [P = 0·0048; HR = 2·21 (95% CI, 1·25–3·89)].
Figure 3.
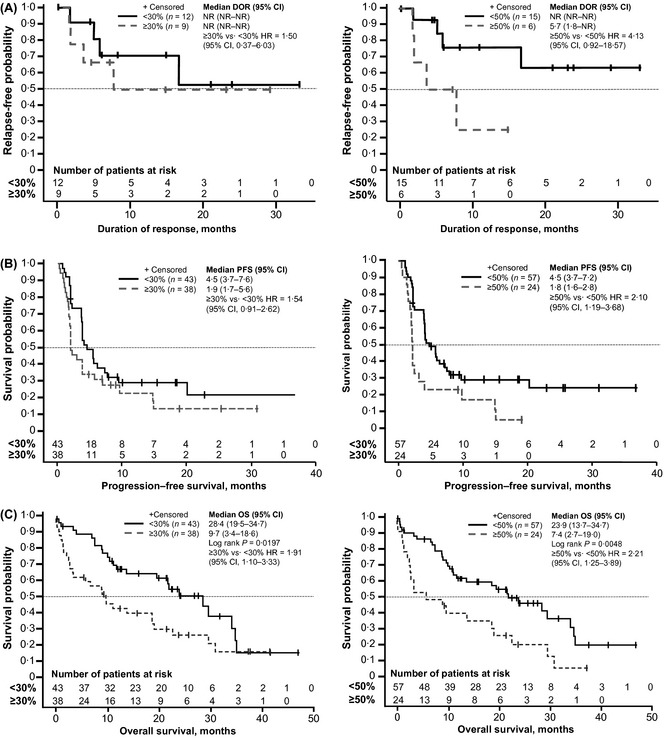
MCL‐001 exploratory analysis of Ki‐67 expression (30% and 50% cut‐offs) and (A) duration of response (DOR), (B) progression‐free survival (PFS) and (C) overall survival (OS). 95% CI, 95% confidence interval; HR, hazard ratio; NR, not reached.
Univariate and multivariate analyses for OS were conducted for Ki‐67 with cut‐offs of 30% and 50%; both showed Ki‐67 as a strong independent prognostic factor after adjusting for known prognostics factors. At the ≥30% vs. <30% Ki‐67 cut‐off, univariate and multivariate analyses showed respective HRs of 1·91 (95% CI, 1·10–3·33; P = 0·022) and 1·87 (95% CI, 1·07–3·25; P = 0·028). For ≥50% vs. <50% Ki‐67 cut‐off, univariate and multivariate analyses showed HR of 2·21 (95% CI, 1·25–3·89; P = 0·006) and 2·02 (95% CI, 1·14–3·60; P = 0·017), respectively. No apparent correlation was identified between Ki‐67 and various prognostic factors.
Discussion
Our exploratory retrospective Ki‐67 analyses showed similar ORR for both low (<30% or <50%) and high (≥30% or ≥50%) Ki‐67–expressing groups, suggesting lenalidomide is active in patients expressing high levels of Ki‐67. Despite similar responses in the low and high Ki‐67‐expressing groups, the DOR, PFS and OS outcomes were worse in those patients with high Ki‐67 expression compared with those with low Ki‐67 expression. This finding is in agreement with reports in the literature for patients with MCL and high Ki‐67 levels; patients demonstrated worse prognosis for survival irrespective of treatment and throughout relapse (Determann et al, 2008; Hoster et al, 2008; Goy et al, 2010). However, it should be noted that the exploratory analysis of Ki‐67 expression and efficacy outcomes in this longer follow‐up of the MCL‐001 study are limited by the small number of patients and lack of prospective evaluation of Ki‐67 expression in correlation with post‐baseline time points when efficacy analyses were performed. Identification of new treatments (as single‐agents or in combination) that improve survival remains a priority for MCL patients with high Ki‐67 levels. The single agent activity of lenalidomide in the MCL‐001 study established the foundation for future combination strategies, as has been shown when lenalidomide is combined with rituximab (R 2) in 31 previously untreated MCL patients (74% with a Ki‐67 index <30%) (Ruan et al, 2013). R 2 treatment led to a 77% ORR (40% CR/CRu) and median PFS and DOR not yet reached. Ki‐67 will be studied prospectively in the ECOG E1411 study (NCT01415752) of lenalidomide/rituximab as maintenance therapy following first‐line bendamustine/rituximab/bortezomib in previously untreated MCL. Additional ongoing studies of lenalidomide combined with chemotherapy or post‐chemotherapy (i.e. maintenance), as well as combinations with other biologicals, will help refine this effect.
Longer median follow‐up for an additional 8 months of patients in the MCL‐001 trial confirmed a consistent safety profile along with rapid and durable activity of lenalidomide in MCL patients who failed multiple prior therapies, including bortezomib, and 63% of whom were ≥65 years of age. At a median follow‐up of 13·2 months, the ORR was 28%, with a CR/CRu rate of 8%. Median TTR was 2·3 months for all patients, with approximately 2 additional months of treatment to achieve a CR/CRu (median TTR of 4·1 months to achieve CR/CRu). Median DOR was 16·6 months, not yet reached for CR/CRu patients (longest ongoing CR at 33·2 months), and median PFS and OS were 4·0 and 20·9 months, respectively. These data are important in demonstrating that rapid and durable activity with lenalidomide may be achieved in patients who were heavily pretreated with a median of 4 prior therapies (range, 2–10), including failure to prior bortezomib, cyclophosphamide, anthracyclines and rituximab.
The safety profile for lenalidomide in this longer‐term evaluation was consistent with previous reports of lenalidomide in MCL (Habermann et al, 2009; Zinzani et al, 2013), as well as the initial report of the MCL‐001 study (Goy et al, 2013). Myelosuppression was the most common toxicity in this longer‐term study and was manageable with dose modifications and/or supportive therapy. Four invasive SPMs were reported at the longer follow‐up and included metastatic colon cancer, metastatic squamous cell carcinoma, myelodysplastic syndrome and an additional bladder cancer that metastasized to the liver.
In summary, these longer‐term analyses of the MCL‐001 study confirmed the rapid and durable efficacy of lenalidomide monotherapy with a consistent safety profile in heavily pretreated patients with MCL. Exploratory analyses of the expression of Ki‐67, a biomarker indicating poor prognosis when elevated, showed that lenalidomide was active in MCL patients with both low and high Ki‐67 expression; however, further study is needed. Through this longer efficacy and safety follow‐up from the MCL‐001 study, lenalidomide continues to demonstrate its activity in heavily pretreated patients with relapsed/refractory MCL, regardless of poor prognostic characteristics, such as high tumour burden, multiple prior therapies, refractoriness to last therapy and high Ki‐67 expression. The favourable and manageable safety profile of lenalidomide in relapsed/refractory MCL offers an opportunity to improve over current therapies in MCL either in combinations with chemoimmunotherapy or as maintenance strategies; these are all currently being tested, including non‐chemotherapy options that are very appealing in a population often comprised of elderly patients.
Author contributions
All authors performed and/or designed the research study, analysed the data, contributed to the development and revision of the manuscript, and approved the final version for submission.
Disclosure and competing interests statement
AG, RR, and TEW have received research funding from Celgene. AG has been a consultant/advisor and part of a speaker's bureau for Celgene. JD has received honoraria from Celgene. LZ, SC, and TF are employees of Celgene and have received Celgene stock and stock options. SKB, MJR, IA, JMR, RH, and AVH have no financial relationships to disclose.
Acknowledgements
We thank the investigators on the MCL‐001 clinical study and all patients, families, and caregivers who participated in this study. This study was supported by Celgene Corporation, Summit, NJ. Medical writing assistance was provided by Julie Kern, PhD with Bio Connections, LLC, and funded by Celgene Corporation; the authors were fully responsible for content and editorial decisions for this manuscript. We also thank Stephen Chen, MS for providing programming support for the manuscript.
Prior presentation: Preliminary results were previously presented at the 2013 American Society of Hematology meeting: Williams, M.E., Besisik, S.K., Drach, J., Ramchandren, R., Robertson, M.J., Avivi, I., Rowe, J.M., Herbrecht, R., Van Hoof, A., Egyed, M., Zhang, L., Cicero, S., Fu, T., Heise, C., Witzig, T.E. (2013) Updated Efficacy and Safety, and Exploratory Ki‐67 Results for the MCL‐001 Study of Lenalidomide in Mantle Cell Lymphoma Patients Who Relapsed or Were Refractory to Bortezomib. Blood, 122, 3057.
References
- Cheson, B.D. , Horning, S.J. , Coiffier, B. , Shipp, M.A. , Fisher, R.I. , Connors, J.M. , Lister, T.A. , Vose, J. , Grillo‐Lopez, A. , Hagenbeek, A. , Cabanillas, F. , Klippensten, D. , Hiddemann, W. , Castellino, R. , Harris, N.L. , Armitage, J.O. , Carter, W. , Hoppe, R. & Canellos, G.P. (1999) Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology, 17, 1244–1253. [DOI] [PubMed] [Google Scholar]
- Determann, O. , Hoster, E. , Ott, G. , Wolfram Bernd, H. , Loddenkemper, C. , Leo Hansmann, M. , Barth, T.E. , Unterhalt, M. , Hiddemann, W. , Dreyling, M. & Klapper, W. ; European Mantle Cell Lymphoma Network and the German Low Grade Lymphoma Study Group . (2008) Ki‐67 predicts outcome in advanced‐stage mantle cell lymphoma patients treated with anti‐CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood, 111, 2385–2387. [DOI] [PubMed] [Google Scholar]
- Fisher, R.I. , Bernstein, S.H. , Kahl, B.S. , Djulbegovic, B. , Robertson, M.J. , de Vos, S. , Epner, E. , Krishnan, A. , Leonard, J.P. , Lonial, S. , Stadtmauer, E.A. , O'Connor, O.A. , Shi, H. , Boral, A.L. & Goy, A. (2006) Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology, 24, 4867–4874. [DOI] [PubMed] [Google Scholar]
- Goy, A. & Kahl, B. (2011) Mantle cell lymphoma: the promise of new treatment options. Critical Reviews in Oncology Hematology, 80, 69–86. [DOI] [PubMed] [Google Scholar]
- Goy, A. , Bernstein, S.H. , McDonald, A. , Pickard, M.D. , Shi, H. , Fleming, M.D. , Bryant, B. , Trepicchio, W. , Fisher, R.I. , Boral, A.L. & Mulligan, G. (2010) Potential biomarkers of bortezomib activity in mantle cell lymphoma from the phase 2 PINNACLE trial. Leukaemia & Lymphoma, 51, 1269–1277. [DOI] [PubMed] [Google Scholar]
- Goy, A. , Sinha, R. , Williams, M.E. , Kalayoglu Besisik, S. , Drach, J. , Ramchandren, R. , Zhang, L. , Cicero, S. , Fu, T. & Witzig, T.E. (2013) Single‐agent lenalidomide in patients with mantle‐cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL‐001 (EMERGE) study. Journal of Clinical Oncology, 31, 3688–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann, T.M. , Lossos, I.S. , Justice, G. , Vose, J.M. , Wiernik, P.H. , McBride, K. , Wride, K. , Ervin‐Haynes, A. , Takeshita, K. , Pietronigro, D. , Zeldis, J.B. & Tuscano, J.M. (2009) Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. British Journal of Haematology, 145, 344–349. [DOI] [PubMed] [Google Scholar]
- Hoster, E. , Dreyling, M. , Klapper, W. , Gisselbrecht, C. , van Hoof, A. , Kluin‐Nelemans, H.C. , Pfreundschuh, M. , Reiser, M. , Metzner, B. , Einsele, H. , Peter, N. , Jung, W. , Wormann, B. , Ludwig, W.D. , Duhrsen, U. , Eimermacher, H. , Wandt, H. , Hasford, J. , Hiddemann, W. & Unterhalt, M. (2008) A new prognostic index (MIPI) for patients with advanced‐stage mantle cell lymphoma. Blood, 111, 558–565. [DOI] [PubMed] [Google Scholar]
- Kane, R.C. , Dagher, R. , Farrell, A. , Ko, C.W. , Sridhara, R. , Justice, R. & Pazdur, R. (2007) Bortezomib for the treatment of mantle cell lymphoma. Clinical Cancer Research, 13, 5291–5294. [DOI] [PubMed] [Google Scholar]
- Kaplan, E.L. & Meier, P. (1958) Nonparametric estimation from incomplete observations. Journal of American Statistical Association, 53, 457–481. [Google Scholar]
- McKay, P. , Leach, M. , Jackson, R. , Cook, G. & Rule, S. (2012) Guidelines for the investigation and management of mantle cell lymphoma. British Journal of Haematology, 159, 405–426. [DOI] [PubMed] [Google Scholar]
- Qian, Z. , Zhang, L. , Cai, Z. , Sun, L. , Wang, H. , Yi, Q. & Wang, M. (2011) Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia Research, 35, 380–386. [DOI] [PubMed] [Google Scholar]
- Ruan, J. , Martin, P. , Shah, B.D. , Schuster, S.J. , Smith, S.M. , Furman, R.R. , Christos, P. , Rodriguez, A. , Wolstencroft, P. , Svoboda, J. , Bender, A. , Lewis, J. , Coleman, M. & Leonard, J.P. (2013) Combination biologic therapy without chemotherapy as initial treatment for mantle cell lymphoma: multi‐center phase II study of lenalidomide plus rituximab. Blood (ASH Annual Meeting Abstracts), 122, Abstract 247. [Google Scholar]
- Salek, D. , Vesela, P. , Boudova, L. , Janikova, A. , Klener, P. , Vokurka, S. , Jankovska, M. , Pytlik, R. , Belada, D. , Pirnos, J. , Moulis, M. , Kodet, R. , Michal, M. , Janousova, E. , Muzik, J. , Mayer, J. & Trneny, M. (2014) Retrospective analysis of 235 unselected patients with mantle cell lymphoma confirms prognostic relevance of Mantle Cell Lymphoma International Prognostic Index and Ki‐67 in the era of rituximab: long‐term data from the Czech Lymphoma Project Database. Leukaemia & Lymphoma, 55, 802–810. [DOI] [PubMed] [Google Scholar]
- The Non‐Hodgkin's Lymphoma Classification Project . (1997) A clinical evaluation of the International Lymphoma Study Group classification of non‐Hodgkin's lymphoma. The Non‐Hodgkin's Lymphoma Classification Project. Blood, 89, 3909–3918. [PubMed] [Google Scholar]
- Tiemann, M. , Schrader, C. , Klapper, W. , Dreyling, M.H. , Campo, E. , Norton, A. , Berger, F. , Kluin, P. , Ott, G. , Pileri, S. , Pedrinis, E. , Feller, A.C. , Merz, H. , Janssen, D. , Hansmann, M.L. , Krieken, H. , Moller, P. , Stein, H. , Unterhalt, M. , Hiddemann, W. & Parwaresch, R. (2005) Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. British Journal of Haematology, 131, 29–38. [DOI] [PubMed] [Google Scholar]
- Turner, J.J. , Hughes, A.M. , Kricker, A. , Milliken, S. , Grulich, A. , Kaldor, J. & Armstrong, B. (2005) WHO non‐Hodgkin's lymphoma classification by criterion‐based report review followed by targeted pathology review: an effective strategy for epidemiology studies. Cancer Epidemiology, Biomarkers & Prevention, 14, 2213–2219. [DOI] [PubMed] [Google Scholar]
- Vose, J.M. (2012) Mantle cell lymphoma: 2012 update on diagnosis, risk‐stratification, and clinical management. American Journal of Hematology, 87, 604–609. [DOI] [PubMed] [Google Scholar]
- Wiernik, P.H. , Lossos, I.S. , Tuscano, J.M. , Justice, G. , Vose, J.M. , Cole, C.E. , Lam, W. , McBride, K. , Wride, K. , Pietronigro, D. , Takeshita, K. , Ervin‐Haynes, A. , Zeldis, J.B. & Habermann, T.M. (2008) Lenal idomide monotherapy in relapsed or refractory aggressive non‐Hodgkin's lymphoma. Journal of Clinical Oncology, 26, 4952–4957. [DOI] [PubMed] [Google Scholar]
- Witzig, T.E. , Vose, J.M. , Zinzani, P.L. , Reeder, C.B. , Buckstein, R. , Polikoff, J.A. , Bouabdallah, R. , Haioun, C. , Tilly, H. , Guo, P. , Pietronigro, D. , Ervin‐Haynes, A.L. & Czuczman, M.S. (2011) An international phase II trial of single‐agent lenalidomide for relapsed or refractory aggressive B‐cell non‐Hodgkin's lymphoma. Annals of Oncology, 22, 1622–1627. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Adams, M. , Carter, T. , Chen, R. , Muller, G. , Stirling, D. , Schafer, P. & Bartlett, J.B. (2008) Lenalidomide enhances natural killer cell and monocyte‐mediated antibody‐dependent cellular cytotoxicity of rituximab‐treated CD20+ tumor cells. Clinical Cancer Research, 14, 4650–4657. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Qian, Z. , Cai, Z. , Sun, L. , Wang, H. , Bartlett, J.B. , Yi, Q. & Wang, M. (2009) Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. American Journal of Hematology, 84, 553–559. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Wang, H. , Fang, W. , Romaguer, J.E. , Zhang, Y. , Delasalle, K.B. , Kwak, L. , Yi, Q. , Du, X.L. & Wang, M. (2008) Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer, 113, 791–798. [DOI] [PubMed] [Google Scholar]
- Zinzani, P.L. , Vose, J.M. , Czuczman, M.S. , Reeder, C.B. , Haioun, C. , Polikoff, J. , Tilly, H. , Zhang, L. , Prandi, K. , Li, J. & Witzig, T.E. (2013) Long‐term follow‐up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL‐003 study. Annals of Oncology, 24, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]


